Abstract
Objectives
Comprehensive epidemiologic data for multiple sclerosis (MS) in Spain are limited. The aim of this study was to collect epidemiologic data on MS in the Northern Seville District of Spain.
Materials and methods
This longitudinal study identified possible MS cases every year from nine centres between 1 January 1991 and 31 December 2011. Patients with a confirmed diagnosis of MS were included. MS data prior to enrolment were collected retrospectively from clinical records and prospectively during quarterly follow‐up clinic visits. Variables included age at onset, age at diagnosis, treatment, follow‐up duration, number of visits, number of relapses, change in the number of relapses over time and Expanded Disability Status Scale score. The incidence and prevalence of MS rate were calculated.
Results
Overall, 156 patients with MS were identified (111 females; mean follow‐up 7.5 years). Most patients had relapsing‐remitting MS (73.7%); primary progressive disease was less frequent than secondary disease (10.9% vs 15.4%). The yearly incidence of MS was 4.6 per 100,000, and the prevalence at 31 December 2011 was 90.2 per 100,000.
Conclusions
The annual MS incidence rate in this southern region of Spain was higher than previously reported rates in Spanish studies.
Keywords: epidemiology, incidence studies, longitudinal studies, multiple sclerosis, Spain
Introduction
Multiple sclerosis (MS) is a complex, chronic inflammatory, progressive neurologic disorder that is the leading cause of non‐traumatic neurologic disability in young adults 1, 2. Its aetiology is unknown, but as for many complex diseases, environmental, genetic and immunologic factors have all been implicated 1. The most common age of onset for MS is 20–40 years, and the disease tends to be more common in women 1, 2. MS affects individuals at a productive time in their lives, has a negative impact on quality of life 3 and is associated with significant economic burden 4.
Studies outside Europe suggest that the distribution of MS is related to latitude (lower MS prevalence in the south versus the north) 5, 6, 7, 8, 9, and similar findings have been reported in most European populations 9, 10. However, the validity and persistence of this trend have been questioned by other researchers 11, 12, particularly in northern Scandinavia and Italy 9.
The estimated mean annual incidence of MS in Europe is 4.3 cases per 100,000, with a prevalence over the last 30 years of 83 per 100,000 10. However, there is marked variation in epidemiologic estimates from different countries throughout Europe 10. In Spain, the estimated incidence of MS is 3.8 per 100,000 and prevalence is 36–55 per 100,000 10. Recent comprehensive epidemiologic data for MS in Spain are limited. To try to address this lack of data, we report results from a study of the incidence and prevalence of MS over a 20‐year period in a southern district of Spain.
Materials and methods
Study design and setting
This epidemiologic, longitudinal study was conducted in the Northern Seville District of Andalucia, Spain (Fig. 1). To reduce the influence of immigration on data collected, the study area excluded the urban area of Seville, where immigration is more prevalent. In Spain, 96% of the population is covered by public health insurance, and approximately 15% also uses private facilities. Furthermore, 96% of individuals in the region of Andalucia are covered by the public healthcare system, which is managed by the information system known as DIRAYA 13.
Figure 1.
Area of the study. Northern Seville District of Andalucia, Spain. Latitude between 37.24 and 38.19.
Every year for 20 years, neurologists from three public hospitals in the North Seville region (Hospital Universitario Virgen Macarena, Hospital Virgen del Rocio, and Hospital Virgen de Valme) and six neurologists from private centres in this region were consulted (see Data collection section) and asked to report cases where patients had a possible diagnosis of MS.
Case ascertainment
Patients with clinically definite MS or laboratory‐supported definite MS were identified and included in this study. Patients were considered to have clinically definite MS if they have had two attacks and two clinical and/or paraclinical lesions (assessed by magnetic resonance imaging [MRI] or electrophysiology [EP] imaging). MRI and EP were performed following routine clinical practice in the same hospital as the treating clinician. There was no requirement to perform the MRI or EP in a certain period of time from diagnosis or from an attack. If a diagnosis of MS was not clinically definite (if the patient presented with a progressive course or has only one MS attack), the testing of cerebrospinal fluid (CSF) was mandatory and had to be positive for immunoglobulin G (IgG) synthesis (tested by immunoelectrophoresis) or oligoclonal banding (OCB; tested by IgG immunophelometry) to be considered a definite laboratory‐supported MS diagnosis. A positive IgG or OCB intrathecal synthesis was required to be considered laboratory‐supported definite MS. The diagnosis of MS was made/confirmed by the study investigators (Dr. Izquierdo and Dr. Navarro) based on history taking, clinical criteria, magnetic resonance imaging (MRI) criteria (including evoked responses) and testing of CSF for IgG synthesis (Tibbling/Link index) and OCB, and verified using the criteria of Poser et al. 14. If some other diagnosis could explain the clinical symptoms, the patient was excluded from the study.
Data collection
The study period was 1 January 1991 to 31 December 2011. Data on MS prior to enrolment in the study were collected by retrospective analysis of patient clinical records by each neurologist. During the study period, MS data from the clinical records of each patient were prospectively collected by each neurologist during scheduled clinic visits, which took place four times each year. A telephone interview of the patient or the patient's family doctor was conducted by the treating neurologist to obtain MS data for patients who were unable to attend the scheduled visits.
Data verification
The MS data for each clinical record were entered into a central database, and each record was verified by the principal investigator on an annual basis. If the data or clinical records were missing/incomplete, the treating neurologist was contacted and, if necessary, the patient was contacted by telephone.
Standard protocol approvals, registrations, and patient consents
Written informed consent was required for participation in the study. The study was approved by the Institutional Review Board at Hospital Universitario Virgen Macarena.
Variables
Information was recorded for the following variables: age at onset, age at study entry, age at diagnosis, age at last follow‐up, follow‐up duration, number of visits (overall and corrected by follow‐up duration), number of relapses (overall and corrected by follow‐up year), change in the number of relapses over time, Expanded Disability Status Scale (EDSS) score at follow‐up (i.e. at each clinic visit) and treatment.
Incidence and prevalence
Incidence was calculated annually (on 31st December) using the number of newly diagnosed cases each year from 1st January to 31st December as the numerator and the size of the population as per the official census of 2001 (consulted on 15 December 2011) as the denominator (163,324 inhabitants, 82,139 women and 81,185 men) 15. The same denominator was used each year because this geographical region does not show great change in population size over time (Fig. 2). The mean incidence was then calculated as a mean of the individual yearly values over the 20‐year period. Prevalence of MS was calculated annually on 31st December each year, including all living patients in the geographical region but excluding deceased patients or those who had moved away from the area by that date. Incidence and prevalence data were adjusted by sex.
Figure 2.
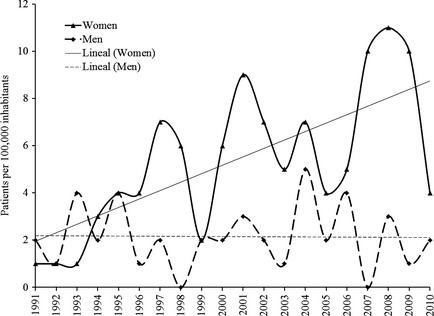
Incidence of multiple sclerosis in men and women by year over a 20‐year period (1 January 1991 to 31 December 2011) in the Northern Seville District of Spain.
Statistical analyses
Distribution frequencies and percentages were calculated for nominal variables. Mean, median and standard error [SE] were calculated for numeric variables using SPSS software (IBM Corporation, Armonk, NY, US). Analysis of raw and adjusted data on incidence and prevalence was conducted using EPIDAT software (Dirección Xeral de Innovación e Xestión da Saúde Pública de la Consellería de Sanidade, Xunta de Galicia, Spain). Incidence data were compared in the first 10 years of the study and the last 10 years of the study in women and in men, and prevalence data were compared between men and women. These comparisons were made using a chi‐squared test.
Results
A total of 169 patients were identified by neurologists as having a possible diagnosis of MS over the 20‐year study period. Overall, 156 of the 169 patients had a confirmed diagnosis of MS; 155 were from one centre (Hospital UniversitarioVirgen Macarena), and one was identified through a private neurologist. The remaining 13 patients were excluded from the study because a diagnosis of MS could not be confirmed; three of these patients were subsequently confirmed not to have MS. The female‐to‐male ratio was 2.5:1. Demographic and clinical characteristics of these patients at baseline are shown in Table 1; patients were followed up for a mean of 7.5 years (range 0–19.4 years), and the mean number of total follow‐up visits per patient was 11.
Table 1.
Demographic and clinical characteristics of study patients
| Patients with multiple sclerosis (n = 156) | |
|---|---|
| Male, n (%) | 45 (28.8) |
| Female, n (%) | 111 (71.2) |
| Age, years, mean ± SE | |
| At study entry | 36.95 ± 0.92 |
| At disease onset | 31.97 ± 0.80 |
| At diagnosis | 36.48 ± 0.89 |
| At follow‐up (end of the study) | 39.55 ± 0.91 |
| Clinical form at study entry, n (%) | |
| Relapsing remitting | 115 (73.7) |
| Primary progressive | 17 (10.9) |
| Secondary progressive | 24 (15.4) |
| Pregnancy, n/N (%)a | 13/111 (11.7) |
| Treatment during the follow‐up period, n/N (%)b | 99/156 (63.5) |
| Interferon beta | 67/156 (42.9) |
| Glatiramer acetate | 20/156 (12.8) |
| Selective immunosuppressants (natalizumab and fingolimod) | 10/156 (6.4) |
| Untreated at study endc | 57/156 (36.5) |
| EDSS, mean ± SE (N = 154)d | 3.61 ± 0.19 |
| EDSS <4, n/N (%)d | 88/154 (57.1) |
| EDSS ≥4, n/N (%)d | 66/154 (42.9) |
| Years of monitoring, mean ± SE, N = 154d | 7.55 ± 0.44 |
| Number of visits, mean ± SE, N = 154d | 11.44 ± 0.73 |
| Visits per year, mean ± SE, N = 154d | 2.23 ± 0.17 |
EDSS, Expanded Disability Status Scale; SE, standard error.
Cumulative incidence of pregnancies over the 20‐year study period in 111 women.
Treatment data were collected at the final study visit (31 December 2011).
Untreated patients were those with benign forms of the disease who did not meet the criteria for disease‐modifying treatments.
Two patients were excluded from the analysis of disability because data were not available for these patients.
Treatment/cumulative drug use
A total of 99 of the 156 patients (63.5%) were treated with immunomodulators (n = 87) or immunosuppressants (n = 12) during the course of the study. The remaining 57 (36.5%) patients were diagnosed with progressive MS, secondary progressive courses without criteria for modifying disease treatment or with benign forms of MS at the moment of the study without criteria for modifying disease treatment. As a result, these patients did not meet the criteria for disease‐modifying treatment and only received symptomatic therapies.
Incidence and prevalence of MS
The incidence of MS in this population was 4.6 (95% CI 4.1–5.1) per 100,000 per year over the 20‐year period. The incidence of MS increased significantly (P < 0.0001) in women from 4.3 (95% CI 3.6–4.9) in the first decade of the study (1991–2000) to 8.8 (95% CI 7.84–9.69) in the second decade (2001–2010). In contrast, there was no significant increase in men (from 2.5 [95% CI 2.0–2.9] to 2.8 [95% CI 2.3–3.4]; Fig. 2). Furthermore, fluctuations in the prevalence of MS over time were more notable in women. While the difference in the incidence of MS between men and women was not considered statistically significant (P = 0.06), the difference between women and men increased every quinquennium from 1:1 in the first to 4:1 in the last (Fig. 3).
Figure 3.
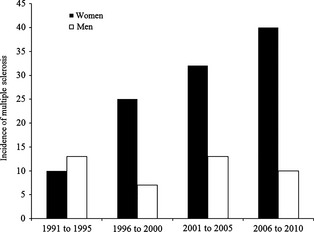
Incidence of multiple sclerosis in men and women by 5‐year increments over a 20‐year period (1 January 1991 to 31 December 2011) in the Northern Seville District of Spain.
The prevalence of MS as of 31 December 2011 was 90.2 (95% CI 75.6–104.8) per 100,000; 127.6 (95% CI 103.5–151.8) per 100,000 in women and 54.2 (95% CI 38.0–70.4) per 100,000 in men, which also differed significantly (P < 0.001).
Discussion
The results of this longitudinal study show that the incidence of MS in the Northern Seville District of southern Spain is 4.6 cases per 100,000 people per year over a 20‐year period. Prevalence of MS is 90.2 cases per 100,000 people. Patients are most likely to present with relapsing‐remitting disease, are more often female (sex ratio female/male = 2.5:1) and are most likely to be diagnosed in their mid‐30s.
Overall, the prevalence of MS appears to be increasing, particularly in Europe and the Mediterranean Basin 16, 17. Prevalence in Spain does appear to be on the increase, with a recent study by Fernandez et al. 18, also in southern Spain, reporting the highest ever figure recorded (125 per 100,000). The current study and the study by Fernandez et al. report a much higher prevalence of MS than in previous epidemiologic studies conducted in the 1990s in various regions of Spain (32–65 per 100,000 population), including an earlier study in southern Spain by Fernandez et al. 19, 20, 21, 22, 23, 24, 25. The higher prevalence compared with earlier studies is probably related to numerous factors, including higher survival in MS and better diagnostic accuracy 26. We can probably expect then that the prevalence of MS in Spain will continue to increase in the future.
The very high prevalence rate in southern Spain reported by Fernandez and colleagues may be attributable in part to differences in study methodology: they used the capture–recapture method where undetected cases of MS were included in the prevalence estimate, thus reducing ascertainment bias 21. These authors noted that their methods of estimating prevalence were different from all of the previous studies in Spain. Nevertheless, the prevalence reported in the Fernandez study remains below that of northern European countries, which are well known to have amongst the highest prevalence rates in Europe 10. However, accuracy of survey methodology, better surveillance and the availability of registries are potential confounding variables.
Likewise, the annual incidence rate of the current study over a 20‐year period was 4.6 per 100,000, a figure much higher than that reported in previous epidemiologic studies in Spain (incidence rates of between 2.2 and 3.8 per 100,000 per year) 19, 22, 23, 25. As the genetic background of the population of Northern Seville District of Spain is not very different from other areas of Spain and the rest of South Europe, this was unexpected. However, these studies analysed incidence over much shorter time frames, the longest being a 10‐year period in a study in northern Spain 23.
Data showing a higher incidence and prevalence of MS in females versus males in the current study are consistent with the published literature 10, 26, 27. Across all of Europe, the prevalence of MS is higher in females, with a female: male ratio of 1.1–3.4 10. The incidence of MS increased significantly over time in women but not in men in the current study. Furthermore, fluctuations in the prevalence of MS over time were more notable in women. The increasing incidence of MS amongst women may explain the increase in prevalence rate observed in this study. An increasing incidence of MS in women (particularly relapsing‐remitting disease) is part of a changing geoepidemiology of MS 26, 28. The underlying reason for this remains to be determined, but vitamin D deficiency has been proposed as one of the most biologically plausible explanations 29.
Previous estimates for Spain have put the number of patients with relapsing‐remitting, those with combined progressive‐relapsing or secondary progressive and those with primary progressive disease at approximately 75%, 7% and 18%, respectively 10. The figures for relapsing‐remitting disease are very similar to those reported in this study (73.7%). However, in the current study, secondary progressive disease was more frequent than primary progressive disease (15.4% vs 10.9%). The reason for this difference from estimates for Spain as a whole is not known exactly. Genetic and environmental factors could be the cause for differences in the prevalence of primary progressive forms in our region, compared with other Spanish data. In addition, the long follow‐up of our patients could increase the number of secondary progressive patients in our study in comparison with other Spanish series. Nevertheless, the rate of primary progressive disease in the current study falls within general estimates for the incidence of this form of MS (10–15%) 30.
With respect to disease severity, patients from the North Seville area of Spain with MS appear to have comparatively mild disease. The proportion of patients with an EDSS score of <4 has been reported as 80% in Spain, compared with 33% in the United Kingdom 10. The results of the current study were a little more conservative, with 56.4% of patients having an EDSS of <4. It has been reported that the progression of disability in MS is slower when age at disease onset is lower 31.
Most patients were treated at some point during follow‐up with immunomodulators/immunosuppressants (63.5% of patients overall; 43% of all patients received interferon beta [IFN β]). A recent study has shown that early treatment with disease‐modifying therapy (in this case with IFN β‐1b) was associated with a significant survival advantage over placebo in patients with MS followed up for 21 years 32. This decreasing mortality trend and the lack of a curative therapy means that patients and their caregivers are living with the consequences of MS for longer, and the associated morbidity can be significant. MS has a significant negative impact on quality of life for the patient 3, 33, 34, 35, 36, but the burden (in terms of cost and care) is shared between healthcare systems and society 4.
A limitation of our study was that we employed limited case ascertainment methods. Also, the restriction of our sample to a small geographical region limits the generalizability of the findings. In addition, accurate information on differences between geographical areas and population demographics helps policymakers to define research priorities and to consider resource allocation and management. Our results do provide an important addition to the body of knowledge about the prevalence and evolution of MS in Spain and may allow for better identification of genetic and environmental factors that influence the occurrence of MS. Furthermore, our study was conducted over 20 years, a much longer period than previous MS epidemiologic studies in Spain, allowing the capture of cases that would otherwise not have been included in conventional epidemiologic studies of shorter duration. This also allowed observation of the change in frequency of the disease over time.
In conclusion, this long‐term epidemiologic study of MS in a single region of southern Spain has confirmed that the incidence of the disease is higher than previously reported in earlier Spanish studies and that this was attributable to a high incidence in women. The prevalence of MS in southern Spain was also higher than expected and given the low mortality rate may be expected to increase in future years. Larger population‐based studies are required to confirm our results.
Conflict of interest
Guillermo Izquierdo and Guillermo Navarro have received research grants from Fondo de Investigaciones Sanitarias (FIS), Consejería de Salud de la Junta de Andalucía, Servicio Andaluz de Salud, Universidad de Sevilla, Fundación Progreso y Salud, Fundación 2000, Teva, Sanofi‐Aventis, Merck‐Serono and Biogen‐Idec; honoraria for acting on scientific advisory boards and giving lectures for Teva, Biogen‐Idec, Sanofi‐Aventis, Genzyme, Merck‐Serono and Bayer‐Schering. He has also served on the Editorial Board of Revista de Neurología for the last 10 years and holds a patent for the digital quantification of IgG oligoclonal bands. Ana Venegas was an employee of Biogen‐Idec from October 2010 until August 2012.
Funding
Statistical analysis and medical writing for this work were funded by Biogen‐Idec Spain. Data collection was conducted as part of routine medical practice in all centres. No grant supported other parts of the study.
Acknowledgements
We would like to acknowledge T Harrison (inScience Communications, Springer Healthcare), N Ryan (formerly of inScience Communications), M Chang (formally of inScience Communications) and M Weitz (inScience Communications) for providing medical writing assistance in the preparation of this manuscript. Finally, we wish to thank R López (Invesalud Consultoría S.L., Madrid, Spain) for performing the statistical analysis and A Prada (inScience Communications) for translating selected portions of the statistical report and F de Pablo (dibujario.com) for providing Figure 1 map.
Izquierdo G, Venegas A, Sanabria C, Navarro G. Long‐term epidemiology of multiple sclerosis in the Northern Seville District. Acta Neurol Scand 2015: 132: 111–117. © 2015 The Authors. Acta Neurologica Scandinavica published by John Wiley & Sons Ltd.
The copyright line for this article was changed on 1 December 2015 after original online publication.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221–31. [DOI] [PubMed] [Google Scholar]
- 2. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000;343:938–52. [DOI] [PubMed] [Google Scholar]
- 3. Aronson KJ. Quality of life among persons with multiple sclerosis and their caregivers. Neurology 1997;48:74–80. [DOI] [PubMed] [Google Scholar]
- 4. Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics 2010;28:363–79. [DOI] [PubMed] [Google Scholar]
- 5. Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–8. [DOI] [PubMed] [Google Scholar]
- 6. Kurtzke JF. Geography in multiple sclerosis. J Neurol 1977;215:1–26. [DOI] [PubMed] [Google Scholar]
- 7. Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology 1985;35:672–8. [DOI] [PubMed] [Google Scholar]
- 8. Miller DH, Hammond SR, McLeod JG, Purdie G, Skegg DC. Multiple sclerosis in Australia and New Zealand: are the determinants genetic or environmental? J Neurol Neurosurg Psychiatry 1990;53:903–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson S Jr, Blizzard L, Otahal P, Van Der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta‐analysis. J Neurol Neurosurg Psychiatry 2011;82:1132–41. [DOI] [PubMed] [Google Scholar]
- 10. Pugliatti M, Rosati G, Carton H et al. The epidemiology of multiple sclerosis in Europe. Eur J Neurol 2006;13:700–22. [DOI] [PubMed] [Google Scholar]
- 11. Koch‐Henriksen N, Sorensen PS. Why does the north–south gradient of incidence of multiple sclerosis seem to have disappeared on the northern hemisphere? J Neurol Sci 2011;311:58–63. [DOI] [PubMed] [Google Scholar]
- 12. Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol 2004;55:65–71. [DOI] [PubMed] [Google Scholar]
- 13. Servicio Andaluz de Salud . Diraya‐Historia clínica electrónica [online]. Available at: http://www.juntadeandalucia.es/servicioandaluzdesalud/principal/documentosacc.asp?pagina=pr_diraya (accessed 19 December 2012).
- 14. Poser CM, Paty DW, Scheinberg L et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31. [DOI] [PubMed] [Google Scholar]
- 15. Instituto Nacional de Estadistica . Censo de población y vivienda [online]. Available at: http://www.ine.es/inebmenu/mnu_cifraspob.htm (accessed 19 December 2012).
- 16. Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg 2002;104:182–91. [DOI] [PubMed] [Google Scholar]
- 18. Fernandez O, Fernandez V, Guerrero M et al. Multiple sclerosis prevalence in Malaga, Southern Spain estimated by the capture–recapture method. Mult Scler 2012;18:372–6. [DOI] [PubMed] [Google Scholar]
- 19. Benito‐Leon J, Martin E, Vela L et al. Multiple sclerosis in Mostoles, central Spain. Acta Neurol Scand 1998;98:238–42. [DOI] [PubMed] [Google Scholar]
- 20. Bufill E, Blesa R, Galan I, Dean G. Prevalence of multiple sclerosis in the region of Osona, Catalonia, northern Spain. J Neurol Neurosurg Psychiatry 1995;58:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez O, Luque G, San Roman C, Bravo M, Dean G. The prevalence of multiple sclerosis in the Sanitary District of Velez‐Malaga, southern Spain. Neurology 1994;44(Pt 1):425–9. [DOI] [PubMed] [Google Scholar]
- 22. Modrego Pardo PJ, Latorre MA, Lopez A, Errea JM. Prevalence of multiple sclerosis in the province of Teruel, Spain. J Neurol 1997;244:182–5. [DOI] [PubMed] [Google Scholar]
- 23. Pina MA, Ara JR, Modrego PJ, Morales F, Capablo JL. Prevalence of multiple sclerosis in the sanitary district of Calatayud, Northern Spain: is Spain a zone of high risk for this disease? Neuroepidemiology 1998;17:258–64. [DOI] [PubMed] [Google Scholar]
- 24. Tola MA, Yugueros MI, Fernandez‐Buey N, Fernandez‐Herranz R. Prevalence of multiple sclerosis in Valladolid, northern Spain. J Neurol 1999;246:170–4. [DOI] [PubMed] [Google Scholar]
- 25. Uria DF, Abad P, Calatayud MT et al. Multiple sclerosis in Gijon health district, Asturias, northern Spain. Acta Neurol Scand 1997;96:375–9. [DOI] [PubMed] [Google Scholar]
- 26. Koch‐Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–32. [DOI] [PubMed] [Google Scholar]
- 27. Zsiros V, Fricska‐Nagy Z, Füvesi J et al. Prevalence of multiple sclerosis in Csongrád County, Hungary. Acta Neurol Scand 2014;130:277–82. [DOI] [PubMed] [Google Scholar]
- 28. Trojano M, Lucchese G, Graziano G et al. Geographical variations in sex ratio trends over time in multiple sclerosis. PLoS One 2012;7:e48078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stuve O. The increasing incidence and prevalence of female multiple sclerosis–a critical analysis of potential environmental factors. Autoimmun Rev 2011;10:495–502. [DOI] [PubMed] [Google Scholar]
- 30. Miller DH, Leary SM. Primary‐progressive multiple sclerosis. Lancet Neurol 2007;6:903–12. [DOI] [PubMed] [Google Scholar]
- 31. Opara JA, Jaracz K, Brola W. Quality of life in multiple sclerosis. J Med Life 2010;3:352–8. [PMC free article] [PubMed] [Google Scholar]
- 32. Goodin DS, Reder AT, Ebers GC et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta‐1b trial. Neurology 2012;78:1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ford HL, Gerry E, Johnson MH, Tennant A. Health status and quality of life of people with multiple sclerosis. Disabil Rehabil 2001;23:516–21. [DOI] [PubMed] [Google Scholar]
- 34. McCabew MP, de Judicibus M. The effects of economic disadvantage on psychological well‐being and quality of life among people with multiple sclerosis. J Health Psychol 2005;10:163–73. [DOI] [PubMed] [Google Scholar]
- 35. Stolp‐Smith KA, Atkinson EJ, Campion ME, O'brien PC, Rodriguez M. Health care utilization in multiple sclerosis: a population‐based study in Olmsted County, MN. Neurology 1998;50:1594–600. [DOI] [PubMed] [Google Scholar]
- 36. Yorkston KM, Johnson K, Klasner ER, Amtmann D, Kuehn CM, Dudgeon B. Getting the work done: a qualitative study of individuals with multiple sclerosis. Disabil Rehabil 2003;25:369–79. [DOI] [PubMed] [Google Scholar]


