Supplemental Digital Content is available in the text
Keywords: chronic kidney disease, estimated glomerular filtration rate, neck circumference, overweight, proteinuria
Abstract
Chronic kidney disease (CKD) is characterized by increased risks of morbidity and mortality. Upper-body subcutaneous fat, which is commonly estimated from the neck circumference (NC), was revealed to be the main reservoir of circulating nonesterified fatty acids in overweight patients. Despite a close association between NC and metabolic complications, the relationship of NC with renal function has not been fully investigated. In this study, the impact of NC on the development of incident CKD was elucidated.
The data were retrieved from the Korean Genome and Epidemiology Study cohort. The subjects were followed at 2-year intervals from 2003 to 2011. Overweight was defined as a body mass index of ≥23 kg/m2. A total of 4298 cohort subjects were screened. After exclusion, 2268 overweight subjects were included for the final analysis. The primary end point was incident CKD, which was defined as a composite of estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or the development of proteinuria.
The mean patient age was 36.3 ± 3.0 years, and 1285 (56.7%) were men. They were divided into 2 groups according to the median NC in male and female subjects, separately. In both sexes, hypertension (men, P < 0.001; women, P = 0.009) and diabetes (men, P = 0.002; women, P < 0.001) were significantly more prevalent in the big NC group than in the small NC group. In contrast, eGFR was significantly lower only in male subjects of the big NC group (P < 0.001), whereas it was comparable between the small and big NC groups (P = 0.167). In multivariate Cox proportional hazards regression analysis, NC values were independently associated with incident CKD development in female subjects after adjusting for multiple confounding factors (per 1 cm increase, hazard ratio [95% confidence interval] = 1.159 [1.024–1.310], P = 0.019) but not in male subjects.
NC is independently associated with the development of CKD in overweight female subjects, suggesting that it could be a practical risk factor for CKD.
1. Introduction
Chronic kidney disease (CKD) is characterized by increased risks of morbidity and mortality.[1] Owing to continuous and relentless decline in renal function, identification of patients at risk may be crucial for reducing the burden of CKD.[2]
Overweight is a well-known predictor of cardiovascular events and mortality. It was also demonstrated that overweight persons were at a greater risk for a rapid decline in renal function.[3] In addition, longitudinal studies found that there was a close association between adiposity and incident CKD in the general population regardless of age or baseline renal function.[4] Among a variety of fat tissues, visceral adipose tissue (VAT) was revealed to be the main pathogenic source of fat responsible for metabolic derangements in overweight patients.[5] The amount of VAT in overweight patients showed close relationships with insulin resistance, type 2 diabetes, and atherosclerosis.[6,7] However, the association between VAT and kidney diseases was not consistently reported, suggesting that some other fat tissues rather than VAT may play key roles in the pathogenesis of the decline in renal function in overweight patients.
Upper-body subcutaneous fat, which is commonly estimated from the neck circumference (NC),[8–10] has recently been demonstrated to be the main reservoir of circulating nonesterified fatty acids (NEFAs) in overweight patients.[11] Moreover, previous studies found that NEFA concentrations were closely related to insulin resistance and endothelial dysfunction, implying that upper-body subcutaneous fat, besides VAT, could be an important modulator of cardiometabolic risks in obese patients.[12,13] Furthermore, several recent studies showed that NC was more significantly associated with cardiometabolic risk factors than the body mass index (BMI) or waist circumference, further supporting this notion.[10,14] To date, however, the relationship of NC with renal function has not been fully investigated.
In this study, the impact of NC, a representative of upper-body subcutaneous fat, on the development of incident CKD was elucidated in Korean overweight subjects who participated in a community-based prospective cohort study.
2. Methods
2.1. Study population
The present study was performed by using the data from the Korean Genome and Epidemiology Study (KoGES), a prospective community-based cohort study. The study cohort consisted of 40- to 69-year-old residents of Ansan or Ansung City, a city nearby the capital city Seoul. Assessment was conducted biennially from 2003 to 2011. Subjects who had missing data or CKD at baseline and were not overweight or obese based on the World Health Organization Asian criteria (BMI < 23 kg/m2) were excluded. Thus, a total of 2268 subjects were included in the final analysis (Fig. 1).
Figure 1.
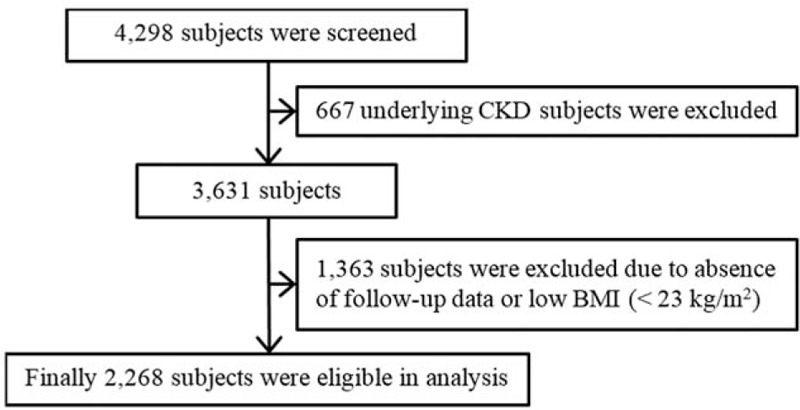
Flow diagram of the study, BMI = body mass index, CKD = chronic kidney disease.
This study was carried out in accordance with the Declaration of Helsinki and approved by the institutional review board of Yonsei University Health System Clinical Trial Center.
2.2. Data collection
A comprehensive health examination was done, and questionnaires on health and lifestyle were filled out by all participants at the time of study entry. With the subjects wearing light clothes, their height, body weight, waist and hip circumference, and NC were measured by experienced research workers through standard methods. The BMI and waist-to-hip ratio were calculated as weight/height (kg/m2) and waist/hip circumference, respectively. Demographic data including age, sex, comorbidities, alcohol intake, smoking, and exercise were also collected. Subjects who had a blood pressure >140/90 mm Hg or were taking antihypertensive medications were considered hypertensive, and those who had a fasting glucose concentration of ≥126 mg/dL or a postload glucose concentration of ≥200 mg/dL after the 75-g oral glucose tolerance test, or were receiving treatment for hyperglycemia were considered diabetic. Cardiovascular disease was defined as a history of arrhythmia, coronary artery or peripheral artery disease, or cerebrovascular disease.
Laboratory data were measured by using fasting blood samples. The following biochemical data were determined with a 747 Chemistry Analyzer (Hitachi, Tokyo, Japan): concentrations of blood glucose, blood urea nitrogen, serum creatinine, total cholesterol, triglyceride, and high-density lipoprotein cholesterol, whereas low-density lipoprotein cholesterol was calculated on the basis of the Friedewald formula.[15] Urine samples were collected in the morning after the first voiding. Urinalysis was performed on fresh urine samples by physicians, by using URISCAN Pro II (YD Diagnostics Corp., Seoul, Korea). The urine test strip results were based on a color scale that quantified proteinuria as absent, trace, 1+, 2+, or 3+. This scale correlated approximately with proteinuria of <10, 10–20, >30, >100, and >500 mg/dL, respectively.[16] The presence of proteinuria was defined as a urinalysis result higher than the trace level, and the estimated glomerular filtration rate (eGFR) was calculated by using the 4-variable Modification of Diet in Renal Disease equation including age, sex, race, and serum creatinine levels.
2.3. Outcome measure
The primary end point was incident CKD, which was defined as a composite of eGFR <60 mL/min/1.73 m2 or the development of proteinuria during the follow-up period.
2.4. Statistical analysis
Statistical analysis was performed by using IBM SPSS software for Windows version 23.0 (IBM Corporation, Armonk, NY). Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables as number (percentage). The normality of the distribution of the measured parameters was ascertained by using the Kolmogorov–Smirnov test. The patients were divided into 2 groups based on the median value of NC (37.6 cm in men and 32.7 cm in women), and the differences between the 2 groups were analyzed by using Student t test or the Mann–Whitney U test for continuous variables, and the Chi-square test for categorical variables. Univariate and multivariate linear regression analyses were performed to determine the significant factors associated with NC. Because of the log-normal distributions of serum triglyceride and high-sensitivity C-reactive protein (hs-CRP) concentrations, natural log values were used in the analysis. Variables with a P value of <0.2 in the univariate linear regression analysis were included in multivariate linear regression analysis. Kaplan–Meier analysis was performed to determine the impact of NC on the development of CKD, and between-group difference was compared by using a log-rank test. The independent predictive value of NC for incident CKD was ascertained with multivariate Cox proportional hazards regression analysis. P values <0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
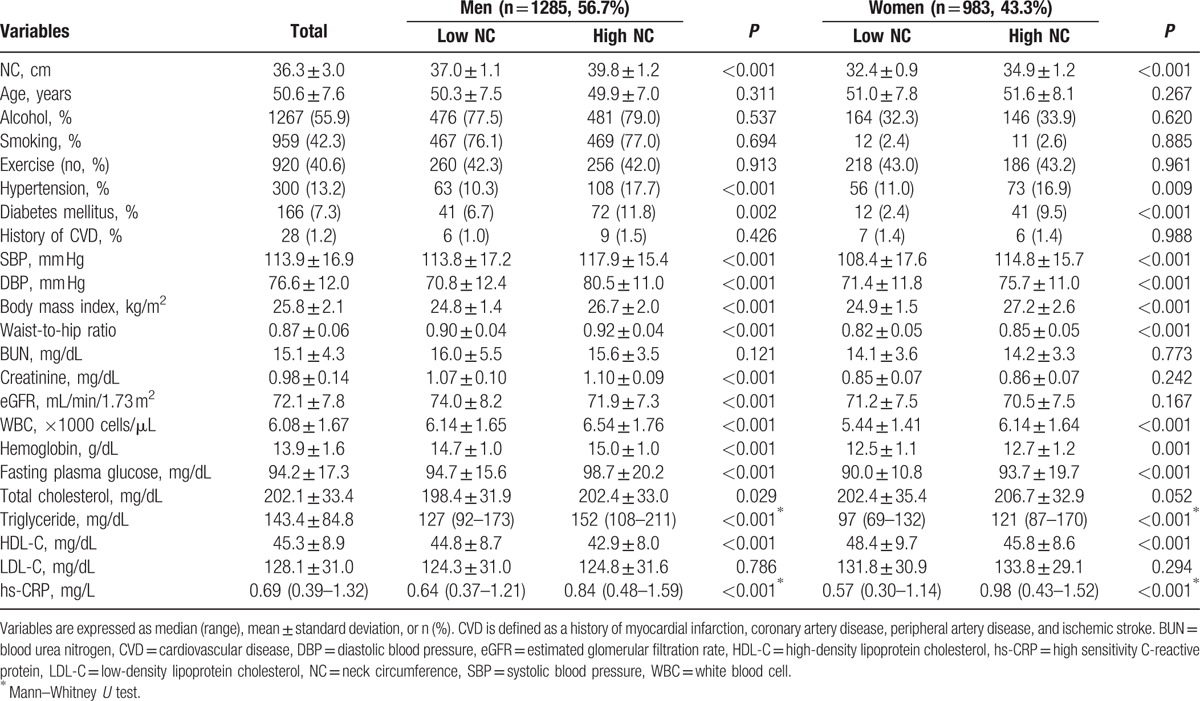
The baseline characteristics of the patients are shown in Table 1. The mean age was 50.6 ± 7.6 years; 1285 patients (56.7%) were men; and the mean eGFR was 72.1 ± 7.8 mL/min/1.73 m2. The mean values of NC were 36.3 ± 3.0, 38.4 ± 1.8, and 33.6 ± 1.6 cm in total, male, and female subjects, respectively. In both sexes, hypertension (men, 108 [17.7%] vs 63 [10.3%], P < 0.001; women, 73 [16.9%] vs 56 [11.0%], P = 0.009) and diabetes (men, 72 [11.8%] vs 41 [6.7%], P = 0.002; women, 41 [9.5%] vs 12 [2.4%], P < 0.001) were significantly more prevalent in the big NC group than in the small NC group. Systolic blood pressure, diastolic blood pressure, BMI, waist-to-hip ratio, white blood cell count, and hemoglobin, fasting blood glucose, and serum triglyceride and hs-CRP levels were also significantly higher in the big NC group in both male and female subjects. In contrast, serum creatinine (1.10 ± 0.09 vs 1.07 ± 0.10 mg/dL, P < 0.001) and total cholesterol concentrations (202.4 ± 33.0 vs 198.4 ± 31.9 mg/dL, P = 0.029) were significantly higher in the big NC group, only in the male sex group.
Table 1.
Baseline characteristics of the study subjects (N = 2268).
3.2. Independent factors associated with NC
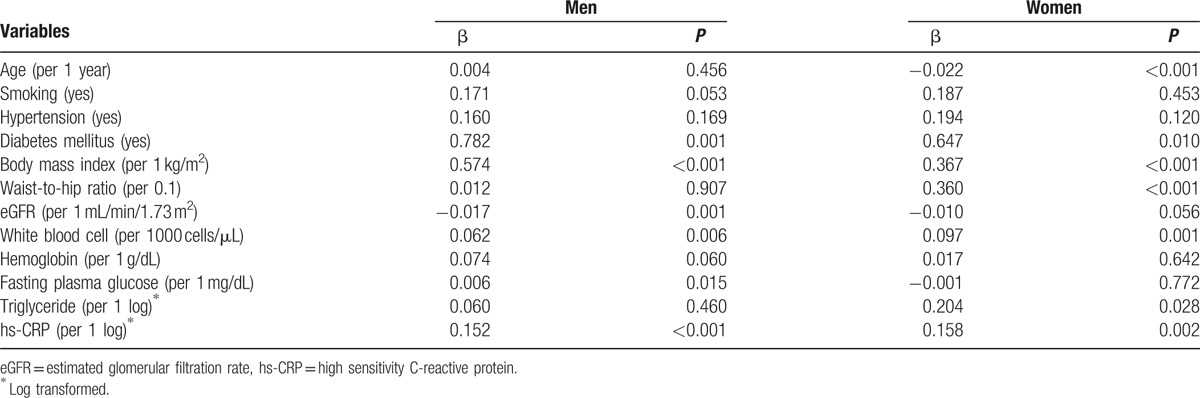
Multivariate linear regression analysis revealed that there were significant positive relationships of NC with the presence of diabetes (β = 0.782, P = 0.001), BMI (β = 0.574, P < 0.001), white blood cell count (β = 0.062, P = 0.006), fasting blood glucose (β = 0.006, P = 0.015), and serum hs-CRP levels (β = 0.152, P < 0.001), whereas NC was significantly and inversely correlated with eGFR (β = −0.017, P = 0.001) in male subjects. In the female sex group, NC was significantly associated with diabetes (β = 0.674, P = 0.010), BMI (β = 0.367, P < 0.001), waist-to-hip ratio (β = 0.360, P < 0.001), white blood cell count (β = 0.097, P = 0.001), serum triglyceride (β = 0.204, P = 0.028), and hs-CRP concentrations (β = 0.158, P < 0.001), whereas there was a significant inverse correlation between NC and age (β = −0.022, P < 0.001) (Table 2).
Table 2.
Association of clinical and biochemical variables with neck circumference.
3.3. Development of incident CKD
During a mean follow-up duration of 95.4 ± 27.6 months in male subjects and 78.3 ± 28.0 months in female subjects, incident CKD events were observed in 243 (18.9%) and 212 (21.6%) subjects in the male and female sex groups, respectively.
3.4. Relationship between NC and incident CKD development
NC was significantly greater in incident CKD subjects than in the non-CKD group in both sex groups (incident CKD vs non-CKD group: men, 38.6 ± 1.8 vs 38.3 ± 1.8 cm, P = 0.016; women, 33.8 ± 1.8 vs 33.5 ± 1.6 cm, P = 0.004) (Fig. 2A, B). When the incidence of newly developed CKD was compared between the big and small NC groups, CKD events were significantly and more frequently observed in the big NC group relative to the low NC group in both male and female subjects (big NC vs small NC group: men, 176 [20.3%] vs 67 [16.0%], P = 0.016; women, 162 [23.9%] vs 50 [16.3%], P = 0.004) (Fig. 2C).
Figure 2.
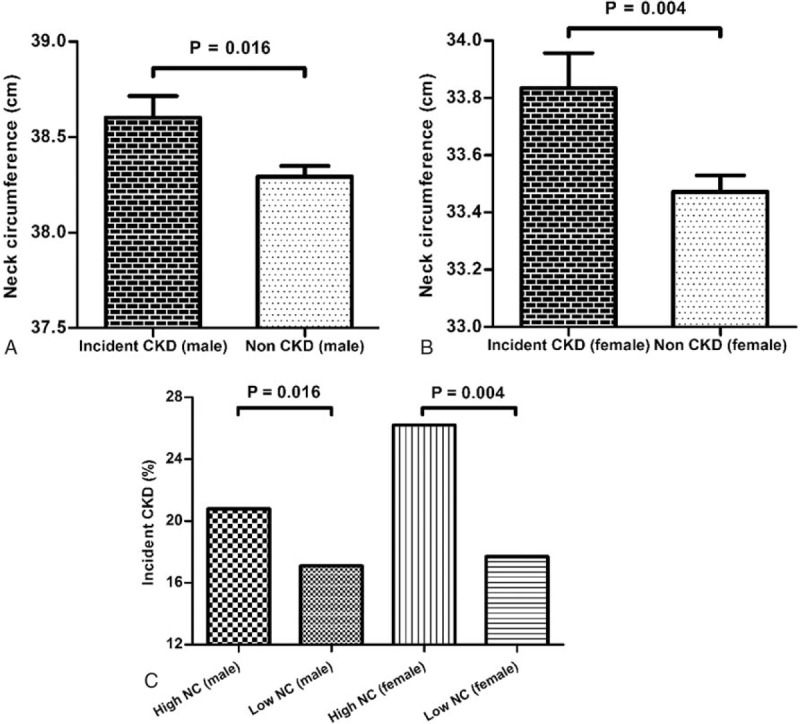
(A, B) Comparison of the mean value of NC according to incident CKD in both sex groups. NC was significantly higher in both male and female subjects with incident CKD than in subjects without CKD. Each bar represents the mean and its standard error. (C) Comparison of the incident rate of CKD between NC groups. The high NC group showed significantly higher incident CKD events than the low NC group in both male and female subjects. CKD = chronic kidney disease, NC = neck circumference.
3.5. Impact of NC on the development of incident CKD
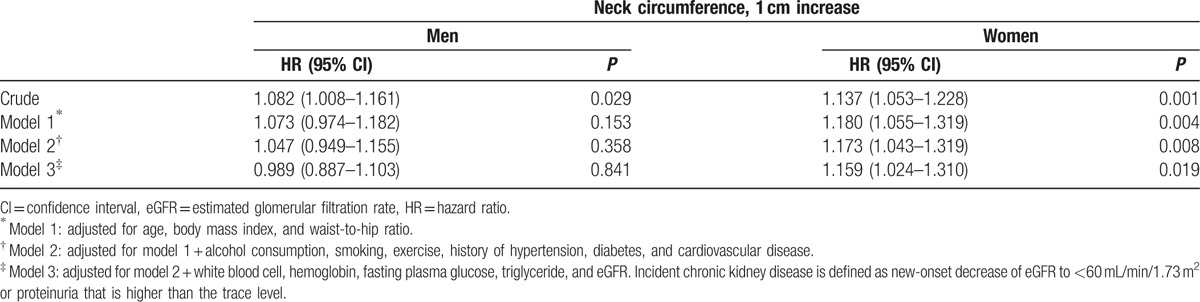
Kaplan–Meier analysis revealed that the big NC group had a significantly worse CKD-free survival rate than the low NC group in female patients (P < 0.001). In contrast, there was no significant difference in the rate of CKD development between the groups in male patients (P = 0.134) (Fig. 3). In Cox proportional hazards analysis, the NC value was a significant risk factor for incident CKD events in the female sex group (per 1 cm increase, hazard ratio [HR] = 1.159, confidence interval [CI] = 1.024–1.310, P = 0.019) even after adjusting for confounding factors, whereas the relationship between the NC value and incident CKD events was not significant in male subjects (per 1 cm increase, HR = 0.989, CI = 0.887–1.103, P = 0.841) (Table 3, Supplementary Tables 1 and 2). However, the significant association of NC with the development of CKD in female subjects disappeared when further adjustment was made for hs-CRP.
Figure 3.
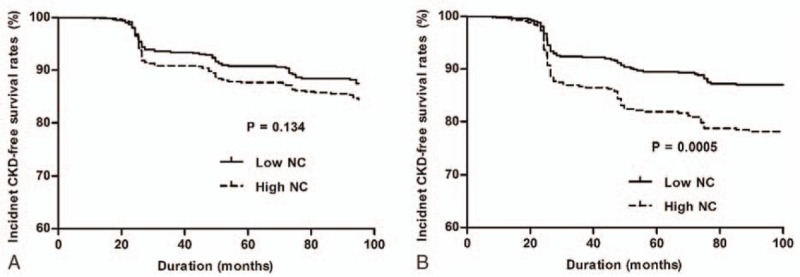
Kaplan–Meier plots for CKD events in the low and high NC groups. (A) No significant difference in CKD incidence was found between the low and high NC group in male subjects. (B) Female subjects in the high NC group showed a significantly higher incidence of CKD. CKD = chronic kidney disease, NC = neck circumference.
Table 3.
Neck circumference is an independent predictor of incident chronic kidney disease.
4. Discussion
Adiposity exerts a significant role in the pathogenesis of various diseases in overweight patients.[17,18] However, the impact of fat tissues of different body parts on the renal outcome is not yet fully investigated. In the current study, the associations between NC and cardiometabolic risk factors and the impact of NC on incident CKD development were elucidated in a prospective cohort of subjects without kidney disease. As a result, NC was revealed to be a significant independent predictor of incident CKD events even after adjusting for confounding factors such as other anthropometric parameters, traditional CKD risk factors, and baseline eGFR.
Previous studies have demonstrated that fat tissues of different body parts have differential roles in several cardiometabolic complications.[5,6,10,19,20] Although VAT, commonly represented as the waist-to-hip ratio, was found to have relationships with metabolic derangements, including insulin resistance,[21] type 2 diabetes,[22] hypertension,[23] and cardiovascular diseases,[24] these associations between VAT and adverse metabolic complications were not strong enough.[25] Similarly, a close association was shown between NC, a proxy of upper-body subcutaneous fat, and metabolic complications.[10,14,26–30] Several recent studies demonstrated that NC was a significant risk factor for type 2 diabetes, cardiovascular diseases, and ischemic stroke.[31] In addition, a cross-sectional association between NC and eGFR was found in a group of patients with or without CKD, suggesting a possible pathogenic role of upper-body subcutaneous fat in the development of renal dysfunction.[29] In the present study, we showed that NC was a significant predictor of incident CKD in a longitudinal analysis of subjects who did not have CKD at baseline, which was consistent with the results of a previous study. It should be noted that the impact of NC on CKD development remained significant even after adjusting for BMI and waist-to-hip ratio. Meanwhile, lower-body subcutaneous fat exerted protective effects on such adverse metabolic outcomes, which were especially more prominent in women.[20,32,33]
The underlying mechanism of the association between NC and the development of incident CKD is currently unclear. The level of circulating NEFA was proposed as one of the factors linking upper-body subcutaneous fat with cardiometabolic complications. Previous studies found that there were significant associations of NEFA levels with insulin resistance, hypertension, and atherosclerosis. Upper-body subcutaneous fat and its representative anthropometric parameter, NC, correlates with circulating NEFA levels, and the clinical implications of NEFA are known to be more prominent in relatively obese subjects.[11,34] Therefore, present study surmised that the association of NC with renal outcomes would be more significant in individuals with higher BMI. In fact, the subjects with higher BMI are more frequently accompanied with CKD risk factors such as diabetes and hypertension. In addition, renal uptake of NEFAs by the kidney proximal tubules induced tubulointerstitial inflammation and fibrosis, suggesting a causal relationship with various renal diseases.[35] Furthermore, NEFAs affected vascular nitric oxide production, promoting endothelial dysfunction, and induced systemic inflammation, impairing vascular reactivity, all of which were potential pathophysiologic mechanisms for kidney damage. As upper-body subcutaneous fat was recently demonstrated to be the main reservoir of circulating NEFAs, and release from upper-body subcutaneous fat was predominantly responsible for this surge in systemic NEFAs especially in obese persons,[19] the excess in NEFA release could be one possible mechanism for the association between NC and incident CKD events observed in this study. Considering that NEFAs are freely converted into triglycerides, the significant positive correlation between plasma triglyceride concentrations and NC in female subjects in the current study might support this notion. In addition, on the basis of the results of the current study revealing that the significant association of NC with incident CKD development disappeared after adjusting for hs-CRP, it was surmised that NEFAs contributed to adverse complications partly through systemic inflammation. The possibility that these coexisting risk factors could have played a role in increasing risks of the incidence of CKD in individuals with larger NC could not be ruled out. However, the fact that larger NC was significantly associated with incident CKD development even after adjustments were made for these confounding factors suggests that NC could have an additive effect on renal function decline over these traditional risk factors.
Another feasible factor linking NC with the development of incident CKD can be obstructive sleep apnea syndrome, which commonly occurs in persons with a large NC.[36] Obstructive sleep apnea syndrome causes complete or incomplete upper airway obstruction, often resulting in hypoxemia and an increase in oxidative stress.[37] Accumulating evidence indicates that sleep apnea is an independent risk factor for metabolic syndrome,[38] hypertension,[39] and premature cardiovascular death.[40] Several recent investigations also found that sleep apnea could mediate renal damage.[41,42] In the present study, simple questionnaires on obstructive sleep apnea symptoms were surveyed; however, the prevalence of sleep apnea-related symptoms was too low and there was no clear association between NC and incident CKD events (data not shown). Nevertheless, as a thorough evaluation for obstructive sleep apnea syndrome was not performed in this study, further investigations on these issues are necessary.
Since the pathophysiologic consequence of adipose tissue is known to differ between fat locations,[5,6,9,11,19,20] this study intended to evaluate the effect of fat distribution on incidental CKD risk rather than the effect of absolute adiposity amount. Therefore, subjects that already had sufficient amount of fat were enrolled for investigation. In addition, upper-body subcutaneous fat amount correlates with circulating NEFA levels,[19,34] and the clinical implications of NEFA are known to be more prominent in relatively obese subjects.[43] Therefore, individuals with higher BMI were investigated assuming that the association of NC with renal outcome would be more significant in this particular group. However, subjects with higher BMI are more frequently accompanied with CKD risk factors such as diabetes and hypertension. The possibility that these coexisting risk factors could have played a role in increasing risks of CKD incidence in individuals with larger NC could not be ruled out. Nonetheless, the fact that larger NC was significantly associated with incident CKD development even after adjustments were made for these confounding factors suggests that NC could have an additive effect on renal function decline over these traditional risk factors.
Interestingly, the results of our study showed that NC was an independent predictor of incident CKD development only in female patients. A previous study demonstrated that obese men stored much smaller amounts of NEFAs in the subcutaneous area than obese women,[33] suggesting that the metabolic derangements accompanied by increased subcutaneous fat might be greater in women. Moreover, the Framingham Heart Study revealed that the association between VAT and adverse cardiovascular risk profiles were closer in women, implying a greater vulnerability to fat-induced metabolic complications in women.[5] On the basis of these findings, the differential impact of NC on the development of incident CKD between male and female subjects in the current study may in part be attributed to the differences in lipid metabolism and the influence of fat tissues between the sexes.
The present study has several limitations. First, as the study subjects were all middle-aged Koreans, the significant impact of NC on incident CKD events may not be generalizable to other populations. Second, although NC is considered a surrogate of upper-body subcutaneous fat and is a simple anthropometric measurement that is not affected by feeding or clothing, there is a possibility of other causes leading to greater NC than fat accumulation. Further investigations such as imaging studies on quantification of the upper-body subcutaneous adipose tissue are mandatory to validate our study findings. Third, to verify that the association between NC and incident CKD was attributed to NEFAs, circulating NEFA levels should be determined; however, these data were not available. Fourth, elevated levels of uric acid are known to independently increase the risk for new-onset kidney disease even in healthy volunteers.[44,45] Unfortunately, circulating uric acid levels were not available in the present cohort. Further investigations including serum uric acid levels as factors influencing renal function should be needed. Last, the cohort was followed biennially, and therefore the exact time of CKD development could not be identified in detail.
In conclusion, NC is independently associated with the development of incident CKD in overweight women, suggesting that upper-body subcutaneous fat could play a relevant role in the pathogenesis of renal disease in this population.
Acknowledgments
The authors thank KoGES of the Korea Centers for Disease Control and Prevention, Republic of Korea for the data used in this study.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, hs-CRP = high-sensitivity C-reactive protein, NC = neck circumference, NEFA = nonesterified fatty acid, VAT = visceral adipose tissue.
C-YY and JTP contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int 2004; 66:1310–1314. [DOI] [PubMed] [Google Scholar]
- 2.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010; 375:1296–1309. [DOI] [PubMed] [Google Scholar]
- 3.Ejerblad E, Fored CM, Lindblad P, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006; 17:1695–1702. [DOI] [PubMed] [Google Scholar]
- 4.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2007; 2:550–562. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116:39–48. [DOI] [PubMed] [Google Scholar]
- 6.Banerji MA, Faridi N, Atluri R, et al. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 1999; 84:137–144. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Shin DH, Kim SJ, et al. Visceral fat thickness is associated with carotid atherosclerosis in peritoneal dialysis patients. Obesity (Silver Spring) 2012; 20:1301–1307. [DOI] [PubMed] [Google Scholar]
- 8.Rosenquist KJ, Therkelsen KE, Massaro JM, et al. Development and reproducibility of a computed tomography-based measurement for upper body subcutaneous neck fat. J Am Heart Assoc 2014; 3:e000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjostrom CD, Hakangard AC, Lissner L, et al. Body compartment and subcutaneous adipose tissue distribution--risk factor patterns in obese subjects. Obes Res 1995; 3:9–22. [DOI] [PubMed] [Google Scholar]
- 10.Preis SR, Massaro JM, Hoffmann U, et al. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab 2010; 95:3701–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilz S, Scharnagl H, Tiran B, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab 2006; 91:2542–2547. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 1997; 100:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou JY, Ge H, Zhu MF, et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc Diabetol 2013; 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 16.Lim D, Lee DY, Cho SH, et al. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res Clin Pract 2014; 33:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA 1998; 280:1843–1848. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DS, Mei Z, Srinivasan SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007; 150: 12-17.e12. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MD, Haymond MW, Rizza RA, et al. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 1989; 83:1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sondergaard E, Gormsen LC, Nellemann B, et al. Body composition determines direct FFA storage pattern in overweight women. Am J Physiol Endocrinol Metab 2012; 302:E1599–1604. [DOI] [PubMed] [Google Scholar]
- 21.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991; 337:382–386. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez G, Duval S, Jacobs DR, Jr, et al. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 2007; 29:115–128. [DOI] [PubMed] [Google Scholar]
- 23.Huxley R, Mendis S, Zheleznyakov E, et al. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk – a review of the literature. Eur J Clin Nutr 2010; 64:16–22. [DOI] [PubMed] [Google Scholar]
- 24.de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007; 28:850–856. [DOI] [PubMed] [Google Scholar]
- 25.Borruel S, Molto JF, Alpanes M, et al. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist hip ratio, model of adipose distribution and visceral adiposity index. PLoS One 2014; 9:e114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Noun L, Laor A. Relationship of neck circumference to cardiovascular risk factors. Obes Res 2003; 11:226–231. [DOI] [PubMed] [Google Scholar]
- 27.Preis SR, Pencina MJ, D’Agostino RB, et al. Neck circumference and the development of cardiovascular disease risk factors in the Framingham Heart Study. Diabetes Care 2013; 36:e3–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Silva Cde C, Zambon MP, Vasques AC, et al. Neck circumference as a new anthropometric indicator for prediction of insulin resistance and components of metabolic syndrome in adolescents: Brazilian Metabolic Syndrome Study. Rev Paul Pediatr 2014; 32:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YF, Chang ST, Lin WS, et al. Neck circumference as a predictive indicator of CKD for high cardiovascular risk patients. Biomed Res Int 2015; 2015:745410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoi S, Miyake T, Iida T, et al. Association of changes in neck circumference with cardiometabolic risk in postmenopausal healthy women. J Atheroscler Thromb 2016; 23:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medeiros CA, Bruin VM, Castro-Silva C, et al. Neck circumference, a bedside clinical feature related to mortality of acute ischemic stroke. Rev Assoc Med Bras 2011; 57:559–564. [DOI] [PubMed] [Google Scholar]
- 32.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006; 291:E1115–E1123. [DOI] [PubMed] [Google Scholar]
- 33.Koutsari C, Ali AH, Mundi MS, et al. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011; 60:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997; 46:3–10. [PubMed] [Google Scholar]
- 35.Kamijo A, Kimura K, Sugaya T, et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int 2002; 62:1628–1637. [DOI] [PubMed] [Google Scholar]
- 36.Castorena-Maldonado A, Espinosa-Morett L, Arredondo Del Bosque F, et al. Diagnostic value of the morphometric model and adjusted neck circumference in adults with obstructive sleep apnea syndrome. Rev Invest Clin 2015; 67:258–265. [PubMed] [Google Scholar]
- 37.Duran-Cantolla J, Aizpuru F, Martinez-Null C, et al. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev 2009; 13:323–331. [DOI] [PubMed] [Google Scholar]
- 38.Alam I, Lewis K, Stephens JW, et al. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev 2007; 8:119–127. [DOI] [PubMed] [Google Scholar]
- 39.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 40.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsioufis C, Thomopoulos C, Dimitriadis K, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis 2008; 52:285–293. [DOI] [PubMed] [Google Scholar]
- 42.Chan GC, Lam B, Yap DY, et al. Proteinuria is associated with sleep apnea in chronic kidney disease. Nephrol Dial Transplant 2015. [DOI] [PubMed] [Google Scholar]
- 43.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444:840–846. [DOI] [PubMed] [Google Scholar]
- 44.Rodenbach KE, Schneider MF, Furth SL, et al. Hyperuricemia and Progression of CKD in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am J Kidney Dis 2015; 66:984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellomo G, Venanzi S, Verdura C, et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis 2010; 56:264–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


