Abstract
Background
The optimal surgical approach for treatment of oesophagogastric junction (OGJ) cancer is controversial. A randomized clinical trial (JCOG9502) comparing transhiatal (TH) and left thoracoabdominal (LTA) approaches was stopped after the first interim analysis owing to limited efficacy for LTA resections. Complete 10‐year follow‐up data are now available.
Methods
Patients with histologically proven adenocarcinoma of the OGJ or gastric cardia with oesophageal invasion of 3 cm or less were randomized to a TH or LTA approach. Both groups underwent total gastrectomy and splenectomy with D2 nodal dissection plus para‐aortic lymphadenectomy above the left renal vein. For LTA, a thorough mediastinal lymphadenectomy below the left inferior pulmonary vein was also mandatory. The primary endpoint was overall survival.
Results
A total of 167 patients (82 TH, 85 LTA) were enrolled. The 10‐year overall survival rate was 37 (95 per cent c.i. 26 to 47) per cent for the TH approach and 24 (15 to 34) per cent for the LTA technique (P = 0·060). The hazard ratio for death was 1·42 (0·98 to 2·05) for the LTA technique. Subgroup analysis based on the Siewert classification indicated non‐significant survival advantages in favour of the TH approach.
Conclusion
LTA resections should be avoided in the treatment of adenocarcinoma of the OGJ or gastric cardia. Registration number: NCT00149266 (https://www.clinicaltrials.gov).
Short abstract
No survival benefit from a more extensive operation
Introduction
The incidence of adenocarcinoma of the oesophagogastric junction (OGJ) has increased in developed countries over the past 20 years1, 2. Although surgery is considered essential as part of a curative treatment strategy for most patients, survival remains poor even in those who undergo R0 resection, with or without additional therapy3. To improve the R0 resection rate and long‐term outcomes, extended surgery with en bloc lymphadenectomy has been attempted for many years. When considering tumours arising from the cardia (Siewert type III4), or those at the OGJ (Siewert type II) with minimal oesophageal extension where total gastrectomy seems appropriate, left thoracoabdominal (LTA) and transhiatal (TH) approaches have been advocated for curative resection. There is no clear information to indicate whether the operative approach influences long‐term outcome.
In East Asian countries, including Japan, the majority of OGJ tumours are Siewert types II and III5. The incidence of lower mediastinal lymph node metastasis from type II and III tumours is reported to range from 10 to 40 per cent6, 7, 8, 9, 10, 11, 12. Some institutions prefer the LTA to the TH approach in order to perform lymph node dissection in the lower mediastinal field and obtain a safe surgical margin6, 7, whereas others prefer the TH technique owing to lower postoperative morbidity and the poor prognosis of patients with metastasis in the lower mediastinum8, 9. To evaluate the survival benefit of these two approaches, the Japan Clinical Oncology Group (JCOG) initiated a phase 3 open‐label randomized clinical trial in 1995.
In this trial, JCOG9502, 167 patients with adenocarcinoma of the OGJ or gastric cardia were enrolled. The first interim analysis conducted in December 2003 showed that the predictive probability of the LTA approach being significantly better than the TH technique was as low as 3·7 per cent, although the LTA operation resulted in increased postoperative morbidity13. The recommendation from the JCOG Data and Safety Monitoring Committee to close study accrual and publish the results was accepted. It seems important, therefore, to report the long‐term data, to ensure that the conclusion reached in the earlier publication remains valid, because many surgeons in Japan and some in the West still recommend the LTA approach14, 15. The present report is the result of the final analysis, based on 10 years of follow‐up data.
Methods
JCOG9502 was designed as a multicentre prospective randomized phase 3 trial. The study protocol was approved by the JCOG Clinical Trial Review Committee and the institutional review boards of all 27 participating Japanese hospitals before study initiation. All patients provided written informed consent. The eligibility criteria for the study consisted of histologically confirmed adenocarcinoma of the gastric body or cardia with oesophageal invasion of 3 cm or less, cT2–4 category, age 75 years or less, no distant metastasis, no lymph nodes larger than 1 cm in the hepatoduodenal ligament or para‐aortic field, a forced expiratory volume in 1 s of at least 50 per cent, and an arterial oxygen tension of at least 9·3 kPa while breathing ambient air.
Procedures
After confirming eligibility, surgeons contacted the JCOG Data Centre by telephone to receive a randomly generated assignment (1 : 1) into one of the treatment groups. A minimization method was used to stratify treatment groups according to institution, cT category (cT2 versus cT3/4) and Borrmann type (0–2 versus 3 or 5) for random number generation.
The TH approach consisted of total gastrectomy with D2 lymphadenectomy including splenectomy. Additional dissection of the lymph nodes along the left inferior phrenic vessels and the para‐aortic nodes lateral to the aorta and above the left renal vein was performed in patients with curable disease. This included patients with positive findings on peritoneal lavage cytology, but without overt peritoneal metastasis. All procedures were undertaken via laparotomy, and the lower mediastinum was accessed transhiatally. Mediastinal resection included the lower oesophagus and perioesophageal lymph nodes only.
An oblique incision over the left thorax and abdomen was made for the LTA approach, followed by the same procedure in the abdominal cavity as for the TH operation. In the thoracic cavity, a thorough mediastinal node dissection below the left inferior pulmonary vein was undertaken with appropriate oesophagectomy.
Each surgeon selected the type of reconstruction. If the operation was considered curative (no macroscopic residual disease), no further treatment was allowed unless recurrence was diagnosed.
Statistical analysis
The primary endpoint was overall survival (OS). Secondary endpoints were disease‐free survival (DFS), morbidity and mortality, postoperative symptoms and postoperative respiratory function. All in‐hospital deaths and deaths within 1 month of surgery were defined as hospital mortality. Operative procedures and pathology results were recorded according to the 12th edition of the Japanese Classification of Gastric Carcinoma (JCGC)16. Tumour stage is reported here using the sixth edition of the TNM classification17. All tumours were classified as Siewert type II, type III, or non‐OGJ if the tumour epicentre was located more than 5 cm distal to the OGJ based on pathological examination of the resected specimen.
The original intention was to recruit 302 patients to achieve a one‐sided α of 0·05 and statistical power of 80 per cent to detect a difference between the two groups, assuming a 5‐year survival rate of 15·5 per cent for the TH approach versus 26·0 per cent for the LTA procedure. The projected accrual period was 4 years. After 8 years of slow accrual, the JCOG Data and Safety Monitoring Committee approved an amendment to the sample size and analysis plan. The amended sample size was 250, with a one‐sided α of 0·1 and power of 80 per cent, with an accrual period of 12 years in total and 8 years of follow‐up. Three interim analyses were planned.
OS was measured from the date of randomization to the date of death from any cause. Among patients who underwent R0 resection, DFS was measured from the date of randomization to the date of the first observation of disease recurrence or death from any cause. OS and DFS curves were estimated using the Kaplan–Meier method and compared with the log rank test. Subgroup analysis was performed by means of Cox regression to assess statistical interactions between treatment approach and 12 patient characteristics. Postoperative factors were also included in the Cox regression analysis to estimate their influence on survival. Two‐sided P values were calculated for all tests. As the study was planned for one‐sided testing, one‐sided P values are presented for the primary endpoint. P < 0·050 was judged to be statistically significant. All analyses were based on an intention‐to‐treat basis. Statistical analyses were performed with SAS® version 9·2 (SAS Institute, Cary, North Carolina, USA).
Results
Between July 1995 and December 2003, 167 patients were enrolled, of whom 82 were randomly assigned to the TH and 85 to the LTA approach (Fig. 1). Baseline characteristics of the two groups were similar, except for Siewert classification (Table 1). There were 95 Siewert type II and 63 type III tumours. Seven patients had large gastric tumours invading the oesophagus that could not be classified by Siewert type. At operation, 141 patients (62 TH, 79 LTA) underwent mediastinal node dissection and 145 (72 TH, 73 LTA) had para‐aortic node dissection. The rate of metastasis in mediastinal nodes was 5 per cent (3 of 62) in the TH group and 11 per cent (9 of 79) in the LTA group. The metastasis rate in para‐aortic nodes was 18 per cent (13 of 72) and 12 per cent (9 of 73) respectively.
Figure 1.
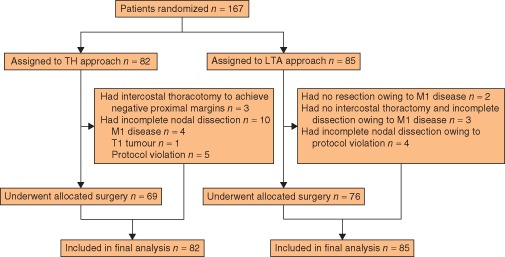
CONSORT diagram for the JCOG9502 trial. TH, transhiatal; LTA, left thoracoabdominal
Table 1.
Patient characteristics
| TH group (n = 82) | LTA group (n = 85) | |
|---|---|---|
| Age (years)* | 60 (36–75) | 63 (38–75) |
| Sex ratio (M : F) | 71 : 11 | 63 : 22 |
| Borrmann type | ||
| 0–2 | 36 | 37 |
| 3 or 5 | 46 | 48 |
| Siewert classification† | ||
| Type II | 52 | 43 |
| Type III | 27 | 36 |
| Non‐OGJ tumour | 3 | 4 |
| Tumour size (cm)*† | 6·2 (2·5–19) | 7·0 (2·0–18) |
| Histological type† | ||
| Differentiated | 42 | 43 |
| Undifferentiated | 40 | 40 |
| Clinical tumour categoryठ| ||
| cT2 | 20 | 20 |
| cT3/4 | 62 | 65 |
| Pathological tumour category†‡§ | ||
| pT1b | 2 | 1 |
| pT2a | 10 | 6 |
| pT2b | 24 | 35 |
| pT3 | 39 | 37 |
| pT4 | 7 | 4 |
| Pathological node category†‡ | ||
| pN0 | 14 | 15 |
| pN1 | 24 | 27 |
| pN2 | 30 | 25 |
| pN3/4 | 14 | 16 |
| Pathological node category†§ | ||
| pN0 | 14 | 15 |
| pN1 | 35 | 28 |
| pN2 | 16 | 26 |
| pN3 | 17 | 14 |
| No. of positive nodes*† | 5 (0–53) | 5 (0–52) |
| Histological oesophageal invasion (cm)*† | 1·6 (0–4·5) | 1·2 (0–7·0) |
| Residual tumour | ||
| R0 | 76 | 75 |
| R1/2 | 6 | 10 |
Values are median (range).
Data not available for two patients in the left thoracoabdominal (LTA) group who did not undergo surgical resection owing to M1 disease.
Japanese Classification of Gastric Carcinoma, 12th edition16;
International Union Against Cancer (UICC) TNM classification, 6th edition17. TH, transhiatal; OGJ, oesophagogastric junction.
Operative details, including morbidity and mortality, postoperative symptoms and postoperative respiratory function, have been reported previously13, 18. Median duration of surgery was 33 min longer for the LTA procedure than for the TH approach (P = 0·127). Median blood loss was similar in the two groups (655 versus 673 ml for LTA and TH group respectively; P = 0·949), but allogeneic blood transfusion was used more frequently in the LTA group (39 of 85, 46 per cent) than in the TH group (25 of 82, 30 per cent) (P = 0·056). Patients in the LTA group had a higher morbidity rate: 42 (49 per cent) versus 28 (34 per cent) (P = 0·060). For six selected major complications (pancreatic fistula, abdominal abscess, pneumonia, anastomotic leak, empyema thoracis and mediastinitis), the incidence was significantly higher following the LTA than the TH procedure: 35 (41 per cent) versus 18 (22 per cent) (P = 0·008). There were two treatment‐related deaths, both in the LTA group.
Median follow‐up for all censored patients was 10·6 (range 5·1–17·1) years at the last follow‐up in December 2012. There had been 52 and 63 deaths in the TH and LTA group respectively, with 42 and 50 patients respectively dying from cancer. The 5‐ and 10‐year OS rates for all randomized patients were 51 (95 per cent c.i. 40 to 61) and 37 (26 to 47) per cent respectively for the TH approach, and 37 (26 to 47) and 24 (15 to 34) per cent for the LTA approach (Fig. 2 a). The log rank test showed marginal differences between the groups (2‐sided P = 0·060, 1‐sided P = 0·970), and the hazard ratio (HR) for the LTA versus the TH approach was 1·42 (95 per cent c.i. 0·98 to 2·05). After adjustment for cT category and Borrmann type, stratified P values were 0·102 in two‐sided and 0·949 in one‐sided log rank tests, and the HR was 1·36 (0·94 to 1·98). In multivariable Cox regression analysis with seven baseline variables (age, sex, Borrmann type, Siewert classification, tumour size, histological type, cT category (JCGC 12th edition)), the HR was essentially unchanged: HR 1·33 (0·91 to 1·95). Per‐protocol analysis (145 patients) also showed a HR of 1·44 (0·96 to 2·15) (2‐sided P = 0·076, 1‐sided P = 0·962) (Fig. S1, supporting information).
Figure 2.
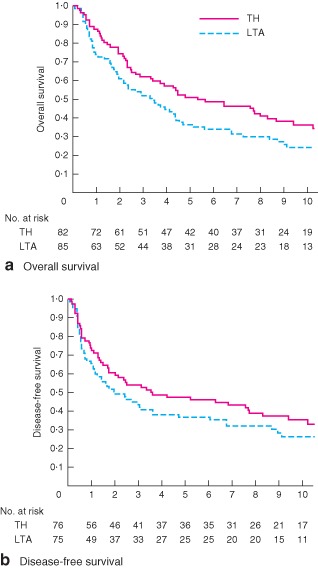
Kaplan–Meier curves of a overall and b disease‐free survival in all randomized patients by treatment group. TH, transhiatal approach; LTA, left thoracoabdominal approach. a Hazard ratio (HR) 1·42 (95 per cent c.i. 0·98 to 2·05; P = 0·970 and P = 0·060, 1‐ and 2‐sided log rank test respectively); b HR 1·28 (0·87 to 1·89; P = 0·892 and P = 0·215, 1‐ and 2‐sided log rank test respectively)
Among patients who underwent R0 or R1 resection, initial sites of recurrence were lymph nodes (31 patients), peritoneum (19), liver (17), lung (10), pleura (4) and other sites (7). The pattern of recurrence was similar in the two groups (Table 2). The 5‐ and 10‐year DFS rates were 47 (95 per cent c.i. 36 to 58) and 36 (25 to 47) per cent for the TH approach, and 37 (26 to 48) and 26 (16 to 37) per cent for the LTA approach (P = 0·215) (Fig. 2 b). The HR for the LTA group compared with the TH group was 1·28 (0·87 to 1·89).
Table 2.
Sites of first recurrence
| TH group (n = 82) | LTA group (n = 85) | P * | |
|---|---|---|---|
| Lymph nodes | 12 (15) | 19 (22) | 0·235 |
| Peritoneum | 9 (11) | 10 (12) | 1·000 |
| Liver | 8 (10) | 9 (11) | 1·000 |
| Lung | 5 (6) | 5 (6) | 1·000 |
| Pleura | 3 (4) | 1 (1) | 0·362 |
| Other | 5 (6) | 2 (2) | 0·271 |
Values in parentheses are percentages. TH, transhiatal; LTA, left thoracoabdominal.
Fisher's exact test, two‐sided.
There were no significant interactions between treatment effects and the patient characteristics examined (Fig. 3). For the 95 patients with Siewert type II tumours, 5‐ and 10‐year OS rates were 50 (95 per cent c.i. 36 to 63) and 35 (22 to 48) per cent respectively for the TH approach, and 42 (27 to 56) and 29 (16 to 43) per cent for the LTA approach (HR 1·19, 95 per cent c.i. 0·72 to 1·95; P = 0·496) (Fig. 4 a). Among 63 patients with Siewert type III tumours, 5‐ and 10‐year OS rates were 59 (39 to 75) and 44 (26 to 62) per cent respectively for the TH approach, and 36 (21 to 51) and 22 (10 to 38) per cent for the LTA approach (HR 1·67, 0·90 to 3·11; P = 0·102) (Fig. 4 b). For the subgroup of patients with type III tumours, excluding those with major complications from the survival analysis, the difference in OS between the groups was significant (HR 2·00, 0·99 to 4·05; P = 0·050).
Figure 3.
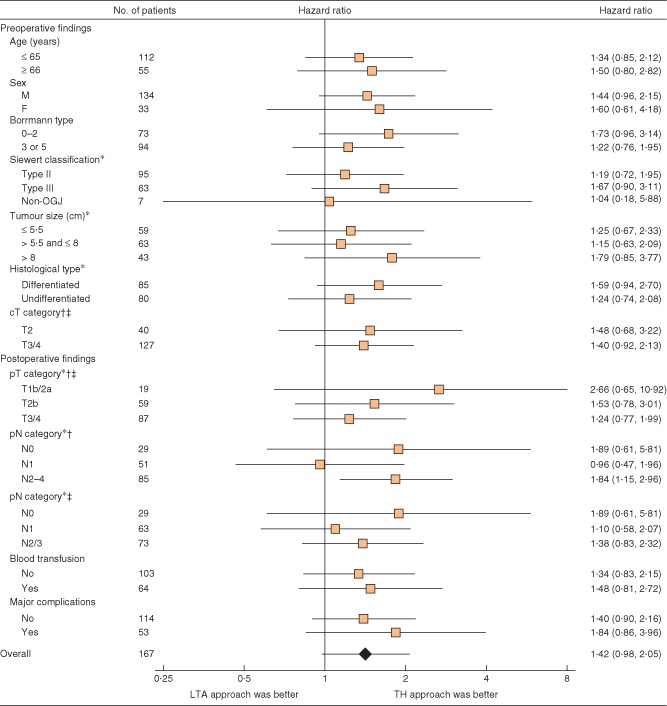
Forest plot for overall survival in the subgroup analysis. *Data not available for two patients in the left thoracoabdominal (LTA) group who did not undergo surgical resection owing to M1 disease. Hazard ratios are shown with 95 per cent c.i. OGJ, oesophagogastric junction; TH, transhiatal. †Japanese Classification of Gastric Carcinoma, 12th edition16; ‡International Union Against Cancer (UICC) TNM classification, 6th edition17
Figure 4.
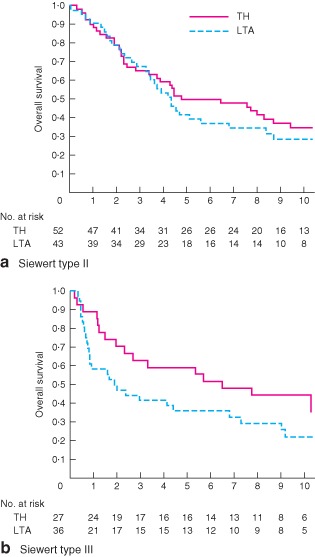
Kaplan–Meier curves of overall survival in patients with a Siewert type II and b Siewert type III tumours by treatment group. TH, transhiatal approach; LTA, left thoracoabdominal approach. a Hazard ratio (HR) 1·19 (95 per cent c.i. 0·72 to 1·95; P = 0·496, 2‐sided log rank test); b HR 1·67 (0·90 to 3·11; P = 0·102, 2‐sided log rank test)
The rate of metastasis in mediastinal lymph nodes was 10 per cent (8 of 81) for type II tumours compared with 5 per cent (3 of 55) for type III. The metastasis rate in para‐aortic lymph nodes was 9 per cent (8 of 86) for type II compared with 22 per cent (12 of 54) for type III tumours. The 5‐year OS rate for 22 patients with pathologically confirmed metastasis in the para‐aortic nodes was 18 (95 per cent c.i. 6 to 36) per cent.
Discussion
This final analysis, based on 10‐year follow‐up data, has confirmed the conclusion reached in the earlier publication of the interim analysis13. Compared with the TH approach, the LTA approach offered no improvement in OS or DFS. The LTA technique did not reduce rates of cancer recurrence in lymph nodes, but was associated with greater morbidity and mortality. Although the LTA approach involved no increase in blood loss, more patients assigned to this approach received allogeneic blood transfusion, mainly to correct haemodynamic instability in the early postoperative period13. LTA cannot, therefore, be justified for the treatment of adenocarcinoma of the OGJ or gastric cardia if the length of oesophageal invasion is 3 cm or less.
Omloo and colleagues19 reported the final results of a Dutch trial comparing a right thoracic with the TH approach for Siewert type I and II tumours. In their trial, subgroup analysis revealed a 14 per cent 5‐year OS advantage with the right thoracic approach for patients with type I tumours, but no difference for patients with type II tumours (27 per cent versus 31 per cent in the TH group). The present study confirms the results of the Dutch study for patients with type II tumours based on surgical approach. For patients with type III tumours, the LTA approach was associated with a 22 per cent lower 10‐year OS rate than the TH technique, and this difference in OS between the two groups occurred within 1 year after surgery. Although the reason for worse survival of patients who underwent LTA in this subgroup is unclear, the greater need for allogeneic blood transfusion and increased postoperative morbidity compared with that in the TH group may be relevant. The earlier results of the trial18 also noted more weight loss, postoperative symptoms and respiratory dysfunction following the LTA operation. In the analysis of patients with type III tumours, excluding those with postoperative complications, OS was significantly better in the TH group despite the biases inherent in this post hoc method. This finding strengthens the recommendation that LTA should be avoided, at least for patients with Siewert type III tumours.
This study is also informative regarding para‐aortic lymph node metastasis from tumours of the OGJ, as prophylactic dissection of the para‐aortic nodes lateral to the aorta and above the left renal vein was required in both treatment groups. The rate of metastasis in the para‐aortic nodes among patients who had dissection was 15·2 per cent (22 of 145), and the 5‐year OS rate of patients with pathologically confirmed metastases in the para‐aortic nodes was 18 (95 per cent c.i. 6 to 36) per cent. Previous studies20, 21 reported metastasis to the para‐aortic field in approximately 15 per cent of OGJ cancers, representing the most frequent site of nodal recurrence. Para‐aortic lymph node dissection has been identified as an independent prognostic factor in patients with pT3/4 Siewert type II tumours22. Although the randomized clinical trial (JCOG9501)23 failed to show a survival advantage for prophylactic para‐aortic lymph node dissection in patients with gastric cancer, the possibility of improving survival with prophylactic para‐aortic lymph node dissection in patients with Siewert type II or III tumours remains unclear, as these patients were excluded from JCOG9501.
Many institutions include neoadjuvant or adjuvant therapy in their standard treatment protocols for patients with oesophageal or gastric cancer. A Dutch randomized clinical trial24 evaluating preoperative chemoradiotherapy using carboplatin and paclitaxel for oesophageal or OGJ tumours demonstrated significantly better OS in a preoperative chemoradiotherapy group versus surgery alone. In East Asia, two large‐scale randomized clinical trials25, 26, each with over 1000 patients, have evaluated postoperative chemotherapy for resectable gastric or OGJ cancer; both demonstrated a significant survival benefit with postoperative chemotherapy. As the present study did not allow neoadjuvant or adjuvant treatment until recurrence, the isolated effect of the TH versus the LTA approach on survival without any treatment interactions associated with perioperative treatment could be evaluated. Selection of the appropriate surgical procedure is still an important issue for OGJ adenocarcinoma irrespective of the addition of other treatments.
There are some limitations to this study. As it was terminated before the planned sample size had been reached, the power to detect a difference between the groups was reduced. Despite this, the interim results were sufficient to reach the conclusion that LTA did not improve survival compared with the TH approach, and this has been validated in this final analysis. Another limitation is that the distribution of Siewert tumour types was not well balanced between the groups. Because the Siewert classification of OGJ tumours had not been proposed at the time of trial initiation4, it was not used for stratification at randomization, and assignment of the Siewert classification was made after surgery. As the results of a post hoc subgroup analysis may include some distortion, caution should be used when interpreting the results regarding Siewert classification.
Supporting information.
Additional supporting information may be found in the online version of this article:
Fig. S1 Kaplan–Meier curves of overall survival in per‐protocol population by treatment group (TIFF file)
Supporting information
FigS1 Kaplan–Meier curves of overall survival in per‐protocol population by treatment group. TH, transhiatal approach; LTA, left thoracoabdominal approach. Hazard ratio (HR) 1·44 (95 per cent c.i. 0·96 to 2·15; P = 0·962 and P = 0·076, 1‐ and 2‐sided log rank test respectively)
Acknowledgements
The authors thank H. Kaba for data management, H. Katayama and K. Kataoka for their comments on drafts of the manuscript, and H. Fukuda for direction of the JCOG Data Centre and oversight of study management. The study was funded in part by Grants‐in‐Aid for Cancer Research and for the Second‐Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare, Japan, and by the National Cancer Centre Research and Development Fund (26‐A‐4).
Disclosure: The authors declare no conflict of interest.
References
- 1. Devesa SS, Blot WJ, Fraumeni JF Jr . Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83: 2049–2053. [PubMed] [Google Scholar]
- 2. Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001; 92: 549–555. [DOI] [PubMed] [Google Scholar]
- 3. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta‐analysis. Ann Thorac Surg 2001; 72: 306–313. [DOI] [PubMed] [Google Scholar]
- 4. Siewert JR, Stein HJ. Carcinoma of the gastroesophageal junction: classification, pathology and extent of resection. Dis Esoph 1996; 9: 173–182. [Google Scholar]
- 5. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K et al Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert's classification applied to 177 cases resected at a single institution. J Am Coll Surg 1999; 189: 594–601. [DOI] [PubMed] [Google Scholar]
- 6. Kawaura Y, Mori Y, Nakajima H, Iwa T. Total gastrectomy with left oblique abdominothoracic approach for gastric cancer involving the esophagus. Arch Surg 1988; 123: 514–518. [DOI] [PubMed] [Google Scholar]
- 7. Kodama I, Kofuji K, Yano S, Shinozaki K, Murakami N, Hori H et al Lymph node metastasis and lymphadenectomy for carcinoma in the gastric cardia: clinical experience. Int Surg 1998; 83: 205–209. [PubMed] [Google Scholar]
- 8. Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol 1996; 23: 360–368. [PubMed] [Google Scholar]
- 9. Yonemura Y, Tsugawa K, Fonseca L, Fushida S, Matsumoto H, Ninomiya I et al Lymph node metastasis and surgical management of gastric cancer invading the esophagus. Hepatogastroenterology 1995; 42: 37–42. [PubMed] [Google Scholar]
- 10. Husemann B. Cardia carcinoma considered as a distinct entity. Br J Surg 1989; 76: 136–139. [DOI] [PubMed] [Google Scholar]
- 11. Wang LS, Wu CW, Hsieh MJ, Fahn HJ, Huang MH, Chien KY. Lymph node metastasis in patients with adenocarcinoma of gastric cardia. Cancer 1993; 71: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 12. Dresner SM, Lamb PJ, Bennett MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001; 129: 103–109. [DOI] [PubMed] [Google Scholar]
- 13. Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T et al Left thoracoabdominal approach versus abdominal–transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006; 7: 644–651. [DOI] [PubMed] [Google Scholar]
- 14. Safranek PM, Sujendran V, Baron R, Warner N, Blesing C, Maynard ND. Oxford experience with neoadjuvant chemotherapy and surgical resection for esophageal adenocarcinomas and squamous cell tumors. Dis Esophagus 2008; 21: 201–206. [DOI] [PubMed] [Google Scholar]
- 15. Gillies RS, Simpkin A, Sgromo B, Marshall RE, Maynard ND. Left thoracoabdominal esophagectomy: results from a single specialist center. Dis Esophagus 2011; 24: 138–144. [DOI] [PubMed] [Google Scholar]
- 16. Japanese Research Society for Gastric Cancer . Japanese Classification of Gastric Carcinoma (1st English edn). Kanehara: Tokyo, 1995. [Google Scholar]
- 17. Sobin LH, Wittekind C. (eds). TNM Classification of Malignant Tumours (6th edn). Wiley‐Liss: New York, 2002. [Google Scholar]
- 18. Kurokawa Y, Sasako M, Sano T, Shibata T, Ito S, Nashimoto A et al Functional outcomes after extended surgery for gastric cancer. Br J Surg 2011; 98: 239–245. [DOI] [PubMed] [Google Scholar]
- 19. Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H et al Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five year survival of a randomized clinical trial. Ann Surg 2007; 246: 992–1000. [DOI] [PubMed] [Google Scholar]
- 20. Yamashita H, Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg 2011; 254: 274–280. [DOI] [PubMed] [Google Scholar]
- 21. Hosokawa Y, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y et al Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol 2012; 19: 677–683. [DOI] [PubMed] [Google Scholar]
- 22. Mine S, Sano T, Hiki N, Yamada K, Nunobe S, Yamaguchi T. Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg 2013; 100: 261–266. [DOI] [PubMed] [Google Scholar]
- 23. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A et al D2 lymphadenectomy alone or with para‐aortic nodal dissection for gastric cancer. N Engl J Med 2008; 359: 453–462. [DOI] [PubMed] [Google Scholar]
- 24. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 25. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T et al Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011; 29: 4387–4393. [DOI] [PubMed] [Google Scholar]
- 26. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH et al Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomized controlled trial. Lancet 2012; 379: 315–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigS1 Kaplan–Meier curves of overall survival in per‐protocol population by treatment group. TH, transhiatal approach; LTA, left thoracoabdominal approach. Hazard ratio (HR) 1·44 (95 per cent c.i. 0·96 to 2·15; P = 0·962 and P = 0·076, 1‐ and 2‐sided log rank test respectively)


