Abstract
Objective
The aim of the study was to investigate the feasibility and preliminary efficacy of an Internet‐based guided self‐help intervention for posttraumatic stress symptoms (PTSS) and related symptoms in parents of children on cancer treatment.
Methods
Parents of children on cancer treatment, who fulfilled the modified symptom criteria on the PTSD Checklist, were randomly allocated to the intervention or to a wait‐list control condition. The intervention group accessed a 10‐week guided self‐help program via the Internet based on principles from cognitve behavior therapy. The primary outcome PTSS and the secondary outcomes depression and anxiety were assessed by self‐report preintervention and postintervention.
Results
Seven hundred forty‐seven parents were approached and informed about the study, 92 were assessed for eligibility, and 58 were included and randomized to the intervention (n = 31) or wait list (n = 27). Eightteen participants completed the intervention. Intention‐to‐treat analyses indicated a significant effect of the intervention on PTSS with a large between‐group effect size at postassessment (Cohen's d = 0.88). The intervention group reported reductions in PTSS with a large within‐group effect size (d = 1.62) compared with a minimal reduction in the wait‐list group (d = 0.09). There was a significant intervention effect on depression and anxiety and reductions in the intervention group with large within‐group effect sizes (d = 0.85–1.09).
Conclusions
Findings indicate a low enrollment rate and considerable attrition but also that Internet‐based guided self‐help shows promise for parents of children on cancer treatment who report a high level of PTSS and would like to take part in an Internet‐based intervention. © 2015 The Authors. Psycho‐Oncology published by John Wiley & Sons, Ltd.
Keywords: cancer, oncology, internet‐based intervention, parents, posttraumatic stress, randomized controlled trial
Background
Survival of childhood cancer has improved and is approaching 80% 5 years after diagnosis 1. Parents of children on cancer treatment report elevated levels of psychological distress such as posttraumatic stress symptoms (PTSS) 2, depression 3, and anxiety 4. Typically, parents report elevated levels of distress shortly after diagnosis and decreasing levels over time 5, 6. However, there is evidence of distinct subgroups reporting a high level of distress. Pöder and colleagues 5 showed that 28% of parents met the criteria for a potential diagnosis of posttraumatic stress disorder (PTSD) 2 months after their child's cancer diagnosis. Furthermore, research indicates that a subgroup of parents reports a clinical level of distress even years after the end of successful treatment 7.
To the best of our knowledge, the most successful psychosocial intervention for parents of children on cancer treatment up to today is an eight‐session protocol of structured problem solving for mothers of children recently diagnosed with cancer, which in two large trials has been shown to be more effective than treatment as usual 8, and an active control condition, that is, nondirective support 9, in reducing negative affectivity such as PTSS and depression. Other intervention studies with this population have been less successful and have not found support for treatment efficacy, for example, Surviving Cancer Competently Intervention Program ‐ Newly Diagnosed 10, and stress management 11. Mentioned studies have not provided interventions according to different needs as suggested by, for example, the Pediatric Psychosocial Preventative Health Model (PPPHM) 12. That is, prior intervention studies have not screened for distress to triage participants to appropriate intensity of intervention. Instead, all participants have been allocated to the same intervention irrespective of reported distress level or perceived need.
There is a need to investigate psychological interventions for parents of children on cancer treatment, especially for those reporting a high level of distress, cohering with the targeted and clinical/treatment groups in the PPPHM model 12. Pediatric cancer care is highly specialized, and the child often receives its treatment at academic medical centers resulting in that many families live far from the center where the child receives its care and may have difficulties in accessing psychological services. For example, in a Swedish study on parents of children with cancer, the families' mean distance from the pediatric cancer center was M = 151 km (standard deviation = 115) 5, and less than half of the parents who reported a need to see a psychologist reported having had the opportunity to do so 13. The geographical distances underscore the need to develop easy accessible interventions for this population. Internet‐based guided self‐help based on principles from cognitive behavior therapy (CBT) is effective for a range of psychiatric and somatic populations 14, including populations with PTSD 15 and parents of children with traumatic brain injury 16. We have recently developed an intervention based on CBT principles for parents of children on cancer treatment that can be administered in an Internet‐based guided self‐help format 17. The intervention was developed for parents reporting a high level of distress, in particular PTSS, consistent with the targeted and clinical/treatment groups in the PPPHM model 12, and its potential feasibility and efficacy have been explored in a case study 17. The purpose of the current study was to investigate the feasibility and potential efficacy of an Internet‐based version of the intervention for parents of children on cancer treatment. It was hypothesized that parents receiving the intervention would show greater reductions in the primary outcome PTSS, and the secondary outcomes depression and anxiety, compared with parents in a wait‐list control condition.
Methods
Design
This was a preassessment, postassessment parallel randomized controlled trial (RCT) comparing an Internet‐based guided self‐help program with a wait‐list control condition. Participants were recruited consecutively from five of the six Swedish pediatric oncology centers. Participants allocated to the intervention condition received the intervention immediately after randomization, and participants in the wait‐list condition received the intervention 12 months later.
Participants and procedure
Swedish‐speaking parents of children on treatment for a cancer disease with access to a computer with an Internet connection, who fulfilled the modified symptom criteria on the PTSD Checklist Civilian Version (PCL‐C) 18, a self‐report instrument corresponding to the DSM‐IV model of PTSD 19, and did not suffer from any psychiatric disorder in immediate need of treatment, were eligible. The modified symptom criteria constitute scoring ≥3 on at least one out of five symptoms of reexperiencing, one out of seven symptoms of avoidance, and one out of five symptoms of hyperarousal, corresponding to partial PTSD 20. A power analysis suggested that a total of 72 participants needed to be included to, with a power of 0.80, detect a large effect size (d = 0.8) on the PCL‐C assuming that p < 0.05. Given that data on health economic variables were collected (not reported here) and that costs generally vary more than clinical efficacy, a total sample of 120 participants was estimated appropriate. However, the participation rate during the 4 years the study was running was considerably lower than expected, and because of administrative reasons, inclusion had to be terminated before this sample size was reached.
Four to 12 weeks after diagnosis,1 potential participants were provided written and oral information about the study by a nurse or physician at the pediatric oncology centers and asked for written consent to participate. Consenting individuals were contacted via telephone by a psychologist from the research group. Participants completed the screening/preassessment online and a clinical interview with a psychologist via telephone. One licensed psychologist and two nonlicensed psychologists with a master's degree from the Psychology program conducted the interviews. Thereafter, included participants were randomized. Participants completed the postassessment online immediately after the intervention and after a corresponding time in the wait‐list condition. The procedure was approved by the regional ethics review board in Uppsala (Dnr 2008/238), and all participants provided written informed consent.
Intervention
The intervention consists of Internet‐based guided self‐help provided during 10 weeks. The material has been described in detail 17 and consists of approximately 100 pages (A4 format) of text and visual material divided in nine modules. The material is based on CBT principles 21, 22, 23 with a focus on psycho‐education and teaching strategies to cope with psychological distress such as relaxation training, coping with distressing thoughts and feelings, behavioral experiments, problem solving, structured emotional writing, values and goal setting, general self‐care, and maintenance of behavior change.
Each participant was assigned a therapist, accessed the intervention material via an online portal, and was instructed to work with each module for 1 week and send completed homework assignments via the portal to the therapist. The therapist sent written feedback on the homework to the participant via the portal. The sequence of modules was fixed, and no customization in this respect was allowed, which enhanced treatment integrity. Therapist feedback was standardized to some extent, but customization to each participant's situation was allowed. There were three therapists in the study. One licensed psychologist and two nonlicensed psychologists with a master's degree from the Psychology program. The two nonlicensed psychologists received supervision from the licensed psychologist. The therapists were affiliated with the research group responsible for the study and independent from centers from which participants were recruited. Logging of therapist time and activities was not supported by the portal, but therapists were instructed to spend 15–20 min per week when providing feedback to each participant. Individuals randomized to the intervention who had their partner included in the study received individual feedback but were encouraged to cooperate with their partner during the intervention if that suited them.
All participants were free to receive standard psychosocial services from the regular health care. These may have differed between centers as there are no standardized psychosocial services within the Swedish pediatric oncology care.
Outcomes
Primary outcome
Posttraumatic stress symptoms related to the child's cancer were assessed with the PCL‐C 18. The PCL‐C consists of 17 items rated on a five‐point scale (1 = Not at all, 5 = Extremely), corresponding to the items assessing the B (reexperiencing), C (avoidance/numbing), and D (hyperarousal) criteria in the DSM‐IV. Items are designed to indicate how much the respondent has been bothered by each symptom during the last month. As an example, item 1 reads as follows ‘Repeated, disturbing memories, thoughts, or images of a stressful experience related to your child's cancer disease’. Ruggiero, Ben, Scotti, and Rabalais 24 report that the instrument has adequate internal consistency, test–retest reliability, and evidence for convergent and discriminant validity when compared with other well‐established measures of PTSS, depression, and general anxiety. A value of 44 or above on the full scale suggests a diagnosis of PTSD 25. Cronbach's α in the current sample was 0.84 at the preassessment.
Secondary outcomes
Depression was assessed with the Beck Depression Inventory‐II (BDI‐II) 26 consisting of 21 items rated on a four‐point scale (0–3). The BDI‐II has shown good concurrent validity with its precursor BDI and the Hamilton Psychiatric Rating Scale, and the suggested cutoffs are 0–13 indicating minimal, 14–19 mild, 20–28 moderate, and 29–63 severe depression. Cronbach's α in the current sample was 0.82 at the preassessment. Anxiety was assessed with the Beck Anxiety Inventory (BAI) 27 consisting of 21 items rated on a four‐point scale (0–3). The BAI has shown good test–retest reliability and convergent validity, and the suggested cutoffs are 0–7 indicating minimal, 8–15 mild, 16–23 moderate, and 24–63 severe anxiety. Cronbach's α in the current sample was 0.87 at the preassessment.
Randomization
Randomization was performed by a consultant independent from the research group. Proc Plan SAS® version 9.1 (SAS Institute Inc., Cary, NC, USA) was used to generate the randomization schedule, and sealed envelopes were prepared by the consultant and handed to the research group. Parents were randomized in 1:1 ratio to intervention or wait list, and the randomization was stratified according to center, parental gender, and whether a participant had a partner in the study or not. Partners were randomized to the same condition.
Statistical analyses
Independent samples t‐test, Mann–Whitney U‐test, χ2‐test, and Fisher exact test were used to test for between‐group differences on demographic characteristics and outcomes at preassessment. Mixed effects modeling with restricted maximum likelihood estimation was used to examine change across assessments and the effects of the intervention 28. A random intercept model was used, and analyses were conducted on the intention‐to‐treat (ITT) principle where all randomized participants are included in the analyses assuming missing data to be missing at random 29. The data missing mechanism was assessed prior to the main analyses by exploring the relationships between characteristics at preassessment and missing data. Standardized effect sizes (Cohen's d) between groups at postassessment were calculated using estimated means and standard deviations from the preassessment 30. Cohen's d for within groups over time was calculated using estimated means and standard deviations adjusted for the correlation between preassessment and postassessment 31. The magnitude of the effect expressed in d was interpreted according to Cohen 32, that is, 0.2 = small effect, 0.5 = medium effect, and 0.8 = large effect. Clinically significant and reliable change was calculated for the primary outcome PCL‐C using the framework by Jacobson and Truax 33. These analyses were performed both on the ITT sample, assuming no change in individuals with missing data using last observation carried forward (LOCF), and for the completers. Because of the small sample size, clustering by center and child was not addressed in the analyses. Analyses were performed in IBM SPSS Statistics 20© (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2010©.
Results
Participant flow through the study is outlined in Figure 1. Between April 2010 and May 2014, 747 potential participants were informed about the study and asked for consent to be contacted again of which 553 declined. One hundred seventy‐four were reached by telephone, and 92 of these completed the screening/preassessment and clinical interview and were assessed for eligibility. Fifty‐eight parents of 46 children were included and randomized. Baseline characteristics are presented in Table 1. There were no differences in baseline characteristics between the groups except for the BAI, which was higher in the intervention group. The final postassessment took place in August 2014.
Figure 1.
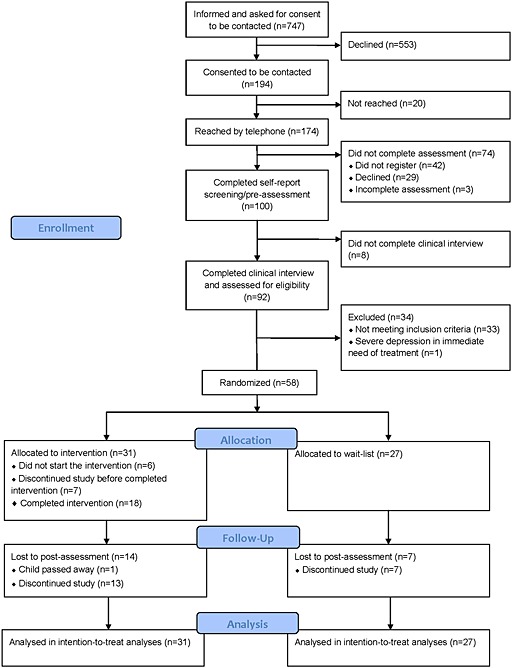
Consort diagram of participant flow through the study
Table 1.
Baseline characteristics
| Total sample | Intervention | Wait list | ||
|---|---|---|---|---|
| Parents' characteristics | n = 58 | n = 31 | n = 27 | p |
| Mothers n (%) | 39 (67) | 21 (68) | 18 (67) | 0.76 |
| Partner also included in study n (%) | 22 (38) | 12 (39) | 10 (37) | 0.90 |
| Mean age (SD) | 38 (7.2)a | 40 (7.4)c | 36 (6.6)e | 0.06 |
| Living with child's biological parent n (%) | 51 (88) | 26 (84) | 25 (93) | 0.31 |
| University studies n (%) | 30 (52) | 17 (55) | 13 (48) | 0.61 |
| Employed n (%) | 47 (81) | 26 (84) | 21 (78) | 0.56 |
| Median distance in kilometers to POC (IQR) | 35 (61) | 39 (62) | 20 (50) | 0.39 |
| Experience of previous traumatic event(s) n (%) | 26 (45) | 14 (45) | 12 (44) | 0.96 |
| Median months since dx (IQR) | 3.0 (3.0)b | 3.0 (2.25)d | 3.0 (3.0) | 0.39 |
| Outcomes, mean (SD) | ||||
| PCL‐C | 49.1 (10.3) | 51.5 (9.4) | 46.6 (10.7) | 0.06 |
| BDI‐II | 20.6 (7.5) | 21.6 (8.1) | 19.3 (6.7) | 0.24 |
| BAI | 14.7 (7.6) | 17.2 (7.8) | 11.9 (6.3) | <0.01 |
| Children's characteristics | n = 46 | n = 25 | n = 21 | |
| Women n (%) | 25 (54) | 16 (64) | 9 (43) | 0.15 |
| Median age (IQR) | 5 (9.0) | 6 (10.5) | 4 (8.0) | 0.26 |
| Diagnosis | 0.33 | |||
| Leukemia n (%) | 24 (52) | 13 (52) | 11 (52) | |
| Sarcoma n (%) | 8 (17) | 5 (20) | 3 (14) | |
| Lymphoma n (%) | 3 (7) | 3 (12) | 0 (0) | |
| CNS tumor n (%) | 7 (15) | 2 (8) | 5 (24) | |
| Other malignant disease n (%) | 4 (9) | 2 (8) | 2 (10) | |
POC, pediatric oncology center; dx, diagnosis; IQR, interquartile range; PCL‐C, PTSD‐Checklist Civilian Version; BDI‐II, Beck Depression Inventory‐II; BAI, Beck Anxiety Inventory; CNS, central nervous system; SD, standard deviation.
n = 55.
n = 57.
n = 29.
n = 30.
n = 26.
Attrition and adherence
Fourteen participants in the intervention group (45%) and seven in the wait‐list group (26%) did not provide postassessments (χ2 = 2.31, d.f. = 1, p = 0.13). At the preassessment, there were no differences in terms of demographic characteristics, whether the participant had a partner in the study or outcome measures between those who provided postassessments and those who did not (p ranging 0.15–0.91); hence, missing data were assumed to be missing at random.
The numbers of treatment modules accessed and logins to the portal by the participants were measured as indicating adherence to the intervention. Six participants did not start the intervention, and seven discontinued before completion. Eighteen participants completed the intervention representing 58% of those allocated to the intervention. For the ITT sample, the median number (interquartile range = IQR) of accessed modules was four (4), and the median number (IQR) of logins was 13 (22). For the completer sample, the median number of accessed modules was 5 (3.5), and the median number (IQR) of logins was 20 (20).
Outcomes
Table 2 presents the results from the mixed effects models and effect sizes.
Table 2.
Estimated outcomes of mixed effects models and effect sizes
| Estimated means (SE) | Time*Group | Effect sizes Cohen's d [95% CI] | ||||
|---|---|---|---|---|---|---|
| Preassessment | Postassessment | F | p | Between‐group postassessment | Within‐group preassessment and postassessment | |
| PCL‐C | 16.5 | 0.000 | 0.88 [1.42–0.34] | |||
| Intervention (n = 31) | 51.5 (2.1) | 35.9 (2.6) | 1.62 [2.56–0.67] | |||
| Wait list (n = 27) | 46.3 (2.2) | 45.1 (2.5) | 0.09 [0.72 to −0.54] | |||
| BDI‐II | 15.1 | 0.000 | 0.92 [1.46–0.37] | |||
| Intervention (n = 31) | 21.6 (1.4) | 12.9 (1.7) | 1.09 [1.88–0.30] | |||
| Wait list (n = 27) | 19.3 (1.5) | 19.8 (1.6) | −0.07 [0.56 to −0.69] | |||
| BAI | 7.1 | 0.011 | 0.17 [0.69 to −0.35] | |||
| Intervention (n = 31) | 17.2 (1.4) | 10.1 (1.8) | 0.85 [1.56–0.14] | |||
| Wait list (n = 27) | 11.9 (1.5) | 11.3 (1.8) | 0.06 [0.67 to −0.54] | |||
PCL‐C, PTSD‐Checklist Civilian Version; BDI‐II, Beck Depression Inventory‐II; BAI, Beck Anxiety Inventory; SE, standard error.
Cohen's d is the standardized mean difference and was calculated using the estimated means and the standard deviation of the complete sample at the preassessment.
Primary outcome
For PCL‐C, there was a significant effect of the intervention, and the estimated between‐group effect size at the postassessment was large. There was a reduction in symptoms in the intervention group with a large within‐group effect size but minimal reduction in the wait‐list group. Twelve participants in the intervention group exhibited clinically significant and reliable change compared with four participants in the control group. This difference was significant both in the ITT sample using LOCF (χ2 = 4.13, d.f. = 1, p < 0.05) and in the completer sample (χ2 = 6.70, d.f. = 1, p < 0.01).
Secondary outcomes
For BDI‐II, there was a significant effect of the intervention, and the estimated between‐group effect size at the postassessment was large. There was a reduction in symptoms in the intervention group with a large within‐group effect size but minimal reduction in the wait‐list group. There was also a significant effect of the intervention on BAI with reduction in symptoms in the intervention group with a large within‐group effect size but minimal reduction in the wait‐list group. However, because of a higher level on BAI in the intervention group at preassessment, the between‐group effect size at postassessment was small.
Discussion
To our knowledge, this is the first study to investigate the feasibility and preliminary efficacy of an Internet‐based guided self‐help intervention aiming to reduce PTSS, depression, and anxiety among parents of children on cancer treatment who report a high level of PTSS. The enrollment rate was poor with 26% of approached parents being interested in participation, and the attrition from the intervention was considerable with a 58% completion rate. These figures question the feasibility of the intervention in its current form. On the other hand, the enrollment figure must be seen in the light of this intervention being designed for and targeting a subgroup reporting a high level of PTSS consistent with the PPPHM model 12. Given this, having 26% of parents approached and asked for interest to participate consenting to be contacted again might adequately reflect the proportion of individuals in need for help and who could consider an Internet‐based intervention as an alternative. As the attrition was high among those allocated to the intervention, it might be the case that the intervention with its current content and format was too demanding or not suitable for many participants. However, the ITT analyses suggested that the intervention was effective with large effect sizes for PTSS, depression, and anxiety. This indicates that the intervention could be effective for a subgroup reporting a high level of PTSS and who accepts an Internet‐based intervention. In sum, the results indicate that the Internet‐based self‐help investigated in this study may have a substantive impact for those who report a high level of PTSS and consider an Internet‐based intervention as an alternative, which agree with a growing body of research suggesting that online psychological treatment based on principles from CBT can be effective for a range of problems 14. The results also tentatively agree with successful evaluations of face‐to‐face problem‐solving therapy for mothers of children with cancer 8, 9 and a face‐to‐face trauma‐focused intervention for mothers of premature infants 34.
The current study has some limitations. As stated previously, the attrition rate was considerable, which warrants caution in the interpretation of the findings. However, exploring the missing data mechanism indicated that data were missing at random and under this assumption, maximum likelihood estimation provides more unbiased estimates compared with, for example, estimation with LOCF 29. The dropout rate is similar to that in a recent RCT investigating the efficacy of an Internet‐based psycho‐educational intervention with the purpose of preventing PTSS/PTSD in parents following an injury in their child, where 61% of participants completed the 6‐week follow‐up 35 but is higher than dropout generally seen in RCTs of Internet‐based interventions for anxiety and depression 36. Furthermore, the current report lacks a follow‐up assessment, so it is still unclear whether the results are maintained over time. Follow‐up assessments at 12 and 24 months are currently underway, and findings from these will be reported. Finally, the current design including a wait‐list control group precludes from firm conclusions regarding the specificity of the effects, that is, we do not know whether it was the components of the intervention that caused the effects. For such conclusions, dismantling designs comparing varying active conditions should be utilized.
To conclude, the enrollment rate and attrition question the feasibility of the intervention with its current format and content and point to difficulties in engaging parents of children on cancer treatment in Internet‐based psychological interventions. Nevertheless, ITT analyses of the preliminary efficacy indicate substantial improvements in PTSS and depression and moderate improvements in anxiety, but these results are only generalizable to a selective subgroup of parents reporting a high level of PTSS and who considers Internet‐based interventions as an alternative. Replication is needed in order to ensure the validity and robustness of the results, and before implementation into standard clinical practice is to be commenced.
Acknowledgements
This research was supported by grants from the Swedish Research Council (grant numbers K2008‐70X‐20836‐01‐3 and K2011‐70X‐20836‐04‐4), the Swedish Cancer Society (grant number 2010/276), and the Swedish Childhood Cancer Foundation (grant numbers PROJ08/010 and PRO12/028).
Cernvall, M. , Carlbring, P. , Ljungman, L. , Ljungman, G. , and von Essen, L. (2015) Internet‐based guided self‐help for parents of children on cancer treatment: a randomized controlled trial. Psycho‐Oncology, 24: 1152–1158. doi: 10.1002/pon.3788.
Footnotes
In the initial protocol, potential participants were to be approached 1–2 weeks after diagnosis. However, during the first months of inclusion, it was evident that parents often, because of administrative reasons, were approached later after diagnosis and the protocol was changed to the time frame reported.
References
- 1. Gustafsson G, Kogner P, Heyman M. Childhood cancer incidence and survival in Sweden 1984‐2010. Report from the Swedish Childhood Cancer Registry. 2013.
- 2. Kazak AE, Boeving CA, Alderfer MA, Hwang WT, Reilly A. Posttraumatic stress symptoms during treatment in parents of children with cancer. J Clin Oncol 2005;23:7405–7410. DOI:10.1200/JCO.2005.09.110. [DOI] [PubMed] [Google Scholar]
- 3. Boman KK, Viksten J, Kogner P, Samuelsson U. Serious illness in childhood: the different threats of cancer and diabetes from a parent perspective. J Pediatr 2004;145:373–379. [DOI] [PubMed] [Google Scholar]
- 4. Fotiadou M, Barlow JH, Powell LA, Langton H. Optimism and psychological well‐being among parents of children with cancer: an exploratory study. Psycho‐Oncology 2008;17:401–409. DOI:10.1002/pon. [DOI] [PubMed] [Google Scholar]
- 5. Pöder U, Ljungman G, von Essen L. Posttraumatic stress disorder among parents of children on cancer treatment: a longitudinal study. Psycho‐Oncology 2008;17:430–437. DOI:10.1002/pon.1263. [DOI] [PubMed] [Google Scholar]
- 6. Dolgin MJ, Phipps S, Fairclough DL, et al. Trajectories of adjustment in mothers of children with newly diagnosed cancer: a natural history investigation. J Pediatr Psychol 2007;32:771–782. DOI:10.1093/jpepsy/jsm013. [DOI] [PubMed] [Google Scholar]
- 7. Ljungman L, Cernvall M, Grönqvist H, Ljótsson B, Ljungman G, von Essen L. Long‐term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS One 2014;9:e103340 DOI:10.1371/journal.pone.0103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahler OJZ, Fairclough DL, Phipps S, et al. Using problem‐solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: report of a multisite randomized trial. J Consult Clin Psychol 2005;73:272–283. [DOI] [PubMed] [Google Scholar]
- 9. Sahler OJZ, Dolgin MJ, Phipps S, et al. Specificity of problem‐solving skills training in mothers of children newly diagnosed with cancer: results of a multisite randomized clinical trial. J Clin Oncol 2013;31:1329–1335. DOI:10.1200/JCO.2011.39.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stehl ML, Kazak AE, Alderfer MA, et al. Conducting a randomized clinical trial of an psychological intervention for parents/caregivers of children with cancer shortly after diagnosis. J Pediatr Psychol 2009;34:803–816. DOI:10.1093/jpepsy/jsn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marsland AL, Long KA, Howe C, Thompson AL, Tersak J, Ewing LJ. A pilot trial of a stress management intervention for primary caregivers of children newly diagnosed with cancer: preliminary evidence that perceived social support moderates the psychosocial benefit of intervention. J Pediatr Psychol 2013;38:449–461. DOI:10.1093/jpepsy/jss173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kazak AE. Pediatric Psychosocial Preventative Health Model (PPPHM): research, practice, and collaboration in pediatric family systems medicine. Fam Syst Heal 2006;24:381–395. [Google Scholar]
- 13. Pöder U, von Essen L. Perceptions of support among Swedish parents of children on cancer treatment: a prospective, longitudinal study. Eur J Cancer Care (Engl) 2009;18:350–357. [DOI] [PubMed] [Google Scholar]
- 14. Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the Internet: a systematic review of applications, clinical efficacy and cost‐effectiveness. Expert Rev Pharmacoecon Outcomes Res 2012;12:745–764. DOI:10.1586/erp.12.67. [DOI] [PubMed] [Google Scholar]
- 15. Ivarsson D, Blom M, Hesser H, et al. Guided internet‐delivered cognitive behavior therapy for post‐traumatic stress disorder: a randomized controlled trial. Internet Interv 2014;1:33–40. DOI:10.1016/j.invent.2014.03.002. [Google Scholar]
- 16. Wade SL, Carey JA, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. J Consult Clin Psychol 2006;74:445–454. [DOI] [PubMed] [Google Scholar]
- 17. Cernvall M, Carlbring P, Ljungman G, von Essen L. Guided self‐help as intervention for traumatic stress in parents of children with cancer: conceptualization, intervention strategies, and a case study. J Psychosoc Oncol 2013;31:13–29. DOI:10.1080/07347332.2012.741095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. Annu. Meet. Int. Soc. Trauma. Stress Stud., San Antonio, TX.: 1993.
- 19. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR, American Psychiatric Association: Washington, DC, 2000. [Google Scholar]
- 20. Breslau N, Lucia VC, Davis GC. Partial PTSD versus full PTSD: an empirical examination of associated impairment. Psychol Med 2004;34:1205–1214. [DOI] [PubMed] [Google Scholar]
- 21. Farmer RF, Chapman AL. Behavioral Interventions in Cognitive Behavior Therapy: Practical Guidance for Putting Theory into Action, American Psychological Association: Washington, DC, 2008. [Google Scholar]
- 22. Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change, The Guilford Press: New York, NY, 1999. [Google Scholar]
- 23. Wells A. Metacognitive Therapy for Anxiety and Depression, The Guilford Press: New York, NY, 2008. [Google Scholar]
- 24. Ruggiero KJ, Ben KD, Scotti JR, Rabalais AE. Psychometric properties of the PTSD checklist—civilian version. J Trauma Stress 2003;16:495–502. [DOI] [PubMed] [Google Scholar]
- 25. Blanchard EB, Jones‐Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther 1996;34:669–673. [DOI] [PubMed] [Google Scholar]
- 26. Beck AT, Steer RA, Brown GK. Beck Depression Inventory‐II, The Psychological Corporation: San Antonio, TX, 1996. [Google Scholar]
- 27. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 28. Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modelling Change and Event Occurence, Oxford University Press: New York, NY, 2003. [Google Scholar]
- 29. Salim A, Mackinnon A, Christensen H, Griffiths K. Comparison of data analysis strategies for intent‐to‐treat analysis in pre‐test‐post‐test designs with substantial dropout rates. Psychiatry Res 2008;160:335–345. DOI:10.1016/j.psychres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30. Feingold A. Effect sizes for growth‐modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods 2009;14:43–53. DOI:10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. John Wiley & Sons, Ltd: Chichester, UK, 2009. doi:10.1002/9780470743386. [Google Scholar]
- 32. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.), Lawrence Erlbaum Associates: Hillsdale, NJ, 1988. [Google Scholar]
- 33. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–19. [DOI] [PubMed] [Google Scholar]
- 34. Shaw RJ, St John N, Lilo EA, et al. Prevention of traumatic stress in mothers with preterm infants: a randomized controlled trial. Pediatrics 2013;132:886–894. DOI:10.1542/peds.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marsac ML, Hildenbrand AK, Kohser KL, Winston FK, Li Y, Kassam‐Adams N. Preventing posttraumatic stress following pediatric injury: a randomized controlled trial of a web‐based psycho‐educational intervention for parents. J Pediatr Psychol 2013;38:1101–1111. DOI:10.1093/jpepsy/jst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christensen H, Griffiths KM, Farrer L. Adherence in internet interventions for anxiety and depression. J Med Internet Res 2009;11: DOI:10.2196/jmir.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]


