Abstract
Background:
Many primary and secondary studies reported the association between Toll-like receptor 4 (TLR4) polymorphism and periodontitis susceptibility, which mainly focused on TLR4–299A>G or TLR4–399C>T of Caucasian, however, these studies had different conclusions. The aim of this study was to reassess relative studies about TLR4 polymorphism and periodontitis susceptibility, and update meta-analysis.
Methods:
We searched the electronic database including CNKI (Chinese National Knowledge Infrastructure), PubMed, Embase, and hand searched relative studies until January 4, 2016. Two authors selected studies according to inclusion and exclusion criteria, assessed studies using Newcastle-Ottawa Scale case control study (NOS), and calculated the combined effect size using STATA software, version 12.0.
Results:
This meta-analysis included 18 studies, containing 2453 healthy participants and 2987 patients with chronic periodontitis (CP) and 462 patients with aggressive periodontitis (AP). There was a significance between TLR4C>G (rs7873784) allele and CP in Asian, and its recessive model was also significant (for C vs G: odds ratio [OR] = 0.72, 95% confidence interval [CI] = 0.54–0.95, I2 = 0%; for CC + CG vs GG: OR = 0.66, 95% CI = 0.49–0.89, I2 = 0%). However, we did not detect any significant relevance between other TLR4 polymorphism and periodontitis susceptibility in overall and subgroup analyses. The sensitive analysis showed that dropping any single studies did not affect the pooled-analysis results. Publication bias was not detected.
Conclusions:
The meta-analysis found association between TLR4C>G (rs7873784) allele and CP in Asian and it may passed on to offsprings in the form of recessiveness. However, further studies about the association between TLR4C>G (rs7873784) and CP is warranted to confirm.
Keywords: meta-analysis, periodontal disease, periodontitis, polymorphism, TLR4
1. Introduction
Periodontitis is a kind of chronic disease affected by multiple factors such as microorganism, host and environment.[1] Periodontitis was identified into 3 types: chronic periodontitis (CP), aggressive periodontitis (AP), and periodontitis as a manifestation of systemic disease.[2] Kassebaum et al[3] predicted an increasing global burden of severe periodontitis on account of growing life expectancy resulted in decreased tooth loss and increasing world population during 1990 to 2010. Susin et al[4] summarized the features of epidemiology and demographics in AP which clarified the prevalence of AP varied significantly in different regions and races. Thus, periodontitis has became one of the hot research fields all over the world.
The traditional method for dealing with periodontitis mainly focused on removing pathogenic bacteria, which resulted in bacteria resistance and disease recurrence. Besides, the host inflammatory response plays a critical role in the destruction of periodontal tissue. In the recent decades, the development of sequencing technology enabled us to discuss whether the variations of host's immune-related Deoxyribose Nucleic Acid molecules affected the occurrence and development of diseases. Thus, there is a great significance to discuss the gene variants of immune-related molecules for the prevention and treatment of periodontitis. Luigi[5] elucidated host genetic variants may work in the occurrence and development of AP through selectively participating in the dysbiotic process. Hajishengallis and Sahingur[6] reported a polymorphic site in the TLR9 gene promoter region differentially expressed and TLR9 gene and protein expression increased in CP. Toll-like receptor 4 (TLR4) was a pattern-recognition receptor, which played an important part in innate immunity by realizing lipid-based structures of bacteria and mediating intracellular signaling.[7,8] Furthermore, Many studies reported the association between TLR4 polymorphism and periodontitis susceptibility, and they mainly focused on TLR4–299A>G or TLR4–399C>T of Caucasian but conducted different conclusions. Therefore, this meta-analysis and subgroup analyses were carried out to further illuminate the relationship between TLR4 polymorphism and periodontitis susceptibility based on the currently available studies.
2. Materials and methods
This review is not a primary research, so ethical approval and informed consent are not necessary. This meta-analysis was reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.[9]
2.1. Inclusion and exclusion criteria
All retrieved literatures fit the following criteria should be included: the study tested TLR4 polymorphism and periodontitis susceptibility, participants in studies must be explicitly diagnosed with CP or AP, the numbers of every genotypes were available both in case and control groups. The study met the following criteria should be excluded: the review or meta-analysis about this theme, the article not described in Chinese or English, no control subjects or without healthy control group, participants who were pregnant or lactating, mutant type not detected.
2.2. Search strategy
The authors performed a systematic search in CNKI, PubMed, and Embase up to January 4, 2016 using key words: “polymorphism OR mutation OR variant” AND “TLR4 OR Toll like receptor 4” AND “periodontal disease OR periodontitis.” We also selected references of the relative reviews by hand searching. The details of search strategy in PubMed are summarized in “Appendix 1.”
2.3. Data extraction
Two authors extracted the useful information independently and any disagreement was solved by discussing until reaching an agreement or consulting a third author if needed. The extracted data were as follows: the first author's name and the publication date, the country or ethnicity of study participants, polymorphisms under investigation, disease type, the numbers of every genotypes both in case and control groups, genotyping method, the Hardy–Weinberg equilibrium (HWE) of the controls.
2.4. Quality assessment
Two of us conducted the quality assessment of included studies according to the Newcastle-Ottawa Scale case control study (NOS).[10] This standard assessed 3 sections (selection, comparability, exposure) and 8 items. In the selection and exposure categories, a quality research item received 1 star, and a comparable category could receive at most 2 stars. The quality assessment values ranged from 0 to 9 stars. Generally, the study which scored at least 5 points was considered to be included in meta-analysis.
2.5. Data analyses
The authors calculated the odds ratios (ORs) and corresponding 95% confidence interval (CI) to estimate the association between TLR4 polymorphism and periodontitis in 5 genetic models: allele comparison (1 vs 2), homozygote comparison (11 vs 22), heterozygote comparison (12 vs 11), dominant model (22 + 12 vs 11), recessive model (11 + 12 vs 22). Heterogeneity was tested using I2 statistics. Values of P < 0.1 or I2 > 50% indicated obvious heterogeneity and the random effects model was considered to use; otherwise, use the fixed effects model.[11] Subgroup analyses were performed according to ethnicity, smoking status. Sensitive analysis was carried out to test the stabilization of the pooled results.[12] Publication bias was detected with funnel plot in visual and with quantitative method of Begg and Egger linear regression.[13,14] All data analyses were conducted by STATA software, version 12.0. Using 2-sided P-values and P < 0.05 was supposed to have a statistically significance.
3. Results
3.1. Study selection and characteristics
Seventy-four studies from database searching and 6 studies from manual retrieval, counting up to 80 studies, were identified in this meta-analysis. After removing 30 duplicates, the remaining 50 studies need further screened. According to inclusion and exclusion criteria, this meta-analysis ultimately included 18 studies,[15–32] which included 2453 healthy participants and 2987 patients with CP and 462 patients with AP. The flow diagram of study selection is shown in Fig. 1.
Figure 1.
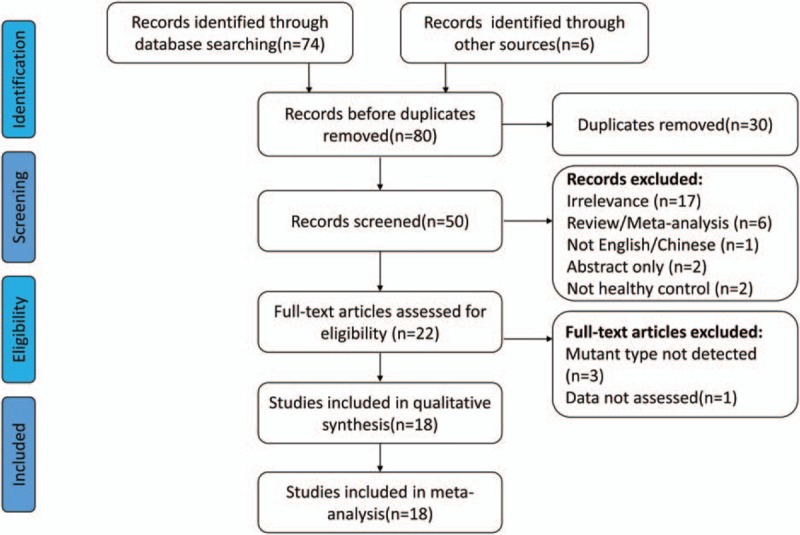
Flow diagram of study selection in the meta-analysis.
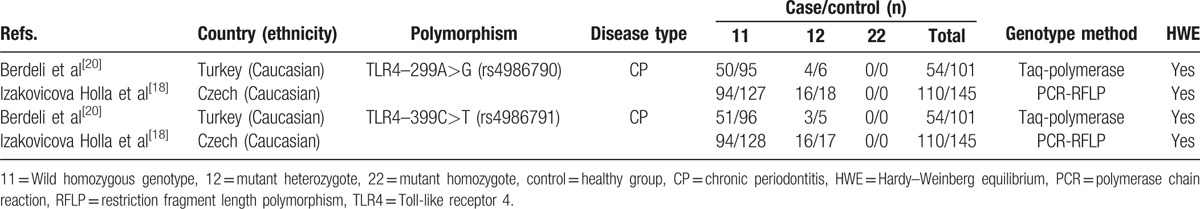
The basic characteristics of included studies are shown in Table 1. Among the 18 included studies, 4 studies were about Asian,[29–32] 12 studies were about Caucasian,[16–27] 1 study was conducted in Brazil (mixed),[15] and 1 in USA (Africa).[28] Only 2 studies[18,20] reported the relationship between gene polymorphism and nonsmokers, and the basic characteristics are summarized in Table 2. The controls of 2 studies’ HWE were not reported, and we could not calculate them in any way.[24,25]
Table 1.
Characteristics of studies included in the meta-analysis.
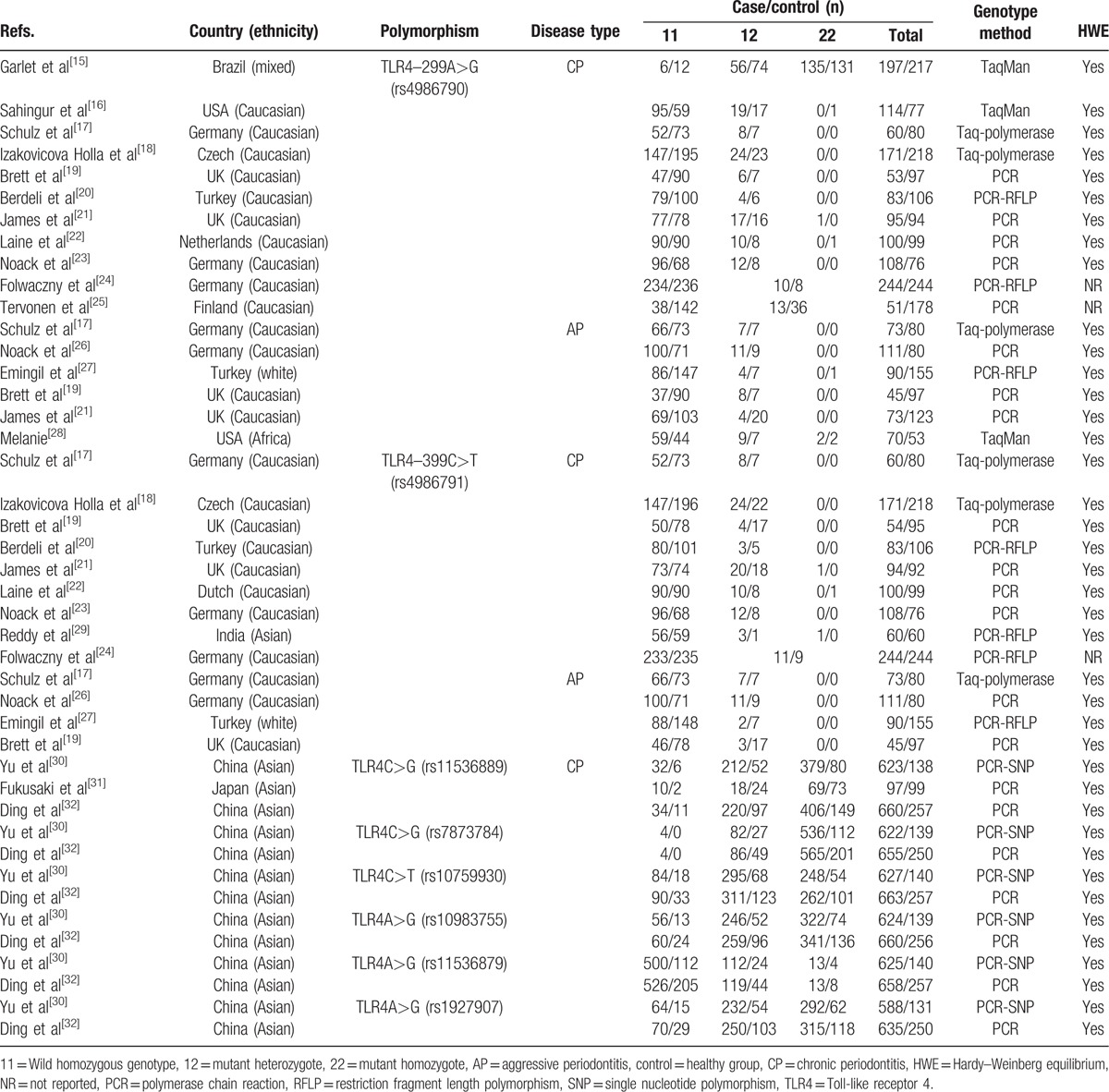
Table 2.
Nonsmokers: characteristics of studies included in the meta-analysis.
3.2. Quality assessment
The details of quality assessment based on the NOS are shown in Table 3. The last column in each row listed the total score of each study. One study had low score of 2 points, which would be considered to conduct the sensitive analysis. The percentage of different star numbers involving in all included studies is revealed in Fig. 2. Except for item “Non-response rate” which the percentage of 0 star was 100%, the remaining items has low percentage of 0 star. In general, the quality of included studies was good and fair.
Table 3.
Evaluations of the qualities of the included studies based on the Newcastle-Ottawa Scale.
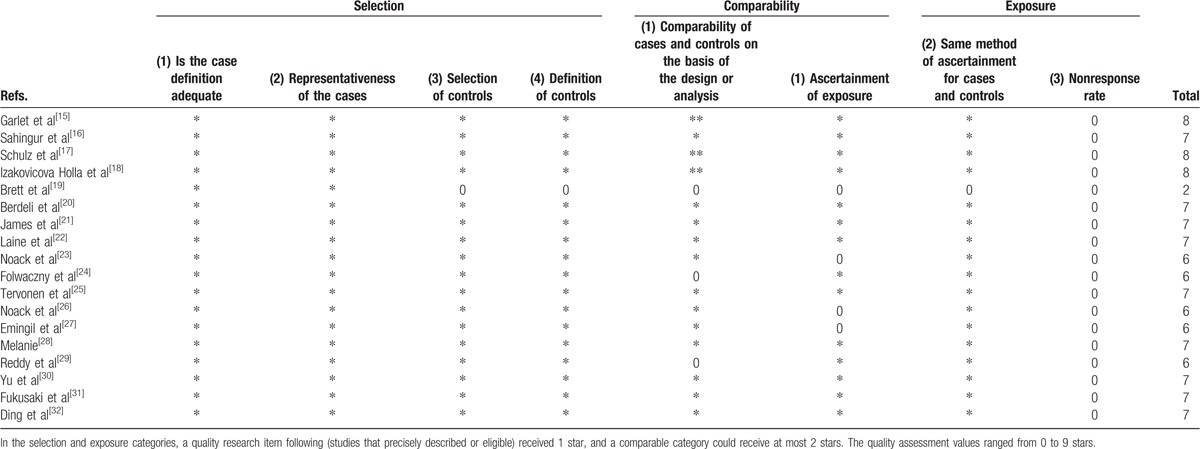
Figure 2.
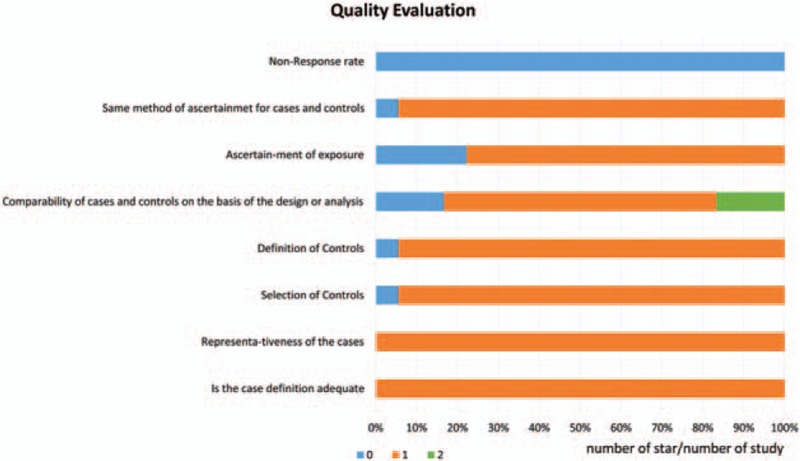
Quality evaluation of the included studies based on the Newcastle-Ottawa Scale.
3.3. Meta-analyses
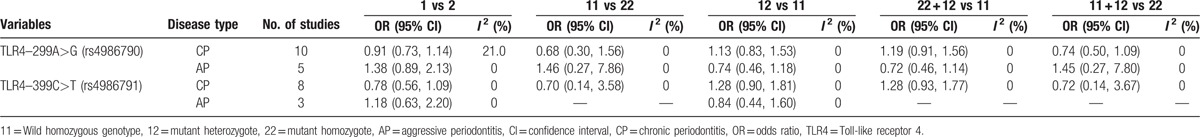
In the association between TLR4C>G (rs7873784) polymorphism and CP susceptibility, there was a significance between TLR4C>G (rs7873784) allele and CP in Asian, and its recessive model was also significant (for C vs G: OR = 0.72, 95% CI = 0.54–0.95, I2 = 0%; for CC + CG vs GG: OR = 0.66, 95% CI = 0.49–0.89, I2 = 0%) (Figs. 3 and 4). All the genetic models of other TLR4 gene polymorphism included in this article in overall and subgroup revealed no significance, and the results are listed in Table 4. The result of sensitive analysis was still nonsignificant but the heterogeneity significantly decreased, which was conducted by removing the study of low quality (Table 5).
Figure 3.
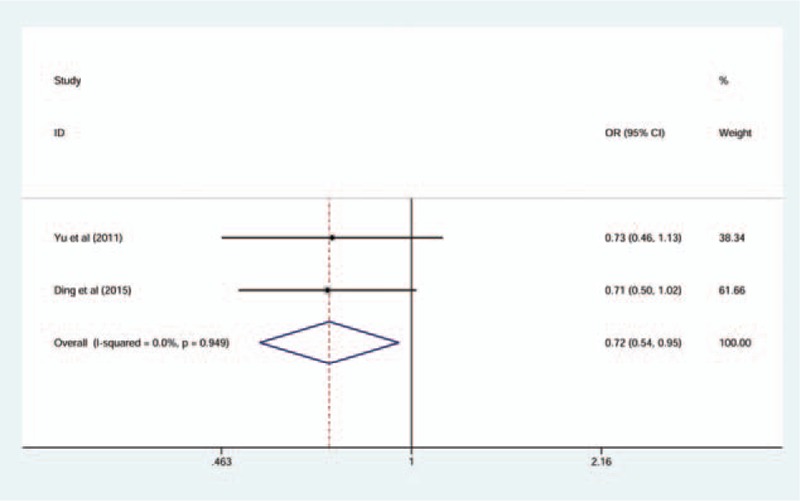
Forest plot for TLR4C>G (rs7873784) associated with chronic periodontitis in C versus G comparison (fixed-effect model).
Figure 4.
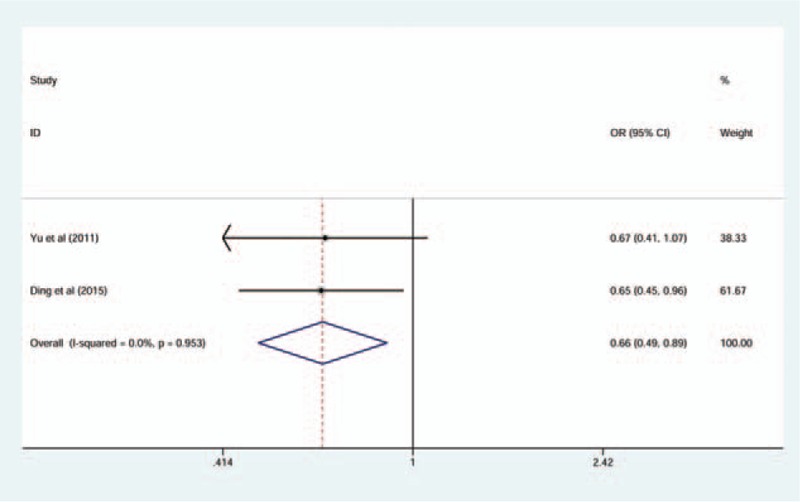
Forest plot for TLR4C>G (rs7873784) associated with chronic periodontitis in CC + CG versus GG comparison (fixed-effect model).
Table 4.
Meta-analysis of the association between the TLR4 gene polymorphism and periodontitis.
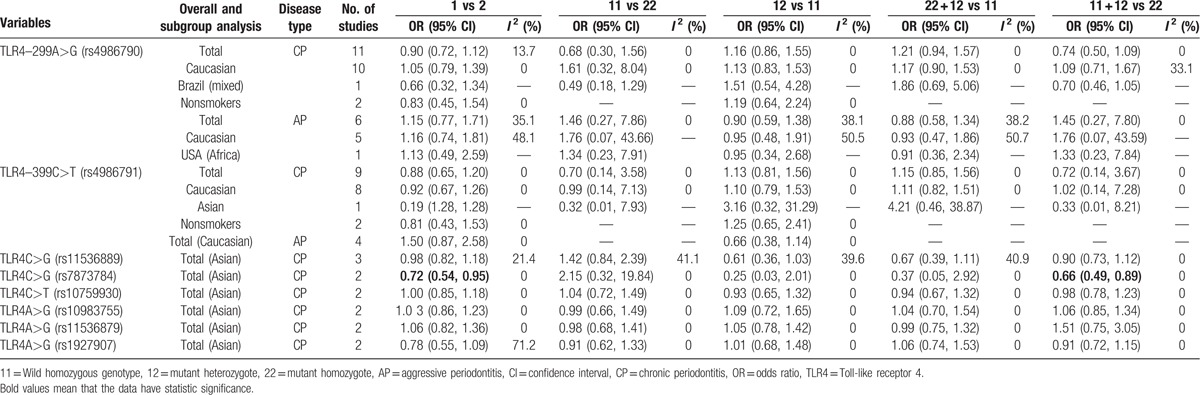
Table 5.
Sensitive analysis by removing the study of low quality.
3.4. Publication bias
The funnel plot method was used when the number of included studies at least 10 at which point it had relatively high test efficiency.[33] The 11 included studies about TLR4–299A>G polymorphism with CP had nearly symmetric funnel plot, which indicated no obvious publication bias (Fig. 5). The studies which had small quantity of 2 could not be detected the publication bias. Egger and Begg test did not indicate obvious publication bias for other studies (for Egger test: for TLR4–299A>G with AP: A vs G: P = 0.64; AG vs AA: P = 0.95; GG + AG vs AA: P = 0.90. for TLR4–399C>T with CP: C vs T: P = 0.88; CC vs TT: P = 0.41; AG vs AA: P = 0.78; GG + AG vs AA: P = 0.49; AA + AG vs GG: P = 0.60. for TLR4–399C>T with AP: C vs T: P = 0.38; AG vs AA: P = 0.36. for TLR4C>G (rs11536889) with CP: C vs G: P = 0.19; CC vs GG: P = 0.23; CG vs CC: P = 0.27; GG + CG vs CC: P = 0.25; CC + CG vs GG: P = 0.15).
Figure 5.
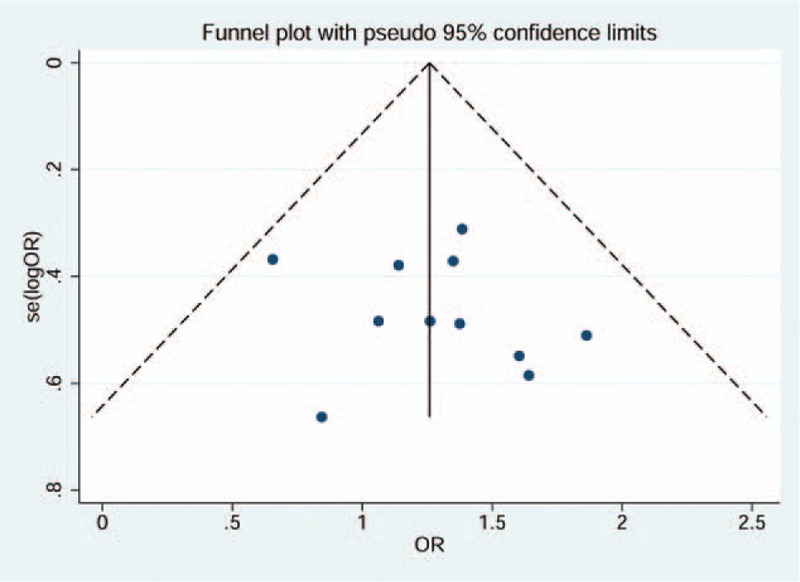
Funnel plot of included studies for TLR4–299A>G associated with chronic periodontitis.
4. Discussion
This meta-analysis systematically collected studies about TLR4 gene polymorphism associated with periodontitis from frequently used databases and manual retrieval. We analyzed the existing different TLR4 gene associated with different periodontitis and ultimately found that there was a significance between TLR4C>G (rs7873784) polymorphism and CP about C and G allele and its recessive model was also significant in Asian, which indicated that the Asian people suffered from CP might due to TLR4C>G (rs7873784) and it possibly passed on to offsprings in the form of recessiveness. On account of only 2 included studies about this gene, the reliability of this conclusion still need more research to demonstrate.
Previous meta-analyses about TLR4 gene polymorphism mainly focused on TLR4–299A>G or TLR4–399C>T of Caucasian, which made different conclusions. Ozturk and Vieira[34] included 7 studies and calculated the OR value of major allele versus minor allele for both TLR4–299A>G and TLR4–399C>T polymorphisms, and concluded that the TLR4–299A>G may be a risk factor against CP (OR = 1.43, 95% CI = 1.04–1.97) and the TLR4–399C>T appeared to be a protective factor to AP (OR = 0.29, 95% CI = 0.13–0.61). Song et al[35] and Zheng et al[36] used differently 4 genetic models, and their overall results associated with ethnic analysis all failed to reveal any association between TLR4–299A>G or TLR4–399C>T and periodontitis. Besides, Zheng et al[36] found a significantly increased risk for periodontitis in recessive models of TLR4–299A>G. Han et al[37] indicated that both TLR4–299A>G and TLR4–399C>T showed elevated risk of CP in Caucasians.
Our meta-analyses have many superiorities. This pooled analysis systematically included 18 studies, excluded studies lack of specific statistics and conducted quality assessment for every included study. Besides, this study analyzed five genetic models to explore inheritance patterns of genes. We also carried out subgroup analyses of participants’ ethnicity and smoking status. Based on the current studies, the authors did not detect any association between TLR4 polymorphisms and periodontitis susceptibility except for TLR4C>G (rs7873784). Through removing the low quality studies, the sensitive analysis only decreased heterogeneity, but could not change the results.
The limitation of this meta-analysis should not be ignored. First, language limitation made us cannot obtain more relative studies. Second, the relatively clinical research in Asian lacked. Third, the case–control study about the association between TLR4 polymorphisms and smoking status was few, which made us cannot extract data directly. Fourth, whether periodontal treatment could influence gene mutation should be seriously considered, but the studies included in this meta-analysis did not make the unified description, which may bring in the bias. Finally, many risk factors of periodontitis were not studied in clinical trials that led to the limitation of subgroup analyses. In conclusion, the unified baseline of studies and more well-designed clinical trials were expected.
5. Conclusions
We found that there was a significance between TLR4C>G (rs7873784) polymorphism and CP about C and G allele and its recessive model was also significant in Asian, which indicated that the Asian people suffered from CP may due to TLR4C>G (rs7873784) and it possibly passed on to offsprings in the form of recessiveness. However, the overall and subgroup analyses in other TLR4 polymorphism included in this study found no significance. Large quantity and high quality researches were expected to explore the pathogenesis of periodontitis.
Acknowledgment
The authors would like to thank editor and anonymous referees for their valuable and informative comments.
Appendix 1: PubMed search terms
#1 Search (((((((Polymorphisms, Genetic[Title/Abstract]) OR Genetic Polymorphism[Title/Abstract]) OR Polymorphism (Genetics)[Title/Abstract]) OR Genetic Polymorphisms[Title/Abstract]) OR gene polymorphism[Title/Abstract]) OR mutation[Title/Abstract]) OR variant[Title/Abstract]) OR “Polymorphism, Genetic”[Mesh]
#2 Search (((((Toll Like Receptor 4[Title/Abstract]) OR Toll4 Receptor[Title/Abstract]) OR Toll 4 Receptor[Title/Abstract]) OR TLR4 Receptor[Title/Abstract]) OR Receptor, TLR4[Title/Abstract]) OR “Toll-Like Receptor4”[Mesh]
#3 Search (((((((((Disease, Periodontal[Title/Abstract]) OR Diseases, Periodontal[Title/Abstract]) OR Periodontal Disease[Title/Abstract]) OR Parodontosis[Title/Abstract]) OR Pyorrhea Alveolaris[Title/Abstract]) OR Periodontitides[Title/Abstract]) OR Pericementitis[Title/Abstract]) OR Pericementitides[Title/Abstract]) OR “Periodontal Diseases”[Mesh]) OR “Periodontitis”[Mesh]
#4 Search #1 AND #2 AND #3
Footnotes
Abbreviations: AP = aggressive periodontitis, CI = confidence interval, CNKI = Chinese National Knowledge Infrastructure, CP = chronic periodontitis, HWE = Hardy–Weinberg equilibrium, NOS = Newcastle-Ottawa Scale case control study, OR = odds ratio, TLR4 = Toll-like receptor 4.
Funding: This study was supported by the NSFC (Natural Science Foundation of China) (81560186), The Program for Science and Technology Innovative Talents Team of Guizhou Province (grant no. Qian Ke He Talents Team[2013]4026), Key Subjects Construction Program for High Institutions in Guizhou Province (grant no. SZXK-201207-04), The Construction Program for Key Characteristic Laboratory in High Institutions of Guizhou Province (grant no. Qian Jiao He KY[2013]109), and Social Development Projects of Guizhou Province (grant no. Qian Ke He SYLKZ[2013]3043).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366:1809–1820. [DOI] [PubMed] [Google Scholar]
- 2.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 2004; 34:9–21. [DOI] [PubMed] [Google Scholar]
- 3.Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 2014; 93:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susin C, Haas AN, Albandar JM. Epidemiology and demographics of aggressive periodontitis. Periodontol 2000 2014; 65:27–45. [DOI] [PubMed] [Google Scholar]
- 5.Luigi N. Aggressive Periodontitis: microbes and host response, who to blame? Virulence 2016; 6:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G, Sahingur SE. Novel inflammatory pathways in periodontitis. Adv Dent Res 2014; 26:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 1997; 9:4–9. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 1992; 13:11–16. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 11.Deeks JJ, Higgins JP, Altman DG. Section 9.5, Heterogeneity. In: Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available at: http://handbook.coch-rane.org/ Accessed January 2016. [Google Scholar]
- 12.Deeks JJ, Higgins JP, Altman DG. Section 9.7, Sensitive analyses. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available at: http://hand-book.cochrane.org/ Accessed January 2016. [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 14.Egger M, Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garlet GP, Trombone APF, Menezes R, et al. The use of chronic gingivitis as reference status increases the power and odds of periodontitis genetic studies-a proposal based in the exposure concept and clearer resistance and susceptibility phenotypes definition. J Clin Periodontol 2012; 39:323–332. [DOI] [PubMed] [Google Scholar]
- 16.Sahingur SE, Xia X-J, Gunsolley J, et al. Single nucleotide polymorphisms of pattern recognition receptors and chronic periodontitis. J Periodont Res 2011; 46:184–192. [DOI] [PubMed] [Google Scholar]
- 17.Schulz S, Zissler N, Altermann W, et al. Impact of genetic variants of CD14 and TLR4 on subgingival periodontopathogens. Int J Immunogenet 2008; 35:457–464. [DOI] [PubMed] [Google Scholar]
- 18.Izakovicova Holla L, Buckova D, Fassmann A, et al. Lack of association between chronic periodontitis and the Toll-like receptor 4 gene polymorphisms in a Czech population. J Periodont Res 2007; 42:340–344. [DOI] [PubMed] [Google Scholar]
- 19.Brett PM, Zygogianni P, Griffiths GS, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res 2005; 84:1149–1153. [DOI] [PubMed] [Google Scholar]
- 20.Berdeli A, Emingil G, Han Saygan B, et al. TLR2 Arg753Gly, TLR4Asp299Gly and Thr399Ile gene polymorphisms are not associated with chronic periodontitis in a Turkish population. J Clin Periodontol 2007; 34:551–557. [DOI] [PubMed] [Google Scholar]
- 21.James JA, Poulton KV, Haworth SE, et al. Polymorphisms of TLR4 but not CD14 are associated with a decreased risk of aggressive periodontitis. J Clin Periodontol 2007; 34:111–117. [DOI] [PubMed] [Google Scholar]
- 22.Laine ML, Morre SA, Murillo LS, et al. CD14 and TLR4 gene polymorphisms in adult periodontitis. J Dent Res 2005; 84:1042–1046. [DOI] [PubMed] [Google Scholar]
- 23.Noack B, Görgens H, Lorenz K, et al. TLR4 and IL-18 gene variants in chronic periodontitis: impact on disease susceptibility and severity. Immunol Invest 2009; 38:297–310. [DOI] [PubMed] [Google Scholar]
- 24.Folwaczny M, Glas J, Török H-P, et al. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol 2004; 135:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tervonen T, Raunio T, Knuuttila M, et al. Polymorphisms in the CD14 and IL-6 genes associated with periodontal disease. J Clin Periodontol 2007; 34:377–383. [DOI] [PubMed] [Google Scholar]
- 26.Noack B, Görgens H, Lorenz K, et al. TLR4 and IL-18 gene variants in aggressive periodontitis. J Clin Periodontol 2008; 35:1020–1026. [DOI] [PubMed] [Google Scholar]
- 27.Emingil G, Berdeli A, Baylas H, et al. Toll-like receptor 2 and 4 gene polymorphisms in generalized aggressive periodontitis. J Periodontol 2007; 78:1968–1977. [DOI] [PubMed] [Google Scholar]
- 28.Melanie C. Frequency of TLR-2, 4, 9 and CD14 Polymorphisms in Aggressive Periodontitis Population in African-Americans. Virginia: Virginia Commonwealth University; 2009. [Google Scholar]
- 29.Reddy BH, Jayakumar ND, Akula SR, et al. Analysis of association between TLR-4 Asp299Gly and Thr399Ile gene polymorphisms and chronic periodontitis in a sample of south Indian population. J Indian Soc Periodontol 2011; 15:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui Y, Mei L, Ting L, et al. The association between Toll-like receptor 2/4 gene polymorphisms and the risk of chronic periodontitis. Chin J Geriatr Dent 2011; 9:333–338. [Google Scholar]
- 31.Fukusaki T, Ohara N, Hara Y, et al. Evidence for association between a Toll-like receptor 4 gene polymorphism and moderate/severe periodontitis in the Japanese population. J Periodont Res 2007; 42:541–545. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y-S, Zhao Y, Xiao Y-Y, et al. Toll-like receptor 4 gene polymorphism is associated with chronic periodontitis. Int J Clin Exp Med 2015; 8:6186–6192. [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JAC, Egger M, Moher D. Section 10.4, Detecting reporting biases. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available at: http://hand-book.cochrane.org/ Accessed January 2016. [Google Scholar]
- 34.Ozturk A, Vieira AR. TLR4 as risk factor for periodontal disease: a reappraisal. J Clin Periodontol 2009; 36:279–286. [DOI] [PubMed] [Google Scholar]
- 35.Song GG, Kim J-H, Lee YH. Toll-like receptor (TLR) and matrix metalloproteinase (MMP) polymorphisms and periodontitis susceptibility: a meta-analysis. Mol Biol Rep 2013; 40:5129–5514. [DOI] [PubMed] [Google Scholar]
- 36.Zheng J, Gao L, Hou T, et al. Association between TLR4 polymorphism and periodontitis susceptibility: a meta-analysis. Crit Rev Eukaryot Genet Expr 2013; 23:257–264. [DOI] [PubMed] [Google Scholar]
- 37.Han M-x, Ding C, Kyung H-M. Genetic polymorphisms in pattern recognition receptors and risk of periodontitis: evidence based on 12,793 subjects. Hum Immunol 2015; 76:496–504. [DOI] [PubMed] [Google Scholar]


