Abstract
Objectives
To evaluate longer‐term safety and efficacy of a single self‐expanding stent up to 20 cm in length in patients with atherosclerotic disease of the superficial femoral (SFA) and proximal popliteal arteries.
Background
Angioplasty and stenting are options for revascularization of symptomatic peripheral artery disease. While angioplasty alone is effective in short lesions, outcomes in longer lesions (i.e., mean 8.7 cm) show suboptimal patency rates of 33% at one year.
Methods
Two hundred eighty‐seven patients (mean age 68 years, 66% male) were treated with the EverFlex™ Self‐Expanding Peripheral Stent System. Patients were followed through 3 years with yearly core lab adjudicated duplex ultrasonography for patency, radiographic assessment of stent fractures, and resting ankle brachial indices.
Results
Overall freedom from loss of primary patency at 3 years was 60.0%. Patency was significantly higher for lesions ≤8 cm compared with lesions >8 cm (71.0 vs. 50.5%, P < 0.0001). There was no significant difference in patency between single‐stent and multistent recipients (60.4 vs. 52.4%, P = 0.343). The three‐year freedom from clinically driven target lesion revascularization was 70.0%. At 3 years, the overall stent fracture rate was 0.9%.
Conclusions
DURABILITY II is the first investigational device exemption trial to report 3‐year duplex Doppler defined stent patency and CD‐TLR outcomes in long SFA and proximal popliteal lesions and demonstrated acceptable stent patency and freedom from CD‐TLR with a low fracture rate. These data suggest that use of a single long stent provides reasonable long‐term outcomes when intervention is required for symptomatic SFA and proximal popliteal arterial disease. © 2015 Wiley Periodicals, Inc.
Keywords: peripheral arterial disease, claudication, stents
INTRODUCTION
There continues to be uncertainty regarding the optimal endovascular treatment of patients with severe lifestyle limiting claudication associated with superficial femoral artery (SFA) and proximal popliteal artery peripheral artery disease (PAD) 1. Previous clinical trials have reported inconsistent data on the benefits of nitinol self‐expanding stents compared to percutaneous transluminal angioplasty for lesions in this challenging arterial segment 2, 3, 4, 5, 6, 7. Invariably, patient demographics and lesion characteristics, including lesion length and the presence of occlusive versus stenotic disease appear to be important factors impacting procedural success and one‐year patency 8. Moreover, concern remains regarding the potential impact of stent fractures in this location and the clinical durability of long stents implanted in patients with diffuse PAD 9, 10, 11, 12.
Despite improved results seen in newer nitinol stent designs, one of the limitations of stenting in the SFA and proximal popliteal artery is the use of multiple stents and the potential associated stent fracture rate and implications for long‐term clinical outcomes. Initial results from the FESTO study reported a stent fracture rate of 37.2% 9; similarly, the superficial Femoral Artery Stenting Trial demonstrated a stent fracture rate at 12 months of 12% 4. Longer‐term data regarding stent fractures in different stent designs have also been reported 11, 13. The RESILIENT trial followed patients for a total of three years after implantation; however, radiographic images of the stented limbs were obtained only at 18‐months to evaluate the likelihood of late stent fractures 13. Additionally, although RESILIENT included a moderate mean lesion length in the stent cohort (mean lesion length 7.0 cm), the trial only assessed clinically driven target lesion revascularization (CD‐TLR) and did not evaluate the commonly reported core laboratory adjudicated duplex ultrasound‐derived binary stent restenosis.
For the first time, we report the late 3‐year clinical outcomes of the DURABILITY II trial, assessing clinical outcomes in patients with severe, life‐style limiting claudication. As previously reported, DURABILITY II included patients with a mean lesion length of 8.9 cm 14. We report the associated CD‐TLR, and core laboratory assessed duplex ultrasound‐derived binary restenosis and stent fracture rates.
METHODS
Study Design
Detailed descriptions of the study design, inclusion and exclusion criteria, and outcomes through one‐year have been previously reported 14. Patients eligible for inclusion in the study were at least 18 years old, had stenotic, restenotic, or occluded lesions of the SFA and proximal popliteal arteries of Rutherford category 2–4. By angiographic examination, target lesion length could be no longer than 18 cm with evidence of ≥50% stenosis and one patent artery runoff to the foot. Written informed consent was obtained from all patients according to the protocols approved by the institutional review and Ethics Boards at each investigational site. Consent was obtained to follow the patients for three years following stent implantation. The study included independent oversight by a Data Safety Monitoring Board, a Clinical Events Committee and a Stent Fracture Adjudication Committee through 3‐years of follow‐up. Angiographic and radiographic (Beth Israel Deaconess Medical Center, Boston, MA), and duplex ultrasound (VasCore Ultrasound Core Laboratory, Boston, MA) core laboratories analyzed all procedural and follow‐up images.
Endpoints
The primary safety endpoint of the study was the major adverse event rate at 30 days, defined as clinically driven target lesion revascularization (CD‐TLR), amputation of the treated limb, or all‐cause mortality. The primary effectiveness endpoint was stent patency rate at 1 year, defined as a duplex ultrasound‐derived peak systolic velocity ratio <2.0 as adjudicated by the independent core laboratory and no clinically driven reintervention within the stented segment. The study primary endpoints have been previously described and reported 14. Secondary endpoints included assisted primary and secondary patency, improvement in the ankle‐brachial index (ABI), absolute claudication distance, and the Walking Impairment Questionnaire (WIQ), a validated measure of patient‐perceived walking capacity. Stent integrity was reported at 2 and 3 years.
Patient Assessments
Rutherford clinical category, resting ABI, the WIQ, laboratory results for serum creatinine and white blood cell count and concomitant medication use were obtained preprocedure. ABI, WIQ scores, and medication were also obtained at 30‐days, 1, 2, and 3 years follow‐up visits. Adverse event evaluation was performed at the end of the index procedure and at each follow‐up visit. Radiographic imaging for the evaluation of stent integrity was performed at 1, 2, and 3 years. A minimum of two images were required, one image in the straight anteroposterior position and the second image a cross table lateral radiograph with flexion at the knee.
Study Device
Study patients received the EverFlex™ Self‐Expanding Peripheral Stent (Covidien, Plymouth MN). The EverFlex™ stent is made of a nickel titanium alloy (nitinol) and is available in diameters 6, 7, and 8 mm and lengths 20, 30, 40, 60, 80, 100, 120, 150, and 200 mm. Although the intent of the study was to evaluate lesions treated with a single stent, multiple stents could be used at the investigator's discretion.
Statistical Analysis
Primary analysis of all baseline characteristics and study outcomes are based on all available data from all enrolled participants, unless otherwise indicated. Discrete variables are presented using frequency distributions and cross tabulations, and presenting the number of observations, mean and standard deviation summarized as continuous variables. Patient counts, not event counts, were used for the primary analysis for adverse event reporting, which includes the primary and secondary safety end points. A patient with more than one event was counted only once toward the event rate based on the total number of participants with adverse events. Kaplan‐Meier analysis was used to evaluate time‐to‐event data, including the primary effectiveness end point. Two‐sided P values of <0.05 and one‐sided P values of <0.025 were deemed statistically significant.
RESULTS
The details of patient characteristics and procedural results have been previously reported 14. Of the 287 patients originally enrolled in the DURABILITY II study, 220 were available for 3‐year follow‐up. Over the course of the study, 26 patients died, 20 patients withdrew consent, and 21 were lost to follow‐up (LTFU). Demographic, clinical and lesion characteristics are shown in Table 1.
Table 1.
Demographic and Lesion Characteristics
| Subject characteristics | N = 287 |
|---|---|
| Age (yrs.) | 67.7 ± 10.7 |
| Male | 66.2% (190/287) |
| Risk Factors | |
| Diabetes | 42.9% (123/287) |
| Hyperlipidemia | 86.1% (247/287) |
| Hypertension | 88.2% (253/287) |
| Renal Insufficiency | 9.8% (28/287) |
| Current smoker | 39.0% (112/287) |
| Myocardial Infarction | 20.9% (60/287) |
| Clinical Characteristics | |
| Rutherford Clinical Category | |
| 2 = Moderate claudication | 39.4% (113/287) |
| 3 = Severe claudication | 55.7% (160/287) |
| 4 = Ischemic rest pain | 4.5% (13/287) |
| 5 = Minor tissue loss | 0.3% (1/287) |
| ABI | 0.69 ± 0.19 (281a) |
| Mean ± SD (N) | |
| Lesion characteristics | |
| Preprocedure reference vessel diameter (mm) | 4.8 ± 0.9 (287) |
| Preprocedure minimum lumen diameter (mm) | 0.7 ± 0.8 (287) |
| Lesion Length (mm; 20‐to‐20 method) | 89.1 ± 44.8 (287) |
| Preprocedure diameter stenosis (%) | 85.8 ± 16.2 (287) |
| Total occlusion | 48.1% (138/287) |
| Calcification (moderate or severe) | 70.0% (201/287) |
ABI not available for six subjects due to noncompressible arteries.
Primary Endpoint at 3‐Years
The cumulative MAE rate consisting of death, amputation of the index extremity and CD‐TLR at three years was 40.9% (Table 2I). In patients who had evaluable patency assessment, defined as a core laboratory‐assessed diagnostic duplex ultrasound evaluation of the stented arterial segment or the presence of TLR, the overall freedom from loss of primary patency was 60.0% (Fig. 1, Table 3) with a freedom from CD‐TLR of 69.7% (Table 3). However, when analyzed based on lesion length (≤8 cm and >8 cm), freedom from loss of primary patency was 71.0% and 50.5%, respectively and the freedom from TLR was 79.6 and 61.2%, respectively. There were 2 amputations (0.7%) and only 7 patients (8 events) required surgical revascularization through the 3‐year follow‐up timepoint. Patients requiring a TLR underwent a variety of endovascular procedures determined at the discretion of the study operator as noted in Table 4V. A post‐hoc subgroup analysis showed that diabetes mellitus had no impact on patency at 3 years; patency was 60.2 and 59.7% for diabetic versus nondiabetic patients, respectively (P = 0.907). Similarly, an analysis was conducted to evaluate the effect of current smoking status and lesion occlusion on patency, and no differences were observed. Interestingly, patency was significantly improved in severely calcified lesions compared to lesions that were not severely calcified (67.3 vs. 54.3%, P = 0.009). Further analysis revealed that the mean lesion length was significantly longer for the nonseverely calcified lesion subset, (93.4 vs. 83.4 mm, P = 0.04). When adjusted for differences in lesion length, the patency rate between the two groups was still statistically significant (P = 0.04). There was no significant difference in patency between single‐stent and multistent recipients (60.4 vs. 52.4%, P = 0.343).
Table 2.
Three‐Year Safety Data
| Major adverse event | MAE at ≤ 30 days | MAE at ≤ 1 year | MAE at ≤ 2 year | MAE at ≤ 3 year | Total |
|---|---|---|---|---|---|
| Major adverse event | 0.0% (0/284) | 17.2% (47/273) | 33.0% (86/261) | 40.9% (105/257) | 37.3% (107/287) |
| Death | 0.0% (0/284) | 2.9% (8/273) | 7.7% (20/261) | 10.1% (26/257) | 9.1% (26/287) |
| Amputation of treated limb | 0.0% (0/284) | 0.0% (0/273) | 0.4% (1/261) | 0.8% (2/257) | 0.7% (2/287) |
| Clinically driven TLR | 0.0% (0/284) | 14.3% (39/273) | 25.7% (67/261) | 31.1% (80/257) | 28.6% (82/287) |
Note: The denominators include patients who had completed the visits for the timeframes listed, or those patients who did not complete visits but had a major adverse event (MAE) prior to that time frame.
Figure 1.
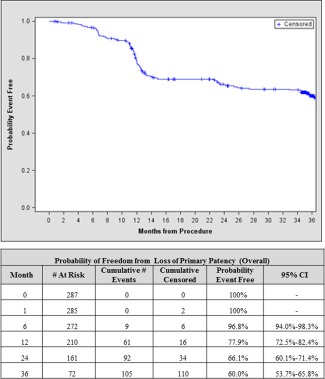
Freedom from Loss of Primary Patency (Overall). Kaplan‐Meier estimate of freedom from loss of primary patency defined as peak systolic velocity ratio (PSVR) <2.0 and no clinically driven reintervention within the stented segment. # At Risk gives the number of patients at risk of an event at the start of the interval, whereas # Censored and # Events are the incremental counts of patients censored or with events during the interval. CI, Confidence interval. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 3.
Patency Outcomes through 3 Years
| Patency outcomes | 1‐Year (N = 287) | 2‐Year (N = 287) | 3‐Year (N = 287) | P‐value |
|---|---|---|---|---|
| Freedom from loss of primary patency (PSVR < 2.0) | ||||
| All Subjects (n = 287) | 77.9% | 66.1% | 60.0% | |
| ≤80 mm (n = 133) | 87.5% | 80.9% | 71.0% | 0.0001 |
| > 80 mm (n = 154) | 69.6% | 53.3% | 50.5% | |
| Freedom from loss of assisted primary patency (PSVR < 2.0) | ||||
| All subjects | 82.2% | 72.2% | 66.1% | |
| ≤80 mm (n = 133) | 89.8% | 85.6% | 76.3% | 0.0003 |
| > 80 mm (n = 154) | 75.5% | 60.2% | 57.0% | |
| Freedom from loss of secondary patency | ||||
| All Subjects | 82.9% | 74.1% | 68.8% | |
| ≤80 mm (n = 133) | 89.8% | 85.6% | 78.1% | 0.001 |
| > 80 mm (n = 154) | 76.8% | 63.8% | 60.6% | |
| Freedom from TLR | ||||
| All Subjects | 86.0% | 75.3% | 69.7% | |
| ≤80 mm (n = 133) | 92.3% | 88.9% | 79.6% | 0.0003 |
| > 80 mm (n = 154) | 80.6% | 63.6% | 61.2% | |
Table 4.
Modes of Reintervention
| Reintervention type | N = 114a |
|---|---|
| PTA only | 63 (55.3%) |
| PTA and stent | 29 (25.4%) |
| Stent only | 7 (6.1%) |
| Surgery | 8 (7%) |
| Without reintervention type data | 7 (6.1%) |
| Atherectomy | 0 (0%) |
Number of TLRs in 82 patients.
Secondary Endpoints at 3‐Years
The proportion of asymptomatic (Rutherford clinical category 0) patients increased from 0% at baseline to 56.1% at one year. Per protocol, further assessments of Rutherford Class were not mandated. The mean baseline ABI of 0.65 increased by 0.23 and 0.24 at 2 and 3 years, respectively, with a corresponding improvement noted in more than 80% of patients (Table 5). We observed significant improvement in WIQ scores at 3 years when compared to baseline (P < 0.0001 for all categories; Table 6I).
Table 5.
Improvement of ABI through 3 Years
| ABI | Baseline | 1‐Year | 2‐Year | 3‐Year |
|---|---|---|---|---|
| Mean ± SD (N) | 0.65 ± 0.15 (246) | 0.89 ± 0.20 (223) | 0.88 ± 0.19 (192) | 0.89 ± 0.19 (181) |
| Changes in ABI | ||||
| % with improvement | – | 86.1% (192/223) | 85.9% (165/192) | 82.9% (150/181) |
| % with no change | – | 0.9% (2/223) | 0.0% (0/192) | 1.7% (3/181) |
| % with decrease | – | 13.0% (29/223) | 14.1% (27/192) | 15.5% (28/181) |
| Mean ± SD (N) | – | 0.24 ± 0.23 (223) | 0.23 ± 0.22 (192) | 0.24 ± 0.23 (181) |
Table 6.
Change in Walking Impairment Score
| Visit | ||
|---|---|---|
| WIQ scores | Baseline | 3‐Yeara |
| Score for pain, aching, or cramps in calves or buttocks (%) | ||
| Mean ± SD (N) | 41.1 ± 31.6 (199) | 74.1 ± 30.8 (199) |
| Changes from baseline (%) | ||
| Mean ± SD (N) | – | 33.0 ± 41.3 (199) |
| Walking Distance Score (%) | ||
| Mean ± SD (N) | 22.1 ± 25.3 (177) | 50.6 ± 38.8 (177) |
| Changes from baseline (%) | ||
| Mean ± SD (N) | – | 28.5 ± 36.9 (177) |
| Walking Speed Score (%) | ||
| Mean ± SD (N) | 21.2 ± 20.7 (143) | 35.9 ± 27.2 (143) |
| Changes from baseline (%) | ||
| Mean ± SD (N) | – | 14.7 ± 27.8 (143) |
| Stair Climbing Score (%) | ||
| Mean ± SD (N) | 40.5 ± 32.5 (163) | 57.1 ± 36.6 (163) |
| Changes from baseline (%) | ||
| Mean ± SD (N) | – | 16.6 ± 38.4 (163) |
Note: For each category, the means and changes from baseline represents paired data.
Changes from baseline to 3 years were statistically significant P < 0.0001 for each category.
A single stent was implanted in 272 patients with a single stent fracture noted at one‐year, classified as a clinically relevant Class V (transaxial spiral fracture) by the Stent Fracture Adjudication Committee. Yearly radiographic stent fracture surveillance was continued through 3‐years. The incidence of stent fractures at 2 and 3 years following implantation was 0.9%, respectively. One additional stent fracture (class III‐ fracture with preserved alignment of the components) was identified at the 2‐year follow‐up. No new fractures were evident at the 3‐year follow‐up visit.
DISCUSSION
The previously published 1‐year results of the DURABILITY II trial established the superiority of using a single, long (up to 20 cm) self‐expanding EverFlex™ stent in reducing duplex‐ultrasound‐defined binary restenosis in patient with severe life‐style limiting claudication compared to a predefined performance goal derived from patient‐level historical data 14. Furthermore, these 1‐year results confirmed an acceptable CD‐TLR rate and improvement in WIQ scores at 12‐months compared to baseline. Importantly, the study confirmed a low 0.4% stent fracture rate in long stents while demonstrating noninferiority to a prespecified safety performance goal.
The long‐term 3‐year data presented here show sustained outcomes in adjudicated primary patency, CD‐TLR, and stent fracture rates and maintenance of functional improvement (ABIs and WIQ scores) with use of the EverFlex™ stent. Three‐year follow‐up from the randomized controlled trial comparing the LifeStent™ nitinol stent (Bard Peripheral Vascular, Tempe, AZ.) to Percutaneous transluminal angioplasty (PTA) in the RESILIENT Trial 13 has been previously reported, noting a freedom from clinically driven TLR rate in the stent treatment arm of 75.5%. However, several important distinctions are notable: first, the core laboratory assessed lesion length of 7.0 ± 4.3 cm in the stent arm was shorter than the mean lesion length of 8.9 ± 4.4 cm in DURABILITY II. Moreover, this mean 7.0 cm lesion length was assessed using the “normal‐to‐normal” lesion assessment methodology that differs from the typically used “20%‐to‐20%” methodology used in DURABILITY II. The “normal‐to‐normal” assessment technique has the effect of extending the mean lesion length to include vascular segments, which are not sufficiently diseased and may typically go untreated in clinical practice. Indeed, if a similar “normal‐to‐normal” assessment was to be made in the DURABILITY II trial, the mean lesion length increased to 10.9 ± 4.5 cm. Second, the RESILIENT trial did not assess core lab adjudicated 3‐year binary restenosis, reporting only CD‐TLR rates. While freedom from revascularization is a primary concern of both the patient and clinician, the binary restenosis rate is a commonly used comparative metric. We note, however, that the association between duplex ultrasound‐defined binary restenosis and the need for revascularization in patients with moderate length SFA lesions and lifestyle limiting claudication is unclear. In fact, of the 106 patients who were deemed “failures” at 3 years, meeting the duplex ultrasound criteria for restenosis or requiring a TLR in DURABILITY II, 23.6% met ultrasound criteria of binary restenosis yet did not undergo a CD‐TLR. The current 3‐year analysis demonstrated the freedom from CD‐TLR rate of 70.0%, comparable to the 75% freedom from TLR rate reported in the RESILIENT trial, taking into account the longer lesions in DURABILITY II. Finally, the RESILIENT investigators did not report stent fracture rates beyond 18 months, noted as 4.1%, with new stent fractures observed between 12 and 18 months. Notably, the incidence of stent fractures at both 2 and 3 years following implantation was 0.9%.
Although not an Investigation device exemption study, VIBRANT reported 3 year patency of 25.9 and 50% fracture rates for the bare metal stent arm of the study 15. Comparison of 3 year data from the VIBRANT study to DURABILITY II study is impossible due to the considerable patient attrition rate. Only 60.8% of enrolled patients were available for the 3‐year follow‐up. Additionally, the VIBRANT study used a variety of off label nitinol stents in the trial. In their assessments of these stents, a variety of grades of stent fractures were reported further diminishing our ability to make direct comparisons.
The extended 3‐year CEC adjudicated follow‐up from the DURABILITY II trial provides several additional insights into the SFA disease process and patient‐related outcomes. Through 3‐years, the probability of survival was 90.9% with none of the reported 26 deaths related to the study device or procedure. Two index limb amputations occurred between year two and year three. Additionally, DURABILITY II is the first trial to report cumulative assisted primary patency rates through 3‐years (Table 3I). While we noted the 3‐year freedom of loss of primary patency was 60%, once a CD‐TLR occurred, we noted the subsequent freedom from loss of assisted primary patency was 66.1%. We were unable to capture the angiographic patterns of restenosis in the 114 CD‐TLR events. However, in re‐treating these lesions, most investigators used PTA alone (55.3%) while 25.4% used a combination of PTA and stenting. No investigators reported the use of stent grafts or atherectomy devices for revascularization.
The WIQ assessed walking improvement through 3‐years and documented maintenance of improved pain scores, increase in walking distance and walking speed in matched pairs. The maintenance of this important patient‐centered metric over 3‐years is encouraging and reinforces a role of this endovascular strategy in patients with lifestyle limiting claudication and suboptimal therapeutic response to more conservative measures.
Limitations
This study has several limitations. It was not a randomized controlled trial although it was the first to utilize a now widely accepted standard to demonstrate one‐year superiority to a pre‐specified performance goal for safety and effectiveness derived from PTA patient‐level data 16. Additionally, this study initially attempted to capture paired baseline and one‐year claudication treadmills, but was only able to analyze this functional endpoint in 33 patients 14. However, objective functional clinical outcomes were captured using the patient‐assessed WIQ of claudication with matched pairs pre‐procedure and at three‐years (Table 6). Finally, this study used a Peak systolic velocity ratio (PSVR) of <2.0 cm as its duplex‐ultrasound criteria to define stent patency at all‐time points as defined by the pre‐specified performance goal to determined SFA nitinol stent efficacy and safety 17, 18. However, other studies of nitinol stents in this vascular bed have used alternative definition for stent patency including <2.4 2, 12 and <2.5 3 Contemporary SFA nitinol stent trials have used the <2.0 endpoint 19; nonetheless, when freedom from loss of stent patency was re‐analyzed using the <2.4 PSVR criteria, we observed no significant difference in loss of patency rates at 3 years.
CONCLUSION
The DURABILITY II is the first and largest investigational device exemption trial to provide 3‐year assessment of adjudicated primary stent patency, CD‐TLR rates and stent fracture assessment in a cohort with severe lifestyle limiting claudication associated with moderate to long length SFA and proximal popliteal artery lesions. We conclude that deployment of a single, long EverFlex™ stent in long femoropopliteal lesions is a safe strategy associated with minimal stent fractures and provides an acceptable and durable clinical result through three‐years.
ACKNOWLEDGMENTS
The authors would like to thank Colleen Holthe for study coordination, Mei Jiang, PhD for statistical analysis and Azah Tabah, PhD for technical review of the manuscript.
Conflict of Interest: MB and GS have no conflicts to report. MJ is a non‐compensated advisor for Abbott Vascular, Boston Scientific, Cordis Corporation, Covidien Vascular, and Medtronic Vascular. He is a Board member of VIVA Physicians (a 501 c 3 not‐for‐profit education and research organization). He has an equity investment in PQ Bypass.
REFERENCES
- 1. Mwipatayi BP, Leong BD, Hockley J, Vijayan V. The pitfalls of femoropopliteal stenting trials. J Endovasc Ther 2012;19:596–598. [DOI] [PubMed] [Google Scholar]
- 2. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879–1888. [DOI] [PubMed] [Google Scholar]
- 3. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve‐month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 2010;3:267–276. [DOI] [PubMed] [Google Scholar]
- 4. Krankenberg H, Schluter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116:285–292. [DOI] [PubMed] [Google Scholar]
- 5. Dick P, Wallner H, Sabeti S, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv 2009;74:1090–1095. [DOI] [PubMed] [Google Scholar]
- 6. Dearing DD, Patel KR, Compoginis JM, Kamel MA, Weaver FA, Katz SG. Primary stenting of the superficial femoral and popliteal artery. J Vasc Surg 2009;50:542–547. [DOI] [PubMed] [Google Scholar]
- 7. Dake MD, Scheinert D, Tepe G, et al. Nitinol stents with polymer‐free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: Twelve‐month safety and effectiveness results from the zilver PTX single‐arm clinical study. J Endovasc Ther 2011;18:613–623. [DOI] [PubMed] [Google Scholar]
- 8. Soga Y, Iida O, Hirano K, Yokoi H, Nanto S, Nobuyoshi M. Mid‐term clinical outcome and predictors of vessel patency after femoropopliteal stenting with self‐expandable nitinol stent. J Vasc Surg 2010;52:608–615. [DOI] [PubMed] [Google Scholar]
- 9. Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol 2005;45:312–315. [DOI] [PubMed] [Google Scholar]
- 10. Schlager O, Dick P, Sabeti S, et al. Long‐segment SFA stenting–the dark sides: in‐stent restenosis, clinical deterioration, and stent fractures. J Endovasc Ther 2005;12:676–684. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki K, Iida O, Soga Y, et al. Long‐term results of the S.M.A.R.T. control(TM) stent for superficial femoral artery lesions, J‐SMART registry. Circ J 2011;75:939–944. [DOI] [PubMed] [Google Scholar]
- 12. Bosiers M, Torsello G, Gissler HM, et al. Nitinol stent implantation in long superficial femoral artery lesions: 12‐month results of the DURABILITY I study. J Endovasc Ther 2009;16:261–269. [DOI] [PubMed] [Google Scholar]
- 13. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. Balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: Three‐year follow‐up from the RESILIENT randomized trial. J Endovasc Ther 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 14. Matsumura JS, Yamanouchi D, Goldstein JA, et al. The United States study for evaluating endovascular treatments of lesions in the superficial femoral artery and proximal popliteal by using the protege everflex nitinol stent system II (DURABILITY II). J Vasc Surg 2013;58:73–83 e1. [DOI] [PubMed] [Google Scholar]
- 15. Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM. Investigators V. Three‐year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg 2013;58:386–395 e4. [DOI] [PubMed] [Google Scholar]
- 16. Rocha‐Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv 2007;69:910–919. [DOI] [PubMed] [Google Scholar]
- 17. Kawarada O, Higashimori A, Noguchi M, et al. Duplex criteria for in‐stent restenosis in the superficial femoral artery. Catheter Cardiovasc Interv 2013;81:E199–205. [DOI] [PubMed] [Google Scholar]
- 18. Higashimori A, Kawarada O, Morioka N, et al. Impact of changing PSVR thresholds on the patency rates of SFA recanalisation with self‐expanding nitinol stents. EuroIntervention 2013;9:964–967. [DOI] [PubMed] [Google Scholar]
- 19. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel‐eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve‐month zilver PTX randomized study results. Circ Cardiovasc Interv 2011;4:495–504. [DOI] [PubMed] [Google Scholar]


