Abstract
Background:
The administration of oral pregabalin preoperatively has been reported to reduce acute postoperative pain. However, no clinical study to date has yet fully investigated whether or not pregabalin premedication affects sensory and motor blocks using spinal anesthesia and its effect upon early postoperative pain management. This prospective, randomized, and double-blind clinical study was designed to evaluate the efficacy of a single dose of pregabalin in terms of spinal blockade duration and its potential opioid-sparing effect during the first 24 hours subsequent to urogenital surgery.
Methods:
Forty-four patients scheduled for urogenital surgery under spinal anesthesia were randomly allocated to 2 groups: group C (no premedication; orally administered placebo 2 hours before surgery) and group P (orally administered 150 mg pregabalin 2 hours before surgery).
Results:
The duration of sensory and motor blockade was significantly prolonged in group P patients when compared with that in group C patients, and the pain scores at postoperative 6 and 24 hours were significantly lower in group P patients. Requests for analgesics during the first postoperative 24 hours were lower among group P patients.
Conclusion:
Premedication with a single dose of 150 mg pregabalin before surgery promoted the efficacy of intrathecal bupivacaine and improved postoperative analgesia in patients undergoing urogenital surgery under spinal anesthesia.
Keywords: preemptive, pregabalin, sensory block, spinal anesthesia
1. Introduction
Various adjuvants have been used to prolong spinal anesthesia, with the additional advantages of delaying the onset of postoperative pain and reducing postoperative analgesic requirements.[1–3] Pregabalin is an r-aminobutyric acid analog that binds to the α2-δ subunit of presynaptic voltage-gated calcium channels. It reduces the depolarization-induced calcium influx at nerve terminals, with a consequent reduction in the release of several excitatory neurotransmitters, including glutamate, noradrenaline, substance P, and gastrin-releasing peptide.[4] The administration of oral pregabalin preoperatively has been reported to reduce acute postoperative pain[5–7] and to prolong the duration of anesthesia produced by single-injection peripheral nerve blockade.[8] However, no clinical study to date has yet fully investigated whether or not pregabalin premedication affects sensory and motor blocks using spinal anesthesia and its effect upon early postoperative pain management.
Preemptive analgesia is analgesic administration that precedes the painful stimulus, thus improving postoperative pain control. It is an antinociceptive treatment that prevents the establishment of altered processing of afferent input, which amplifies postoperative pain.[9] This technique is utilized in acute postsurgical pain management to improve the efficacy of analgesics and thereby reduce the requirement for opioids.[10]
In this prospective, randomized, double-blind, and placebo-controlled study, we hypothesized that single dose 150 mg pregabalin premedication would prolong the sensory blockade of spinal bupivacaine anesthesia in urogenital surgery. A secondary objective of this study was to determine if premedication with pregabalin also reduces the need for medication to relieve postoperative pain.
2. Methods
2.1. Patients and exclusion criteria
Patients between the ages of 19 and 70 years with American Society of Anesthesiologists grade I or II scheduled to undergo either transurethral resection of the bladder (TUR-B) or transurethral resection of the prostate (TUR-P) under spinal anesthesia were included in this study. Patients were excluded if they were known to be allergic to any medicines, had a history of drug or alcohol abuse, were taking opioids or sedative medications, and had a history of psychiatric conditions. Patients with a history of taking pregabalin or gabapentin were also excluded.
2.2. Anesthesia and data collection
After receiving Kyungpook National University Institutional Review Board approval (KNUH 2014-05-027), all patients were provided with a written informed consent. This study was registered at www.clinicaltrials.gov (NCT 02690506). Forty-four consecutive patients scheduled to undergo elective urogenital surgery (TUR-B and TUR-P) under spinal anesthesia were enrolled in this randomized, placebo-controlled, and double-blind trial. Cases were divided into 2 randomized groups of 22 patients. Patients were randomized to a treatment group using a computer-generated randomization sequence (Fig. 1). Both the patient and anesthesiologist were double-blinded to the treatment, and all records were recorded by an anesthesiologist blinded to group allocation. Identical capsules of either pregabalin or placebo were prepared by the hospital pharmacy, and a doctor who was not involved in the perioperative evaluation administered the capsule according to the randomization sequence.
Figure 1.
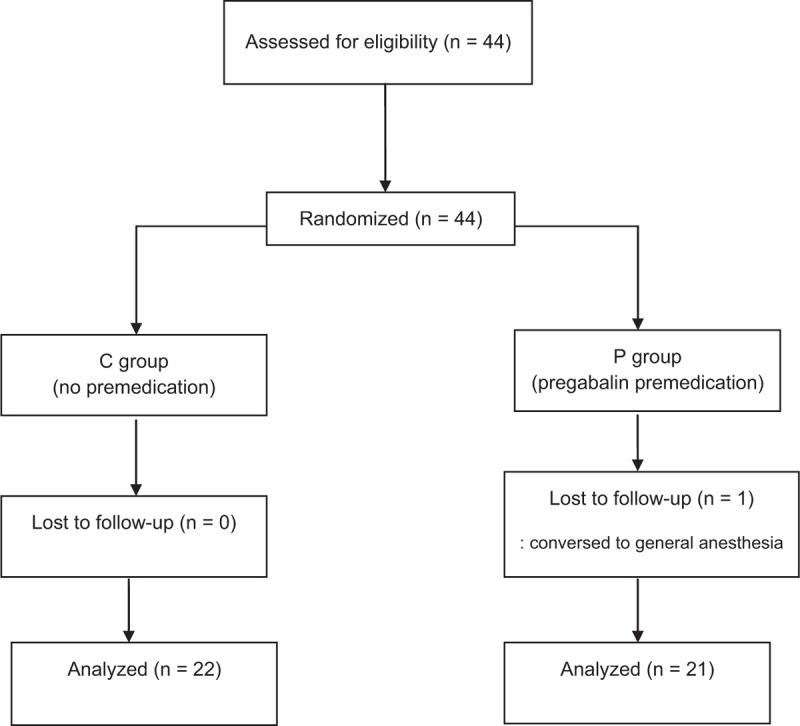
Flow diagram of the study.
Two hours before surgery, the control patients (group C) received capsules containing placebo, and pregabalin patients (group P) received capsules containing 150 mg of pregabalin in the ward. Electrocardiography, blood pressure, and oxygen saturation were monitored upon arrival to the operating room and subsequently every 5 minutes. A transverse line connecting the tops of the right and left iliac crests was defined as the level of L 4 to L 5. First, 2% lidocaine for the purpose of local anesthesia was administered via infiltration at the L 3 to L 4 in the lateral decubitus position. Then, all patients received spinal anesthesia with 2.4 mL of 0.5% hyperbaric bupivacaine through the L 3 to L 4 interspace using a midline approach with a 25-G Quincke needle. Before completion of intrathecal injection, intravenous crystalloid was administrated at 6 mL/kg. After the injection, the patient was returned to the supine position and remained in that position for at least 20 minutes. All patients in this study were positioned similarly during the entire surgical procedure. When the patient experienced either a decrease in systolic blood pressure >30% from baseline values or a mean arterial blood pressure <60 mm Hg, intravenous ephedrine 4 mg or phenylephrine 50 mcg was administered. When the heart rate decreased to <45 bpm, intravenous atropine 0.5 mg was administered. Intraoperative sedation was not provided. Sensory blockade was assessed using a pinprick test in at the midaxillary line on both sides of the chest. Pinprick tests were performed every 1 minute until maximum sensory blockade was achieved in the relevant body segment and subsequently every 5 minutes for the next 30 minutes. Thereafter, assessments were performed every 15 minutes until recovery of sensation in the L2 segment. The time to T10 sensory block, peak sensory level, and time from the injection to the peak level were recorded.
Recovery time from the sensory blockade was defined as a 2-dermatome regression of anesthesia from the maximum level. In addition, immediately after sensory block assessment, the motor block was evaluated using a modified Bromage scale as reported in previous studies (grade 0: no paralysis; grade 1: unable to raise an extended leg but able to move the knees and ankles; grade 2: unable to flex knees, can flex ankle, grade 3: no movement).[11] The time to reach Bromage 1 was recorded. Motor block duration was defined as the time for return to Bromage 2.
Postoperative pain was controlled by rescue analgesics administered by hospital personnel without patient-controlled analgesia. Postoperative pain was assessed by the patient using the visual analog scale (VAS, 0 = no pain; 10 = worst possible pain) at 6 and 24 hours after the operation. Patients with a VAS score of 4 or more received 25 mg pethidine intravenously. If, after pethidine was injected twice, the patient complained of a VAS score of 4 or more, then 50 mg tramadol was given intravenously. The times of the first request for postoperative analgesia and the number of injections were recorded.
The presence of any of the following possible complications during the first 24 hours postoperative was recorded by the nurses in the relevant hospital ward: drowsiness (no eye opening in response to a verbal command), dizziness, dry mouth, and nausea/vomiting.
2.3. Sample size
The sample size for this study was calculated based on a pilot study. We determined that if the difference between the 2 groups was 15 minutes (±standard deviation of 17 minutes) to achieve a sensory regression of 2 dermatomes with an α-error = 0.05 at 80% power, at least 21 patients per group were needed.
2.4. Statistical analysis
Data were analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL). Demographic data and clinical variables were compared using the Student t test, X2 test, or Mann–Whitney–Wilcoxon test as appropriate. Continuous parameters such as age, weight, surgery time, time of the first request for analgesics, and the duration of blockade were compared between 2 groups using the unpaired Student t test. Categorical scales such as adverse effects and the number of postoperative analgesics were analyzed using Fisher test. Associations between the duration of the sensory blockade and the time to the first analgesic request, as well as the time to the first analgesic request and the dosage of analgesics during the first 24 hours postoperative, were analyzed using Pearson correlation. A P value <0.05 was considered significant.
3. Results
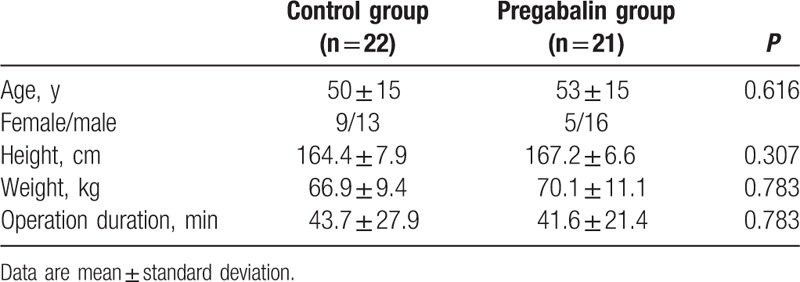
Forty-three patients completed the study according to the protocol and were included in the analysis. One patient received general anesthesia in addition to the initial spinal block because of prolonged surgery. When the 2 groups were compared in terms of age, gender, height, weight, and operation duration, no significant differences were found (Table 1). All surgical procedures were completed without complications.
Table 1.
Demographic characteristics and duration of surgery.
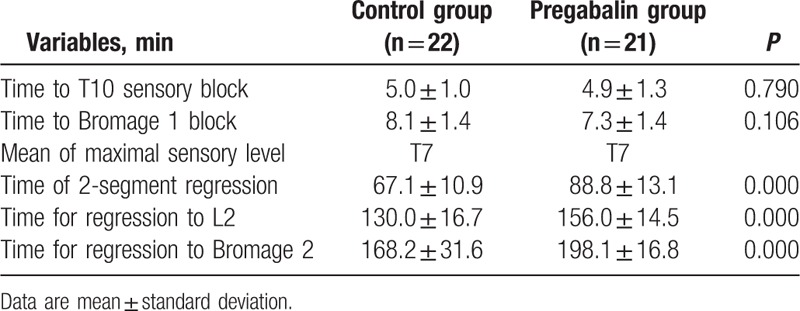
The mean duration of 2-dermatome regression from peak sensory block levels in group P (88.8 ± 13.1 minutes) was significantly longer than in group C (67.1 ± 10.9 minutes) (P = 0.000). The time for regression to L2 sensory block levels was significantly prolonged in group P as well. In addition, the regression time from Bromage 1 to Bromage 2 was prolonged in group P (198 ± 16.8 minutes) than group C (168.2 ± 31.6 minutes) (P = 0.000). The mean time of onset for T10 sensory blockade was similar between the 2 groups. The time to reach Bromage score 1 motor block was not significantly different (P = 0.106), and the maximum level was similar in both groups (Table 2).
Table 2.
Onset time and duration of sensory and motor blocks.
The postoperative 6- and 24-hour VAS pain scores were decreased in group P. The time to the first request for postoperative supplemental analgesia was significantly prolonged in group P (404.0 ± 123.2 minutes) when compared with group C (204.8 ± 37.6 minutes) (P = 0.000). The total rescue dosages of pethidine and tramadol were lower in group P (P = 0.000) (Table 3) (Fig. 2).
Table 3.
Pain score, cumulative analgesics consumption, and time to first postoperative analgesics request during the first 24 hours.
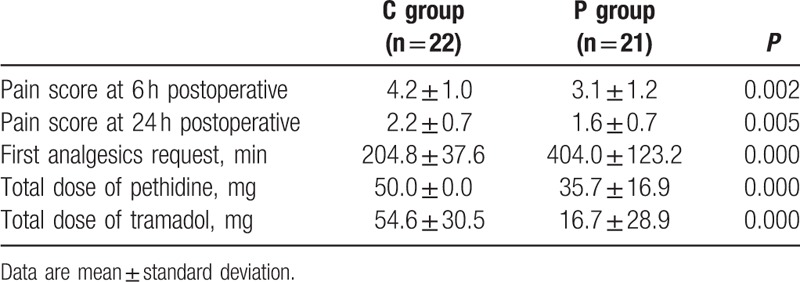
Figure 2.
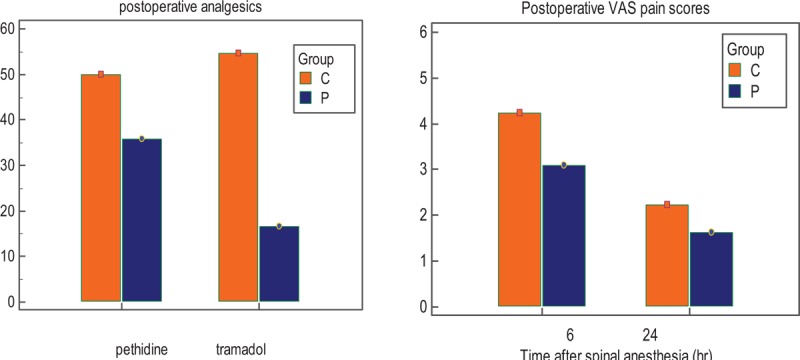
Visual analog scale pain scores and analgesics consumption in the first 24 postoperative hours.
The time for 2-dermatome regression from peak sensory block levels was positively correlated with the time to the first request for postoperative analgesics (r = 0.365, P = 0.016). Further, the time to first request for postoperative analgesics was significantly negatively correlated with the total rescue dosages of pethidine (r = −0.729, P = 0.000) and tramadol (r = −0.670, P = 0.000).
The frequencies of bradycardia and hypotension during intraoperative time did not differ between the groups. No differences were observed in the postoperative adverse effects between the 2 groups during the first 24 hours following surgery (Table 4).
Table 4.
Postoperative adverse effects during the first 24 hours.
4. Discussion
We investigated whether pregabalin premedication prolonged the duration of a sensory block as well as the time to the first request for postoperative analgesics. This study showed that oral pregabalin administered 2 hours before spinal anesthesia prolongs both sensory and motor blocks induced by spinal bupivacaine anesthesia. The time to the first request for postoperative analgesics was delayed, and lower rescue analgesic requirements were observed during the early postoperative 24 hours. The length of the delay to the first request for postoperative analgesics was significantly related to the total dose of postoperative analgesics required.
Meta-analyses have demonstrated that pregabalin leads to a reduction in postoperative pain scores.[6,7] However, only a limited number of studies have been published to date about the efficacy of pregabalin in regional anesthesia,[8,12] and the effect of administration of a single dose of preemptive pregabalin on the effectiveness of spinal anesthesia has not been fully reported to date.
Bafna et al[12] compared the time to the first analgesic request for the groups of single dose 600 mg gabapentin and 150 mg pregabalin premedication in gynecological surgeries under spinal anesthesia. Both medications prolonged the mean duration of effective analgesia of a spinal bupivacaine block, but pregabalin showed significantly longer duration of effective analgesia. In the pregabalin group, the analgesic effect was maintained for 535 minutes (±32.8), which was a longer duration than our result (404 minutes [±123.2]). This might be because of the dose of bupivacaine and the difference between the types of surgery. The efficacy of perioperative administration of gabapentinoids (gabapentin and pregabalin) has been investigated in various surgeries, and clinical studies have confirmed the potential of gabapentinoids as an adjuvant for pain treatment, mostly in acute and persistent postoperative pain.[5,13–16] Gabapentin is a useful adjunct for the management of postoperative pain since it provides analgesia through a different mechanism than opioids and therefore makes a reasonable addition to a multimodal analgesic treatment plan.[17] The use of gabapentin might be limited by its negative side effects, such as dizziness, somnolence, confusion, and ataxia.[16] Pregabalin has antihyperalgesic, anticonvulsant, and anxiolytic properties similar to those of gabapentin, but with fewer side effects as well as dose-independent absorption.
Preemptive usage of pregabalin in infraclavicular nerve blocks has been reported to result in early initiation of the motor block and prolongation of sensory block.[8] Unlike in our study in spinal anesthesia, the duration of the motor block was not prolonged. In addition, 150 and 300 mg doses of pregabalin premedication showed no differences as anxiolytic agents in peripheral nerve blocks.[8] White et al[18] found that preoperative pregabalin administration (70–300 mg) increased perioperative sedation in a dose-related fashion. Single dose of preemptive pregabalin administration was also shown to reduce postoperative pain in third molar dental surgery (75 mg) as well as double-jaw surgery (150 mg) under general anesthesia.[13,15] However, greater side effects were seen with 300 mg pregabalin in patients for dental surgery under general anesthesia.[19] Thus, the basis of previously published studies as well as our own pilot study, we determined that a dose of 150 mg pregabalin premedication was most appropriate for this study. In addition, we observed no significant difference in the postoperative adverse effects between the 2 groups during the first 24 hours following surgery.
The mechanisms by which pregabalin premedication prolongs motor and sensory blocks using local anesthetics in spinal anesthesia are not fully understood. There may be several reasons for the prolongation of spinal anesthesia. Gabapentinoids are an r-aminobutyric acid analog that binds to α2-δ subunit of presynaptic voltage-gated calcium channels, and this inhibition decreases postsynaptic excitability by reducing potassium-evoked excitatory transmitter release. These medications provide antiepileptic, anxiolytic, and analgesic features by modulating both GABAergic neurotransmission and calcium influx. Gabapentinoid compounds produce a significant and clinically important improvement in preoperative anxiety scores.[8,14,20] Since patients may be anxious in the perioperative period, the anxiolytic effects and euphorigenic effects of pregabalin may be beneficial.
In this study, a single dose of 150 mg pregabalin 2 hours before spinal anesthesia showed sufficient efficacy during the first postoperative 24 hours. Pregabalin has an elimination half-time estimated to range from 5.5 to 6.7 hours, which is independent of the dose and frequency of administration.[21] On the other hand, the duration of bupivacaine is approximately 2 to 3 hours. Buvanendran et al[22] found that 6 hours after a single dose of 300 mg pregablain oral administration, the cerebrospinal fluid pregabalin level is high enough to reduce central nervous system hypersensitivity. Evoked pain during movement is enhanced by central neuronal sensitization,[23] and the persistent pregabalin effects observed in our study were likely the result of preoperative pregabalin preventing central nervous system sensitization.
There were limitations to the present study. First, since only 1 dosage of pregabalin was evaluated, we could not determine the most effective dosage. Second, clinically meaningful improvements in recovery were not assessed. Adequate postoperative pain control provides early postsurgical mobilization, shortened hospitalization, and increased patient satisfaction. Third, preoperative pain and anxiety scores were not recorded. Pregabalin might affect the preoperative pain, mood, and anxiety scores, and these factors can be related to the postoperative pain score.
We investigated the effects of single dose preoperative administration of 150 mg pregabalin 2 hours before spinal anesthesia and demonstrated that it prolonged the duration of both sensory and motor blocks. The mean time to the first postoperative analgesic request was delayed by 200 minutes, and the dosage of postoperative analgesics was significantly decreased in the first 24 hours following urogenital surgery relative to the control group. After further assessment of individual pregabalin dosages and types of surgery, these results can be used to improve the quality of acute postoperative recovery.
Footnotes
Abbreviations: TUR-B = transurethral resection of the bladder, TUR-P = transurethral resection of the prostate, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- 1.Kaya FN, Yavascaoglu B, Turker G, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth 2010; 57:39–45. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Li M, Zhang SY, et al. Intravenous dexmedetomidine promotes spinal bupivacaine anesthesia and postoperative analgesia in lower limb surgery: a double-blind, randomized clinical CONSORT study. Medicine 2016; 95:e2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ota K, Namiki A, Iwasaki H, et al. Dose-related prolongation of tetracaine spinal anesthesia by oral clonidine in humans. Anesth Analg 1994; 79:1121–1125. [PubMed] [Google Scholar]
- 4.Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 2004; 45 (suppl 6):13–18. [DOI] [PubMed] [Google Scholar]
- 5.Clarke H, Page GM, McCartney CJ, et al. Pregabalin reduces postoperative opioid consumption and pain for 1 week after hospital discharge, but does not affect function at 6 weeks or 3 months after total hip arthroplasty. Br J Anaesth 2015; 115:903–911. [DOI] [PubMed] [Google Scholar]
- 6.Lam DM, Choi SW, Wong SS, et al. Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine 2015; 94:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015; 114:10–31. [DOI] [PubMed] [Google Scholar]
- 8.Cegin MB, Soyoral L, Yuzkat N, et al. Pregabalin administered as an anxiolytic agent in ultrasound-guided infraclavicular block: a controlled, double-blind, dose-ranging trial. Eur Rev Med Pharmacol Sci 2016; 20:568–574. [PubMed] [Google Scholar]
- 9.Kissin I. Preemptive analgesia. Anesthesiology 2000; 93:1138–1143. [DOI] [PubMed] [Google Scholar]
- 10.Bromley L. Pre-emptive analgesia and protective premedication. What is the difference? Biomed Pharmacother 2006; 60:336–340. [DOI] [PubMed] [Google Scholar]
- 11.Bromage PR, Burfoot MF, Crowell DE, et al. Quality of epidural blockade. I. Influence of physical factors. Br J Anaesth 1964; 36:342–352. [DOI] [PubMed] [Google Scholar]
- 12.Bafna U, Rajarajeshwaran K, Khandelwal M, et al. A comparison of effect of preemptive use of oral gabapentin and pregabalin for acute post-operative pain after surgery under spinal anesthesia. J Anaesthesiol Clin Pharmacol 2014; 30:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahiskalioglu A, Ince I, Aksoy M, et al. Effects of a single-dose of pre-emptive pregabalin on postoperative pain and opioid consumption after double-jaw surgery: a randomized controlled trial. Int J Oral Maxillofac Surg 2016; 74:53.e1–53.e7. [DOI] [PubMed] [Google Scholar]
- 14.Shimony N, Amit U, Minz B, et al. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double-blind, and controlled clinical study. J Neurosurg 2016; 1–10.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CW, Choi WS, Leung YY, et al. A double-blind randomized crossover study to evaluate the timing of pregabalin for third molar surgery under local anesthesia. Int J Oral Maxillofac Surg 2012; 70:25–30. [DOI] [PubMed] [Google Scholar]
- 16.Tiippana EM, Hamunen K, Kontinen VK, et al. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg 2007; 104:1545–1556.table of contents. [DOI] [PubMed] [Google Scholar]
- 17.Short J, Downey K, Bernstein P, et al. A single preoperative dose of gabapentin does not improve postcesarean delivery pain management: a randomized, double-blind, placebo-controlled dose-finding trial. Anesth Analg 2012; 115:1336–1342. [DOI] [PubMed] [Google Scholar]
- 18.White PF, Tufanogullari B, Taylor J, et al. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg 2009; 108:1140–1145. [DOI] [PubMed] [Google Scholar]
- 19.Hill CM, Balkenohl M, Thomas DW, et al. Pregabalin in patients with postoperative dental pain. Eur J Pain 2001; 5:119–124. [DOI] [PubMed] [Google Scholar]
- 20.Menigaux C, Adam F, Guignard B, et al. Preoperative gabapentin decreases anxiety and improves early functional recovery from knee surgery. Anesth Analg 2005; 100:1394–1399.table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg 2007; 105:1805–1815. [DOI] [PubMed] [Google Scholar]
- 22.Buvanendran A, Kroin JS, Kari M, et al. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system? Reg Anesth Pain Med 2010; 35:535–538. [DOI] [PubMed] [Google Scholar]
- 23.Brennan TJ. Frontiers in translational research: the etiology of incisional and postoperative pain. Anesthesiology 2002; 97:535–537. [DOI] [PubMed] [Google Scholar]


