Abstract
Purpose. Gastric emptying (GE) is often reported to be slower and more irregular in premature neonates than in older children and adults. The aim of this study was to investigate the impact of age and other covariates on the rate of GE. Methods. The effect of age on the mean gastric residence times (MGRT) of liquid and solid food was assessed by analysing 49 published studies of 1457 individuals, aged from 28 weeks gestation to adults. The data were modelled using the nonlinear mixed‐effects approach within NONMEM version 7.2 (ICON, Dublin, Ireland), with evaluation of postnatal age, gestational age and meal type as covariates. A double Weibull function was selected as a suitable model since it could account for the typical biphasic nature of GE. Results. Age was not a significant covariate for GE but meal type was. Aqueous solutions were associated with the fastest emptying time (mean simulated gastric residence time of 45 min) and solid food was associated with the slowest (98 min). Conclusions. These findings challenge the assertion that GE is different in neonates, as compared with older children and adults due to age, and they reinforce the significance of food type in modulating GE. © 2015 The Authors. Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Keywords: gastric emptying, neonatal gut, paediatric gastroenterology, infant gut
Introduction
The stomach controls the rate at which nutrients and xenobiotics (including drugs) reach the duodenum and are absorbed into the systemic circulation. In the fasted state, gastric motility occurs in continuity with the rest of the intestine as part of the migrating motor complex (MMC) 1. In the fed state, gastric motility is highly dependent on meal composition 1, and may be slowed by diseases such as diabetes 2, in people with high body mass index (BMI) 3 and in the elderly 4.
Scintigraphy (typically using a solid meal labelled with 99mTc‐sulphur colloid or a liquid meal labelled with indium) is generally considered to be the gold standard method for measuring gastric emptying 5, 6, and correlations have been established with results of the 13C‐acetate breath test 7, PEG dilution 8, acetaminophen (paracetamol) kinetics 8 and ultrasound 3, 9. Gastric emptying profiles are often noted to be biphasic, especially after a solid and/or high‐fat meal, due to inhibitory feedback from the small intestine 1. The first phase is indicated either by a lag time 10, 11, 12 or an initial faster process 4, 12, 13.
It is often stated that gastric emptying is slower in neonates, especially premature ones, compared with older infants and children 14, 15, 16, 17, 18, 19. This is usually assigned to immaturity of the neuro‐regulation of motility, although data on the postnatal development of gastric motility are equivocal. Gastric emptying cycles have been observed in fetuses of 24 weeks gestation 20, and premature neonates born at 30 to 38 weeks' gestation have been shown to have the isolated pyloric pressure wave patterns necessary to coordinate gastric functions including stomach emptying 21. In addition, stable patterns of slow gastric waves have been reported in premature neonates at 28 to 36 weeks after gestation and starting at postnatal day 3 22. However, in term and pre‐term infants a 30–40% incidence of normal slow waves (between two and four counts per minute) was found to increase with age to nearly 90% in adults in both the fasting and fed states, indicating significant development of post‐natal coordination of gastric emptying 23, 24.
Although premature neonates have been noted to be more likely than term neonates to suffer from feed intolerance, regurgitation and gastro‐oesophageal reflux, the causes of this may be altered feedback from the proximal small intestine and intolerance to the high fat content of preterm infant formulas rather than any impairment of gastric motility 25, 26, 27. Lange et al. found no difference in the half‐emptying time between preterm and term infants after a test meal of water 28. However, Riezzo et al. observed a significantly longer half‐emptying time at 1 week after birth in preterm infants (28–32 weeks' gestation) compared with preterm neonates born at a later gestational age and term neonates after a meal of formula 18. No differences in gastric emptying half‐life have been observed between older children, adolescents and adults 29. However, it appears that there have been no studies comparing gastric emptying times between ages ranging from preterm neonates through to adults.
A complication in evaluating whether or not gastric emptying is impaired in term or preterm neonates is that in the fed state the process is known to be strongly influenced by meal type, which may differ depending on the ages of the subjects studied. While older children and adults may be tested using a variety of liquids and solids, neonates can only consume liquid test meals, usually breast milk or formula (and occasionally water or sugar solutions), which confounds comparisons with studies done in older adults and children using solid meals. Liquids are known to empty more rapidly from the stomach than solids and breast milk empties more quickly than formula 13, 30, 31. Aqueous solutions empty more quickly than liquids containing fat and/or protein content which, in turn, empty more rapidly than solids 1.
Understanding changes in gastric emptying with age is important both clinically in terms of the pathophysiology of disease and for predicting the absorption of orally administered drugs 32. Gastric emptying is a primary determinant of the rate at which drugs are presented to the small intestinal mucosa for absorption and, along with a multitude of other age related changes, may influence the design of suitable dosage forms for administration to neonates and infants. This study is part of a wider effort to develop a comprehensive paediatric drug absorption model.
The aim of this study was to investigate the impact of age and other covariates on the rate of gastric emptying. Accordingly, to document a more complete understanding of the impact of development on gastric emptying, a meta‐analysis of the literature with respect to studies on preterm neonates through to adults was done using a mixed effect modelling approach.
Methods
Search strategy and data selection
A literature search was undertaken using the PubMed and Embase databases. Keywords were 'gastric emptying' (PubMed) and 'gastric emptying AND neonates' or 'gastric emptying AND pediatrics' with limits (PubMed) or filters (Embase) of 'human' and 'English'. In PubMed, initial searches were conducted in each of the paediatric age groups individually, i.e. 'Newborn: birth–1 month', 'Infant: birth–23 months', 'Pre‐school child: 2–5 years', 'Child: 6–12 years', 'Adolescent: 13–18 years', and the adult age categories. The last data search was in June 2014. Some references were also obtained from the bibliographies of published papers.
The inclusion criterion was studies in healthy preterm neonatal through to adult subjects that reported the % remaining of gastric contents at time points after administration of a test meal. Subject groups were excluded if they were obese, receiving drugs affecting GI motility (such as metoclopramide or cisapride), had disease (except for apnoea) and were stated to have regurgitation or vomiting. Paediatric and adult subjects were excluded if they had proven gastro‐oesophageal reflux (GOR), but not if referred for testing for suspected GOR. The study database used to obtain the final model comprised 49 studies of 1457 subjects. The final model was validated using an independent dataset comprising 17 studies of 468 subjects. The latter were selected randomly after grouping them for similar age ranges.
Data extraction
The data extracted from the studies consisted of either the mean or median % of a test meal remaining in the stomach at various time points after administration. When not listed in manuscript tables or text, data on the percentage remaining in the stomach after administration of food were retrieved from figures by computer digitalization (Enguage Digitizer, Version 4.1, Free Software Foundation, Inc., Boston, MA).
Model building and selection criteria
The data were modelled using a nonlinear mixed effects approach using NONMEM, Version 7.2 (ICON, Dublin, Ireland) with the first‐order conditional estimation method (FOCE) with interaction. The model building process was guided by graphical analysis of goodness of fit plots in Xpose 4 Version 4.3.5 33 and changes in the Akaike information criterion (AIC) calculated from the objective function values (OFV) obtained from NONMEM and the number of model parameters. Uncertainty in estimated model parameters is indicated by the relative standard error values (RSE) obtained from NONMEM.
Based on visual inspection of the % remaining versus time data, three structural models were tested: namely exponential decay 34, exponential decay with a lag time 34 and a double Weibull function 35. An advantage of the latter model (Eq. 1) is the flexibility it provides with respect to describing the relative speed of two emptying phases.
| (1) |
Where GE ij is the percentage of the test meal remaining in the stomach at time t ij for the ith publication at jth time point. The parameters γ 1,i and γ 2,i define the scatter and β 1,i and β 2,i define the shape of the distribution. If the condition γ 1,i < γ 2,i is satisfied, then the parameter PR i represents the percentage of test meal remaining in the stomach when the emptying study period is complete. The value of PR i was constrained between 0 and 100 by using a logit transformation 36.
Inter‐study variability (η i) in the parameters was described using an exponential error model (Eq. 2).
| (2) |
The ith study's parameter θ i was assumed to differ from the population mean parameter by a value of exp (η i). The value of η i was assumed to have a normal distribution with a mean of 0 and variance of ω2.
The residual error structure (Eq. 3) was weighted based on the number of patients represented at each time point and the method used to measure the GE ij, to account for heteroscedasticity and any inaccuracies in the methods.
| (3) |
Where, is the model prediction based on the data, θ w is the standard deviation, N ij is the sample size, T i is the test type used to measure the % remaining and ε ij is the additive‐error component of residual variability. T was given a value of 2 when the data were obtained by scintigraphy and 1 when another method was used in order to give more weight to scintigraphy. The variance of ε was fixed at 1.
Mean gastric residence times (MGRT) were calculated using Equation (4)
| (4) |
where Г( ) is the gamma function.
Covariate selection and evaluation
Since the aim of the work was to assess the influence of age on gastric emptying, age was tested as a covariate after allowance for type of test meal as a known significant covariate. To facilitate the analysis, test meals used in the studies were divided into five categories: namely aqueous solution (water, sugar solutions, fruit juice), breast milk, formula (any variety, including nutritional shakes), semi‐solid meals (pudding, rice cereal or oatmeal) and solid meals. The meal type was entered into the model as a binomial variable, either 0 (absent) or 1 (present). The effect of meal type was tested on different parameters of the model shown in Equation (1). Covariate selection was based on visual inspection of the η and covariate plots and AIC values.
After allowance for the effect of test meal type, the postnatal age and gestational age were each tested as covariates. Postmenstrual age was used for graphical purposes and was calculated as the mean or median gestational age of the subjects in the study plus the mean or median postnatal age in weeks. For preterm infants, their gestational age at birth (in weeks) was added to their postnatal age in weeks. For term infants, a standard full‐term gestational age of 40 weeks was added to postnatal age in weeks. For adults, age in years was converted to weeks and 40 weeks was added. The method of gastric emptying determination was accounted for in the residual error model and thus was not included as a separate covariate. Having selected the best model it was subjected to visual predictive checks in PLT tools Version 4.0 (PLTsoft, San Francisco, CA).
Simulations based on the final model
The final model was coded in MATLAB Version 2010.2 (MathWorks, Natick, MA) and, using the associated parameter estimates, % remaining versus time plots was simulated for 1000 individuals covering the age range of 0.01 to 800 months for the five different meal types. The resulting MGRT values for the simulated individuals were calculated using Equation (4).
Results
Data characteristics
The initial literature search yielded a total of 7514 articles. Ultimately, 66 papers that reported the percentage of the test meal remaining at one or more time points were identified. The initial modelling was done using a dataset of 49 of these gastric emptying studies (representing 1457 individuals) covering an age range from preterm neonates at 28 weeks' gestation to adults. General characteristics of the studies are listed in Table 1 with more details in the supplementary material. Sampling times ranged from 20 to 300 min after meal ingestion, the number of time points per study ranged from 1 to 19, and the number of subjects per study ranged from 6 to 186. Sex demographics of subjects are shown in Figure 1. Of the 1457 subjects in the original data set, 637 were paediatric subjects (mostly neonates) in whom sex was not specified. Of the remaining subjects, 495 were paediatric and 325 were adults. Of the 495 paediatric subjects in whom sex was specified, 256 were male and 228 were female, and 403 were preterm neonates. Of the 325 adult subjects in whom sex was specified, 160 were male and 165 were female. The group of 165 adult women included those of both pre‐ and postmenopausal ages, but in most studies it was not possible to make this distinction. In addition, in one study containing premenopausal females, all 44 subjects were studied in the first half of the menstrual cycle, which should have negated any potential hormonal effects on gastric emptying.
Table 1.
Studies used to construct and (*) validate the final model. References for the studies can be found in the supplementary material for the online version of this article
| Study no | Year | Study population | Age in months Mean (range) | Method | Meal | Feed category in analysis | Duration of sampling (min) | No of subjects | No of time points per subject |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1980 | Preterm neonates | (0–0.25) | D (PR) | 10% dextrose solution | Aqueous | 30 | 20 | 1 |
| 2 | 1980 | Preterm neonates | 0.5 | D (PR) | Sugar solutions | Aqueous | 30 | 16 | 1 |
| 3 | 2004 | Preterm neonates | 0.57 (0.20–1.2) | S (Tc) | Breast milk | Breast milk | 120 | 10 | 2 |
| 4 | 1996 | Preterm neonates | 1.47 (0.5–2.77) | U/S | Breast milk | Breast milk | 150 | 11 | 16 |
| 5 | 2000 | Preterm neonates | 1 | D (PEG) | Breast milk/formula | Breast milk/formula | 120 | 11 | 2 |
| 6 | 1997 | Preterm neonates | 0.77 | U/S | Breast milk/formula | Breast milk/formula | 120 | 32 | 9 |
| 7 | Preterm neonates | 0.73 | D | Breast milk/formula | Breast milk/formula | 180 | 31 | 2 | |
| 8 | Preterm neonates | 0.87 | D (PEG) | Breast milk/formula | Breast milk/formula | 20 | 27 | 1 | |
| 9 | 1994 | Preterm neonates | 0.36 (0.13–0.87) | U/S | Breast milk/formula | Breast milk/formula | 200 | 14 | 17 |
| 10 | 2008 | Preterm neonates | 0.13 | U/S | Breast milk/formula | Breast milk/formula | 120 | 20 | 1 |
| 11* | 1992 | Preterm neonates | 0.233 | D | Breast milk/formula | Breast milk/formula | 240 | 50 | 1 |
| 12 | 1999 | Preterm neonates | 0.9 (0.47–1.23) | U/S | Breast milk/formula | Breast milk/formula | 120 | 21 | 6 |
| 13 | 1983 | Preterm neonates | 0.41 (0.20–1.1) | S (Tc) | Formula | Formula | 30 | 16 | 1 |
| 14 | 1994 | Preterm neonates | 0.043 | S (Tc) | Formula | Formula | 120 | 20 | 16 |
| 15 | 1991 | Preterm neonates | 0.33 | D (PR) | Formula | Formula | 30 | 10 | 1 |
| 16 | 1978 | Preterm/term neonates | 0.011/0.04/0.071 | D (PR) | 5% dextrose solution | Aqueous | 30 | 47 | 1 |
| 17 | 1975 | Preterm/term neonates | 0.033 | D (PR) | 10% glucose solution | Aqueous | 30 | 12 | 1 |
| 18 | 2008 | VLBW neonates | (0.2–1) | U/S | Breast milk | Breast milk | 180 | 20 | 18 |
| 19 | 1996 | Preterm neonates/infants | 0.67 (0.2–2.23) | U/S | Breast milk | Breast milk | 120 | 22 | 9 |
| 20 | 2007 | Preterm neonates/infants | 1.33 | D | Formula | Formula | 180 | 9 | 6 |
| 21 | 1982 | Preterm neonates/infants | 1.2 (0.23–2.1) | D (PEG) | Formula | Formula | 120 | 10 | 6 |
| 22 | 1984 | Preterm neonates/infants | 1.5 (0.23–2.8) | D (PEG) | Formula | Formula | 100 | 10 | 5 |
| 23 | 1985 | Preterm neonates/infants | 1.4 (0.47–2.3) | D (PEG) | Formula | Formula | 100 | 11 | 5 |
| 24 | 1984 | Term neonates | (0.1–0.17) | D (PR) | Sugar solutions | Aqueous | 30 | 13 | 1 |
| 25 | 1980 | Term neonates | 0.30 (0.17–0.87) | D (PR) | 10% dextrose solution | Aqueous | 180 | 14 | 9 |
| 26 | 1992 | Infants | 9 (6–14) | D (PEG) | 5% dextrose solution | Aqueous | 80 | 10 | 5 |
| 27 | 1990 | Infants | (0–12) | S (Tc) | Breast milk/formula | Breast milk/formula | 2/3 | 90 | 2 |
| 28* | 2011 | Infants | 1.57 | U/S | Formula | Formula | 3 | 21 | 1 |
| 29 | 1989 | Infants | 7.56 (1.68–19.0) | D (PEG) | Formula | Formula | 3 | 12 | 4 |
| 30 | 2006 | Infants | 4.6 | U/S | Formula | Formula | 3 | 14 | 5 |
| 31 | 1993 | Infants | 4.3 (1–11) | S (Tc) | Formula | Formula | 3 | 28 | 1 |
| 32 | 2007 | Infants | 3 | S (Tc) | Formula | Formula | 3 | 81 | 2 |
| 33 | 1988 | Infants | 5.7 (2–14) | S (Tc) | Rice cereal | Semi‐solid | 120 | 10 | 1 |
| 34 | 1988 | Neonates/infants | 1.2 (0.47–1.63) | U/S | Formula | Formula | 180 | 6 | 12 |
| 35 | 1995 | Preterm/term infants | 0.8 (pre)/1.5 (term) | APT | Dioralyte/formula | Aqueous/formula | 90 | 82 | 10 |
| 36 | 1983 | Infants/children | 35.7 (0.75–168) | D (PEG) | Water | Aqueous | 60 | 30 | 4 |
| 37* | 2012 | Infants/children | 18 | S (Tc) | Breast milk/formula | Breast milk/formula | 60 | 81 | 1 |
| 38* | 2012 | Infants/children | 5 (infants), 84 (children) | U/S | 'Milk' | Formula | 20/120 | 44/11 | 4/13 |
| 39 | 1983 | Infants/children | 5.7 (1–23)/109 (24–174) | S (Tc) | 'Milk formula' | Formula | 60 | 49 | 1 |
| 40 | 2011 | Children | 110 (76.8–154) | MRI | Raspberry syrup soln | Aqueous | 120 | 16 | 4 |
| 41 | 2012 | Children | 133 (98.4–150) | MRI | Raspberry syrup soln | Aqueous | 60 | 14 | 2 |
| 42 | 1998 | Children | 84 (60–132) | S (Tc) | Pancakes | Solid | 120 | 11 | 8 |
| 43 | 1987 | Neon/inf/child/adolesc | 10 (0.5–192) | S (Tc) | Apple juice | Aqueous | 60 | 14 | 2 |
| 44 | 1984 | Neon/inf/child/adolesc | 1/8/19/42/120 | S (Tc) | Breast milk/for/water | Breast milk/for/aq | 120 | 44 | 2 |
| 45 | 1987 | Neon/inf/child/adolesc | 1.8/4.9/8.9/20.9/52.8/134 | S (Tc) | Formula or pudding | Formula/semi‐solid | 60 | 186 | 1 |
| 46 | 1997 | Children/adolescents | 153.6 | S (Tc) | Pancakes | Solid | 120 | 17 | 11 |
| 47* | 2011 | Adults | 840 | S (Tc) | Sucrose solution | Aqueous | 300 | 10 | 19 |
| 48* | 2012 | Adults | 418 | S (Tc) | Orange juice | Aqueous | 60 | 7 | 1 |
| 49* | 2011 | Adults | 598 | S (Tc) | Formula/lemonade | Aqueous/formula | 120 | 8 | 8 |
| 50 | 2011 | Adults | 744 (228–1008) | S (Tc) | Formula | Formula | 240 | 14 | 4 |
| 51 | 1981 | Adults | 340 (240–432) | S (Tc) | Cow's milk | Formula | 60 | 6 | 4 |
| 52 | 1989 | Adults | 504 (240–912) | S (Tc) | Liquid prot, fat, carb | Formula | 60 | 88 | 8 |
| 53 | 2012 | Adults | 564 | MRI | Liquid prot, fat, carb | Formula | 60 | 6 | 2 |
| 54 | 2011 | Adults | 468 | S (Tc) | Eggs/bread/milk | Solid | 240 | 19 | 12 |
| 55 | 1989 | Adults | 336 | S (Tc) | Tuna sandwich/milk | Solid | 90 | 10 | 1 |
| 56 | 2000 | Adults | 372 | S (Tc) | Egg/ham/bread/OJ | Solid | 165 | 15 | 11 |
| 57 | 1985 | Adults | 320 (252–432) | S (Tc/In) | Chicken liver/water | Solid | 120 | 13 | 12 |
| 58 | 2000 | Adults | (204–960) | S (Tc) | Omelette | Solid | 120 | 160 | 17 |
| 59* | 2012 | Adults | 439 | S (Tc) | Omelette | Solid | 120 | 18 | 11 |
| 60 | 2004 | Adults | (240–600) | S (In/Tc) | Water/omelette | Aqueous/solid | 120 | 11 | 8 |
| 61* | 2000 | Adults | 397.2 | S (Tc) | Omelette | Solid | 180 | 7 | 18 |
| 62* | 1994 | Adults | 342 (240–564) | S (Tc) | Scrambled egg/bread | Solid | 180 | 20 | 12 |
| 63 | 2011 | Adults | 565 | S (Tc) | Egg white sandwich | Solid | 240 | 23 | 3 |
| 64* | 2000 | Adults | 355 | S (Tc) | Egg sandwich | Solid | 120 | 9 | 8 |
| 65* | 2000 | Adults | 492 | S (Tc) | Egg subst/bread/jam | Solid | 240 | 123 | 3 |
| 66* | 1992 | Adults | 444 | S (Tc) | Egg sandwich | Solid | 90 | 10 | 1 |
Data used in model validation only.
neon, neonate; inf, infant; VLBW, very low birth weight; S, scintigraphy; Tc, 99technetium; In, 111indium; D, dilution method; PR, phenol red; PEG, polyethylene glycol; U/S, ultrasound; MRI, magnetic resonance imaging.
Figure 1.

Age and sex of the subjects in the study data set
In the original dataset, the test meal was given orally in 22 studies, by nasogastric tube in 21 studies, by orogastric tube in four studies, and either orally or via tube in two studies. With regard to body position, the subjects were supine in 20 studies, prone in six, in the right lateral position in ten, sitting or standing in five, sitting or supine in one, prone or supine in one, and the subject position was not specified in six studies.
Final model selection
The objective functions for the three models that were tested are listed in Table 2. Based on these values and goodness of fit plots, the double Weibull function was selected as the final model. The results of the visual predictive check for the original data set are shown in Figure 2, and indicate that the majority of the observed data points fall within the 2.5th and 97.5th percentiles of the simulations with model parameters.
Table 2.
Models evaluated and their objective function values (OFVs). The final model is indicated by a bold italic font
| Model | Attributes | OFV |
|---|---|---|
| Exponential models | Combined error | 2801.212 |
| Exponential + lag time | Additive error | 2696.632 |
| Exponential + lag time | Combined error | 2681.434 |
| Double Weibull | Combined error | 2176.032 |
| Double Weibull (BASE MODEL) | Combined error + Accounting for number of individuals in study + Test type | 2138.568 |
| Covariate model | BASE MODEL + Food types | 1875.252 |
| Covariate model | BASE MODEL + Food types + postnatal Age | 1875.121 |
| Covariate model | Food types + Gestational age | 1875.211 |
Figure 2.
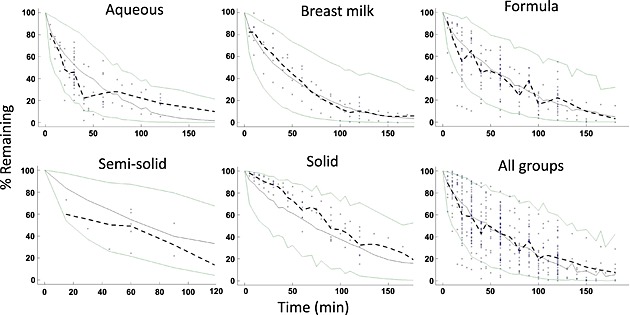
Visual predictive check plots. The green lines represent 2.5th and 97.5th percentiles of model‐predicted data. The solid grey line represents the 50th percentile of model‐predicted data. The dashed black line represents the median of the observed data
Covariates
Test meal type had a significant influence on the mean gastric residence time (as indicated by the objective function value). Addition of gestational or postnatal age in the final model did not change the objective function value, indicating they are not significant covariates.
The simulations with the final model indicated mean gastric residence times of 45 min for aqueous solutions, 57 min for breast milk, 64 min for formula, 87 min for semi‐solid food and 98 min for solid test meals (Figure 3). The lack of a relationship between MGRT and age irrespective of meal type is illustrated in Figure 4A, B.
Figure 3.
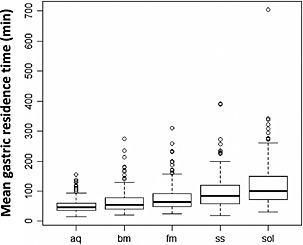
Box‐whisker plots indicating a relationship between model‐based simulated mean gastric residence time and meal type: aq, aqueous solution; bm, breast milk; fm, formula; ss, semi‐solid meal; sol, solid meal
Figure 4.
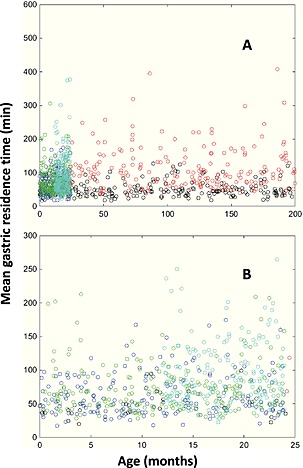
Model‐based simulation of the relationship between postnatal age and mean gastric residence time, allowing for differences in the meal type. (A) whole 0–17 age range and (B) more detailed view for the 0–25 month age range. Open circles represent meal types: black, aqueous; blue, breast milk; green, formula milk; cyan, semi‐solid; red, solid
Model evaluation
The final model parameter estimates and their precision are shown in Table 3, and the goodness of fit plots, including population and individual model predictions, individual weighted residuals against individual predictions and weighted residuals with time are shown in Figure 5. The residual plots indicate that no systematic error was detected in the final model. The result of the visual predictive check for the final model with respect to the independent validation dataset is shown in Figure 6. This shows that the majority of the data points in the independent data set also fall within the 2.5th and 97.5th percentiles of the simulated data produced by the model. This indicates that the model predicts independent gastric emptying data well.
Table 3.
Final population model parameters
| Model parameter | Estimate (RSE) | Variability, ω2 (RSE) |
|---|---|---|
| PR (%) | 0.26 (17.7%) | 114 (11.6%) |
| β 1 | 0.816 (6.1%) | 38.6 (10.5%) |
| β 2 | 2.48 (11.3%) | 14.1 (15.2%) |
| γ 1(min) | 37.6 (21%) | 58.7 (8.1%) |
| γ 2 (min) | 63.7 (7.6%) | 19.2 (28.1%) |
| θ w | 11.1 (15.9%) | NA |
| θ Aqueous | 0.697 (25.3%) | NA |
| θ Breast milk | 0.959 (35.7%) | NA |
| θ Form | 1.15 (21.7%) | NA |
| θ Semi solid | 1.61 (37.5%) | NA |
| θ Solid | 1.99 (22.4%) | NA |
The parameters γ 1 and γ 2 define the scatter and β 1 and β 2 define the shape of the Weibull distribution function; PR represents the remaining percentage of test meal in the stomach when emptying is temporarily halted (see Eq. 1); θ w is the weighted standard deviation (see Eq. 3); θ Aqueous, θ Breast Milk,θ Form, θ Semi solid and θ Solid are the coefficient estimates for the following food types as covariates, aqueous solution, breast milk, formula, semi‐solid meals and solid meals, respectively. RES (%) is the relative standard error (%). NA, not applicable.
Figure 5.
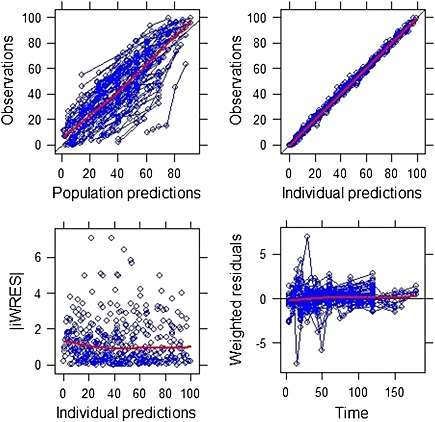
Goodness of fit plots showing population and individual predictions, individual weighted residuals against individual predictions and weighted residuals against time. Lines are the best fit to the data
Figure 6.
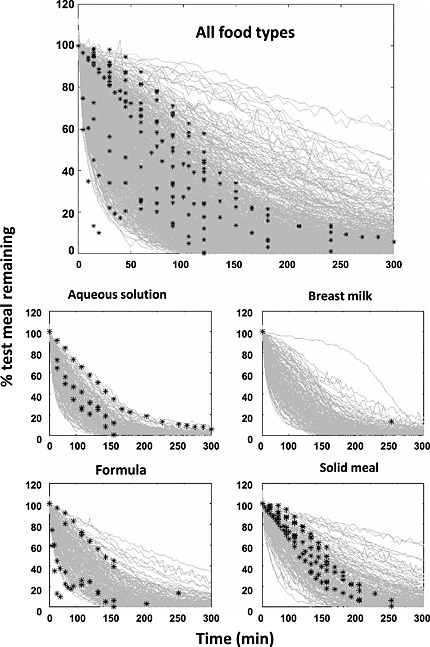
Visual predictive check (VPC) of the final model against the validation dataset in relation to meal type. Black stars are the observed data
Discussion
This study has been the largest evaluation of covariates for gastric emptying involving paediatrics data. Our meta‐analysis of 66 publications provided 'no evidence' for an effect of postnatal age or gestational age on the mean gastric emptying time, although a significant influence of meal type was confirmed, with aqueous solutions emptying most rapidly and solid meals most slowly and with liquid meals falling in between. This is consistent with previous reports 2, 37, 38. Strengths of our meta‐analysis include the large number of subjects represented in the initial dataset (1457) that contained a significant number of preterm neonates of a variety of gestational ages, as well as a weighting method that gave a higher weight to scintigraphic measurement as the gold standard and to time points with data from individuals. In addition, the type of gastric emptying data used in this study (% remaining at various time points) is likely to be more reliable than the often‐reported measure of gastric emptying half‐life, which is subject to bias from the method of calculation and by extrapolation beyond the testing time.
There are also limitations to the analysis, given the diversity of study protocols. These include the relatively small number of subjects (24%) between the ages of 1 and 10 years. Further issues are the differences in body position across studies, the wide range of calorific, fat and protein contents and volumes of test meals and their method of administration, and the presence of some premenopausal women among the adult subjects in the database. Gastric emptying is reported to be faster in the prone and right lateral positions than when supine 39, although one study in neonates that specifically addressed the effect of body position showed no influence 40. Ultimately body position was not included in the analysis because of the wide range of posture in the studies and the lack of convincing evidence of a strong effect. Significant variability in and missing information on the calorific, fat and protein contents and volumes of test meals and their method of administration across studies precluded any meaningful evaluation of these variables. Gastric emptying has been shown to be faster after a fatty compared with a protein based meal 41, and with greater feed calorific density and volume 41, 42. In the limited number of studies where information on feed volume was available a sub‐analysis was performed but showed no significant correlation with measured gastric emptying (data not shown).
It should also be noted that age and test meal types were somewhat confounded in the analysis because semi‐solid/solid meals were not tested in neonates such that the results only represent the age groups older than 2 months. However, aqueous and liquid meals were tested across all ages including around 150 adults spread over eight studies. A recent study in 700 children (which was published after the present analysis) has shown significantly faster gastric emptying associated with tube feeding relative to oral feeding 43. As many newborns are tube fed this may have obscured an impact of age in this study. However, the same study also found no effect of age on gastric emptying when allowing for the feeding method.
With regard to the effects of differences in gender, although a slower gastric emptying rate has been noted in some studies in premenopausal females compared with postmenopausal females and males 44, likely due to the effects of oestrogens in the luteal phase of the menstrual cycle 45, most of the adult subjects in the dataset that was evaluated were men and postmenopausal women. Given the likely hormonal causes of slower gastric emptying in adult women of reproductive age, the gender of the subject was assumed to have no effect on gastric emptying before puberty. Therefore, it was not accounted for in the overall analysis given that 1132 of the 1457 subjects (approximately 78%) in the dataset were paediatric. Since only 165 of the total 1457 subjects (577 adult subjects) were adult women it is unlikely that gastric emptying differences between males and females could have been shown from the data.
This study is part of a wider project to understand the underlying age related physiological changes that may affect oral drug absorption in the paediatric population with a view to the development of a paediatric in silico absorption model. Such models will not only increase our understanding of the influence of specific GI system parameters on drug absorption but may also aid in the development of new oral formulations for this population.
Conclusion
Overall, the findings challenge the assertion that gastric emptying times are different in neonates, including premature neonates, compared with older children and adults, and reinforce the significance of food type in modulating gastric emptying time. Because of the limits of the available data, further prospective studies of gastric emptying times across a wide age range, using a liquid meal of consistent volume and nutritional content and using scintigraphy are warranted. However, for the purpose of developing a general model of paediatric drug absorption, the initial model will define gastric emptying time to be independent of age, although it will vary according to meal type in the fed state.
Conflict of Interest
The authors have no conflicts of interest to declare.
Supporting information
Supporting into item
Supporting into item
Acknowledgements
The authors gratefully acknowledge the assistance of James Kay and Eleanor Savill with manuscript formatting and Emma Booker with image formatting. Jennifer Bonner's salary was funded by a European Framework 7 grant entitled 'Treatment of Adrenal Insufficiency in Neonates' (TAIN).
Bonner, J. J. , Vajjah, P. , Abduljalil, K. , Jamei, M. , Rostami‐Hodjegan, A. , Tucker, G. T. , and Johnson, T. N. (2015), Does age affect gastric emptying time? A model‐based meta‐analysis of data from premature neonates through to adults. Biopharm. Drug Dispos., 36, 245–257. doi: 10.1002/bdd.1937.
The copyright line for this article was changed on 5 March 2015 after original online publication.
References
- 1. Barrett KE. Gastrointestinal Physiology. McGraw‐Hill Medical: New York, 2006. [Google Scholar]
- 2. Ziessman HA, Fahey FH, Collen MJ. Biphasic solid and liquid gastric emptying in normal controls and diabetics using continuous acquisition in LAO view. Digestive Diseases and Sciences 1992; 37: 744–750. [DOI] [PubMed] [Google Scholar]
- 3. Brogna A, Ferrara R, Bucceri AM, et al. Gastric emptying rates of solid food in relation to body mass index: an ultrasonographic and scintigraphic study. European Journal of Radiology 1998; 27: 258–263. [DOI] [PubMed] [Google Scholar]
- 4. Brogna A, Ferrara R, Bucceri AM, et al. Influence of aging on gastrointestinal transit time. An ultrasonographic and radiologic study. Investigative Radiology 1999; 34: 357–359. [DOI] [PubMed] [Google Scholar]
- 5. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterology and Motility 2011; 23: 8–23. [DOI] [PubMed] [Google Scholar]
- 6. Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Journal of Nuclear Medicine Technology 2008; 36: 44–54. [DOI] [PubMed] [Google Scholar]
- 7. Braden B, Peterknecht A, Piepho T, et al. Measuring gastric emptying of semisolids in children using the 13C‐acetate breath test: a validation study. Digestive and Liver Disease 2004; 36: 260–264. [DOI] [PubMed] [Google Scholar]
- 8. Naslund E, Bogefors J, Gryback P, et al. Gastric emptying: comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scandinavian Journal of Gastroenterology 2000; 35: 375–379. [DOI] [PubMed] [Google Scholar]
- 9. Gomes H, Hornoy P, Liehn JC. Ultrasonography and gastric emptying in children: validation of a sonographic method and determination of physiological and pathological patterns. Pediatric Radiology 2003; 33: 522–529. [DOI] [PubMed] [Google Scholar]
- 10. Siegel JA, Urbain JL, Adler LP, et al. Biphasic nature of gastric emptying. Gut 1988; 29: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Couturier O, Le Rest C, Gournay J, et al. Gastric emptying of solids: estimates of lag phase and constant emptying times. Nuclear Medicine Communications 2000; 21: 665–675. [DOI] [PubMed] [Google Scholar]
- 12. Lawaetz O, Dige‐Petersen H. Gastric emptying of liquid meals. A study in 88 normal persons. Annales Chirurgiae et Gynaecologiae 1989; 78: 267–276. [PubMed] [Google Scholar]
- 13. Cavell B. Gastric emptying in preterm infants. Acta Paediatrica Scandinavica 1979; 68: 725–730. [DOI] [PubMed] [Google Scholar]
- 14. Gupta M, Brans YW. Gastric retention in neonates. Pediatrics 1978; 62: 26–29. [PubMed] [Google Scholar]
- 15. Yigit S, Akgoz A, Memisoglu A, et al. Breast milk fortification: effect on gastric emptying. The Journal of Maternal‐Fetal & Neonatal Medicine 2008; 21: 843–846. [DOI] [PubMed] [Google Scholar]
- 16. Cavell B. Reservoir and emptying function of the stomach of the premature infant. Acta Paediatrica Scandinavica. Supplement 1982; 296: 60–61. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez A, Wong WW, Shulman RJ. Factors regulating gastric emptying in preterm infants. The Journal of Pediatrics 2006; 149: 475–479. [DOI] [PubMed] [Google Scholar]
- 18. Riezzo G, Indrio F, Montagna O, et al. Gastric electrical activity and gastric emptying in term and preterm newborns. Neurogastroenterology and Motility 2000; 12: 223–229. [DOI] [PubMed] [Google Scholar]
- 19. Riezzo G, Castellana RM, De Bellis T, et al. Gastric electrical activity in normal neonates during the first year of life: effect of feeding with breast milk and formula. Journal of Gastroenterology 2003; 38: 836–843. [DOI] [PubMed] [Google Scholar]
- 20. Sase M, Miwa I, Sumie M, et al. Gastric emptying cycles in the human fetus. American Journal of Obstetrics and Gynecology 2005; 193: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 21. Hassan BB, Butler R, Davidson GP, et al. Patterns of antropyloric motility in fed healthy preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002; 87: F95–F99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riezzo G, Indrio F, Raimondi F, et al. Maturation of gastric electrical activity, gastric emptying and intestinal permeability in preterm newborns during the first month of life. Italian Journal of Pediatrics 2009; 35: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen JD, Co E, Liang J, et al. Patterns of gastric myoelectrical activity in human subjects of different ages. The American Journal of Physiology 1997; 272: G1022–G1027. [DOI] [PubMed] [Google Scholar]
- 24. Liang J, Co E, Zhang M, et al. Development of gastric slow waves in preterm infants measured by electrogastrography. The American Journal of Physiology 1998; 274: G503–G508. [DOI] [PubMed] [Google Scholar]
- 25. Tomomasa T, Itoh Z, Kiozumi T, et al. Nonmigrating rhythmic activity in the stomach and duodenum of neonates. Biology of the Neonate 1985; 48: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Barnett CP, Omari T, Davidson GP, et al. Effect of cisapride on gastric emptying in premature infants with feed intolerance. Journal of Paediatrics and Child Health 2001; 37: 559–563. [DOI] [PubMed] [Google Scholar]
- 27. Berseth CL. Gastrointestinal motility in the neonate. Neo Gastroenterol 1996; 23: 179–190. [PubMed] [Google Scholar]
- 28. Lange A, Funch‐Jensen P, Thommesen P, et al. Gastric emptying patterns of a liquid meal in newborn infants measured by epigastric impedance. Neurogastroenterology and Motility 1997; 9: 55–62. [DOI] [PubMed] [Google Scholar]
- 29. Maes BD, Ghoos YF, Geypens BJ, et al. Relation between gastric emptying rate and energy intake in children compared with adults. Gut 1995; 36: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ewer AK, Durbin GM, Morgan ME, et al. Gastric emptying in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1994; 71: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cavell B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatrica Scandinavica 1981; 70: 639–641. [PubMed] [Google Scholar]
- 32. Mooij MG, de Koning BAE, Huijsman ML, et al. Ontogeny of oral drug absorption processes in children. Expert Opinion on Drug Metabolism & Toxicology 2012; 8: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 33. Jonsson EN, Karlsson MO. Xpose – an S‐PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Computer Methods Programs Biomed 1999; 58: 51–64. [DOI] [PubMed] [Google Scholar]
- 34. Gibaldi M, Perrier D. Pharmacokinetics. Marcel Dekker: New York, 1982. [Google Scholar]
- 35. Locatelli I, Mrhar A, Bogataj M. Gastric emptying of pellets under fasting conditions: a mathematical model. Pharmaceutical Research 2009; 26: 1607–1617. [DOI] [PubMed] [Google Scholar]
- 36. Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research (4th edn). Wiley: Malden, MA, 2002. [Google Scholar]
- 37. Nour S, Mangnall YF, Dickson JA, et al. Applied potential tomography in the measurement of gastric emptying in infants. Journal of Pediatric Gastroenterology and Nutrition 1995; 20: 65–72. [DOI] [PubMed] [Google Scholar]
- 38. Spiegel TA, Fried H, Hubert CD, et al. Effects of posture on gastric emptying and satiety ratings after a nutritive liquid and solid meal. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 2000; 279: R684–R694. [DOI] [PubMed] [Google Scholar]
- 39. Yu VYH. Effect of body position on gastric emptying in the neonate. Archives of Disease in Childhood 1975; 50: 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pildes RS, Blumenthal I, Ebel A. Stomach emptying in the newborn. Pediatrics 1980; 66: 482–483. [PubMed] [Google Scholar]
- 41. Hunt JN, Smith JL, Jiang CL. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology 1985; 89: 1326–1330. [DOI] [PubMed] [Google Scholar]
- 42. Fisher RS, Rock E, Malmud LS. Effects of meal composition on gallbladder and gastric emptying in man. Digest Dis Sci 1987; 32: 1337–1344. [DOI] [PubMed] [Google Scholar]
- 43. Chen W, Codreanu I, Yang J, et al. Tube feeding increases the gastric‐emptying rate determined by gastroesophageal scintigraphy. Clinical Nuclear Medicine 2013; 38: 962–965. [DOI] [PubMed] [Google Scholar]
- 44. Nusynowitz ML, Benedetto AR. The lag phase of gastric emptying: clinical, mathematical and in vitro studies. Journal of Nuclear Medicine 1994; 35: 1023–1027. [PubMed] [Google Scholar]
- 45. Gryback P, Hermansson G, Lyrenas E, et al. Nationwide standardisation and evaluation of scintigraphic gastric emptying: reference values and comparisons between subgroups in a multicentre trial. European Journal of Nuclear Medicine 2000; 27: 647–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting into item
Supporting into item


