Abstract
BACKGROUND
Petroselinum crispum (English parsley) is a common herb of the Apiaceae family that is cultivated throughout the world and is widely used as a seasoning condiment. Studies have shown its potential as a medicinal herb. In this study, P. crispum leaf and stem extracts were evaluated for their antioxidant properties, protection against DNA damage in normal 3T3‐L1 cells, and the inhibition of proliferation and migration of the MCF‐7 cells.
RESULTS
The dichloromethane extract of P. crispum exhibited the highest phenolic content (42.31 ± 0.50 mg GAE g−1) and ferric reducing ability (0.360 ± 0.009 mmol g−1) of the various extractions performed. The extract showed DPPH radical scavenging activity with an IC50 value of 3310.0 ± 80.5 µg mL −1. Mouse fibroblasts (3T3‐L1) pre‐treated with 400 µg mL −1 of the extract showed 50.9% protection against H2O2‐induced DNA damage, suggesting its potential in cancer prevention. The extract (300 µg mL −1) inhibited H2O2‐induced MCF‐7 cell migration by 41% ± 4%. As cell migration is necessary for metastasis of cancer cells, inhibition of migration is an indication of protection against metastasis.
CONCLUSION
Petroselinum crispum has health‐promoting properties with the potential to prevent oxidative stress‐related diseases and can be developed into functional food. © 2015 The Authors. Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Petroselinum crispum, antioxidant activity, antiproliferative activity, DNA protection, MCF‐7 cell migration, hydrogen peroxide
INTRODUCTION
Petroselinum crispum (Mill) Nyman ex AW Hill, commonly known as English parsley, is a culinary and medicinal herb of the Apiaceae family that grows up to 30–100 cm high.1 The herb has been used to flavor the cuisines of South East Asia, China, India, South America and Mexico.2 Although native to Europe and western Asia, the herb is now cultivated and consumed throughout the world.3 The leaves and stems, either fresh or dried, as well as the seeds, have been employed in the food, pharmaceutical and cosmetic industries.4 In folk medicine, the aerial part of P. crispum is used to treat hemorrhoids, the stem for urethral inflammation, and the root is used to pass kidney stones5 and improve brain function and memory.6 Additionally, P. crispum is used as a carminative, stomachic, emmenagogic, abortifacient and nutritive agent.7 Studies have shown that P. crispum has hypoglycemic, diuretic, hypolipidemic, antimicrobial, anticoagulant and hepatoprotective activities.8
The chemical composition and pharmacological properties of P. crispum have been previously reported in various studies. The herb contains flavonol glycosides of quercetin, apiol, myristicin and luteolin. Terpenes, phthalides, furanocoumarins, apiin, carotenoids, ascorbic acid and tocopherol are also present in P. crispum.9, 10 Supplementation of diets with fresh P. crispum leaves increases the antioxidant capacity of plasma in rats11 and decreases oxidative stress in humans.12 Zheng et al. 13 reported the inhibition of benzo[a]pyrene‐induced tumorigenesis in the lungs of mice by myristicin, a major volatile aromatic constituent of parsley leaf oil. The vast health‐promoting properties associated with P. crispum warrant further study. Previous investigations on P. crispum mostly focused on its antioxidant properties.14 As phenolic compounds and antioxidant activities depend on variety, location and growth conditions of the plant, data on the antioxidant activity of P. crispum are still relevant and useful. The effect of P. crispum leaves and stems on the two most common cancers in humans – breast cancer and colon cancer – are unclear and lacking thus far. The main aim of this work was to investigate the antioxidant activities and protection against DNA damage by extracts of P. crispum leaves and stems and their inhibition of the proliferation and H2O2‐induced migration of the breast cancer cell line MCF‐7. To the best of our knowledge, this is the first study reporting on the effects of P. crispum on DNA protection and inhibition of MCF‐7 cell migration.
EXPERIMENTAL
Materials and methods
Analytical‐grade solvents were purchased from Fisher Scientific (Loughborough, UK). Dimethyl sulfoxide (DMSO) and H2O2 were purchased from Univar (Ingleburn, NSW, Australia). Chemicals, polyphenolic standards (gallic acid, quercetin, rutin), proteinase K and RPMI‐1640 were obtained from Sigma‐Aldrich (St Louis, MO, USA). Dulbecco's Modified Eagle Medium (DMEM) was purchased from Lonza (Basel, Switzerland) and fetal bovine serum (FBS) was obtained from iDNA Biotechnology, Singapore. 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT), Folin–Ciocalteu phenol reagent, tris(hydroxymethyl)aminomethane and ethidium bromide were purchased from Merck (Darmstadt, Germany). Sodium dodecyl sulfate (SDS) was from Bio‐Rad (Hercules, CA, USA). TRIzol® reagent was purchased from Life Technologies (Carlsbad, CA, USA). RNase A and DNA ladders were obtained from Thermo Scientific (Carlsbad, CA, USA). Ultrapure water from a Milli‐Q‐plus filter system (Millipore, Billerica, MA, USA) was used throughout the experiments.
Cell lines and cell culture conditions
Two human breast adenocarcinoma cell lines – MCF‐7 and MDA‐MB‐231 – and the human colorectal adenocarcinoma cell line HT‐29 were used in the antiproliferative study. The MCF‐7 cell line expresses the estrogen receptor whereas MDA‐MB‐231 cells do not. The study on the inhibition of cell migration, trypan blue dye exclusion assay and DNA fragmentation analysis was performed using MCF‐7 cells. Mouse fibroblasts (3 T3‐L1) were used in the comet assay to estimate protection against H2O2‐induced DNA damage. All cells were purchased from the American Type Culture Collection (Manassas, VA, USA). MCF‐7 and HT‐29 cancer cells were routinely cultured in RPMI‐1640. MDA‐MB‐231 and 3 T3‐L1 were grown in DMEM. The cells were supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin and 100 µg mL−1 streptomycin. Cells were grown at 37 °C in a humidified incubator with 5% CO2.
Plant material
Fresh P. crispum leaves and stems were purchased from the local market in Kuala Lumpur, Malaysia. The plant was identified by Dr M Sugumaran, Institute of Biological Sciences, University of Malaya, and the voucher specimen (KLU47745) was deposited in the University of Malaya herbarium. The leaves and stems were washed under running tap water and finally rinsed with distilled water. The plant parts were then freeze dried, weighed, ground into fine powder and stored at −20 °C until extraction.
Preparation of P. crispum extracts
Powdered leaves and stems of P. crispum were extracted through sequential extraction using hexane, dichloromethane, ethyl acetate, methanol and water. Briefly, 120 g powdered leaves and stems were extracted in 600 mL hexane (1:5 w/v) for 6 h at 40 °C on a hotplate stirrer. Extracts were then filtered through Whatman no. 1 filter paper and the resulting residue was re‐extracted twice with fresh hexane. The remaining residue was subsequently extracted three times each with dichloromethane, followed by ethyl acetate, methanol and water. Each filtrate (except for the aqueous extract, which was concentrated to dryness in a freeze‐dryer) was concentrated to dryness under reduced pressure at 40 °C using a rotary evaporator. The dried extracts were stored at −20 °C. For bioassays, the dried extracts were dissolved in DMSO and diluted in ultrapure water to make appropriate extract concentrations. The final concentration of DMSO in reaction mixtures was less than 1%. All dissolved extracts were kept at 4 °C throughout the experiments.
Determination of total phenolic content
The total phenolic content of P. crispum extracts was determined using the Folin‐Ciocalteau method,15 with some modifications. Briefly, 500 µL of 1:10 Folin–Ciocalteau phenol reagent was added to 10 µL of sample (dissolved in 10% DMSO), standard or positive control. The mixture was mixed and allowed to stand for 5 min before the addition of 350 µL of 10% sodium carbonate. The resulting reaction mixture was incubated in the dark at room temperature for a further 2 h. Absorbance was then measured at 765 nm using a spectrophotometer. Gallic acid (50–500 mg L−1 in 10% DMSO) was used as the standard. Rutin and quercetin were used as positive controls. Results were expressed in milligrams of gallic acid equivalents (GAE) per gram of dried extract. All experiments were carried out in triplicate.
Ferric reducing antioxidant power (FRAP) assay
Ferric reducing activity of P. crispum extracts was estimated based on the assay by Benzie and Strain16 with slight modifications. A working reagent was prepared fresh by mixing 10 mL of 300 mmol L−1 acetate buffer with 1 mL of 10 mmol L−1 2,4,6‐tripyridyl‐s‐triazine (TPTZ) in 40 mmol L−1 hydrochloric acid and 1 mL of 20 mmol L−1 ferric chloride hexahydrate (FeCl3.6H2O). The freshly prepared FRAP reagent was pre‐warmed at 37 °C for 5 min, after which a blank reading was taken at 595 nm using a plate reader. Subsequently, 3 µL sample (dissolved in 10% DMSO), standard or positive control and 9 µL water were added to 90 µL of the FRAP reagent. Absorbance readings were measured instantly upon addition of the FRAP reagent and again at 4 min after the start of the reaction. The change in absorbance in the 4 min reaction was calculated by comparison with a FeSO4.7H2O standard curve (100–1000 µmol L−1) tested in parallel. Rutin and quercetin were used as positive controls. Results were expressed as millimoles of ferric reducing activity of the extracts per gram of dried extract. All experiments were carried out in triplicate.
1,1‐Diphenyl‐2‐picrylhydrazyl (DPPH) radical scavenging assay
The radical scavenging activity of P. crispum extracts was determined by the DPPH radical scavenging assay,17 with some modifications. Petroselinum crispum extract (20 µL) was added to 120 µL of 0.04 mg mL−1 DPPH solution in methanol. The extracts tested ranged from 0 to 5000 µg mL−1 (dissolved in 10% DMSO). The solutions were mixed well and incubated in the dark for 30 min. The reduction of DPPH absorption was measured at 515 nm using a plate reader. Rutin and quercetin were used as positive controls. All determinations were performed in triplicate. The DPPH radical scavenging activity was calculated according to the following equation:
 |
The results were expressed as half‐maximal inhibitory concentration (IC50), i.e. the concentration of the plant extract required to scavenge 50% of the total DPPH radicals available.
Inhibition of proliferation (MTT assay)
The antiproliferative activity of P. crispum extracts on MCF‐7, MDA‐MB‐231 and HT‐29 cancer cell lines was estimated using the MTT assay as described by Mosmann.18 Briefly, cells supplemented with 5% FBS were seeded (5 × 103 cells per well) in 96‐well plates and were allowed to grow at 37 °C in a humidified atmosphere with 5% CO2. After 24 h incubation, the cells were treated with different concentrations of extract (0–500 µg mL−1) for a further 48 h. Vehicle‐control wells with cells only and diluent‐control wells with similar DMSO concentrations as in the treatment were included. After incubation, 10 µL of 5 mg mL−1 MTT bromide in phosphate‐buffered saline (PBS) were added to each well. The plates were reincubated for 4 h, after which media and MTT were removed by aspiration. DMSO (100 µL) was added to each well to dissolve the formazan crystals. Absorbance was read using a microtiter plate reader at 595 nm. All measurements were performed in triplicate. The percentage inhibition of cell proliferation was calculated using the following formula:
 |
Comet assay
The DNA protective effect of P. crispum was estimated using the comet assay.19 Mouse fibroblasts (3 T3‐L1) were cultured in 12‐well culture plates (1 × 105 cells per well) for 24 h. The cells were then pre‐treated with dichloromethane extracts of P. crispum, at concentrations of 100–400 µg mL−1 for a further 24 h. The control contained DMSO instead of extract. After pre‐treatment, cells were treated with 100 µmol L−1 of H2O2 (final concentration in the well) for 60 min on ice to induce DNA damage. Cells were then harvested using a cell scraper, centrifuged and resuspended in 1 mL PBS. The cell suspension (25 µL) was mixed with 75 µL of 0.6% low‐melting agarose and the suspension was spread on a frosted microscopic slide pre‐coated with 250 µL of 0.8% normal melting agarose, covered with a cover slip, and then allowed to solidify on ice for 10 min. The cover slips were removed and the slides were immersed in cold lysis solution containing 1% SDS, 2.5 mol L−1 NaCl, 100 mmol L−1 Na2 ethylenediaminetetraacetic acid (Na2EDTA), 1% Triton X‐100 and 10% DMSO (with the DMSO added just before use) for 1 h at 4 °C in the dark. The slides were arranged in an electrophoresis tank filled with pre‐chilled electrophoretic buffer (1 mmol L−1 Na2EDTA and 300 mmol L−1 NaOH) and incubated for 20 min. Electrophoresis was conducted in the same buffer at 25 V (300 mA) for 20 min. The slides were washed with 0.4 mol L−1 Tris–HCl (pH 7.5) and stained with 20 µg mL−1 ethidium bromide for viewing under a fluorescence microscope. The comet tail length was measured using an ocular micrometer. A total of 50 individual cells were screened per slide.20 The assay was carried out in triplicate. Results were expressed in percent DNA protection, calculated using the following formula:
 |
Scratch motility assay
The inhibitory effect of P. crispum on MCF‐7 cell migration was tested using the scratch motility assay. MCF‐7 cells (3.5 × 105 cells per well) were seeded in a 24‐well plate and grown for 24 h. The confluent cell monolayer was then scratched vertically with a pipette tip, washed twice with PBS and incubated with media containing P. crispum dichloromethane extract (0, 200, 300, 400, 500 and 600 µg mL−1) with 5% FBS. H2O2 was added into each well at a final concentration of 1 µmol L−1 in the cell suspension to stimulate the proliferation and migration of MCF‐7 cells. The number of cells in the denuded area were photographed and counted at 0 and 24 h incubation. The experiment was performed in triplicate. The percentage inhibition was calculated as described by Sato and Rifkin.21 Percentage inhibition = 100 − [(cell no. in denuded area of sample / cell no. in denuded area of control) × 100].
Trypan blue dye exclusion assay
A hemocytometer‐based trypan blue dye exclusion cell quantitation and viability assay was used to confirm the antiproliferative activity of P. crispum. MCF‐7 cells were seeded in a six‐well plate (1.5 × 106 cells per well) supplemented with 5% FBS and allowed to grow at 37 °C in a humidified atmosphere with 5% CO2. After 24 h incubation, the cells were treated with different concentrations of extract (0–800 µg mL−1) for a further 48 h. Diluent‐control wells with similar DMSO concentrations as in the treatment were included. Following treatment, the cells were collected using 0.25% trypsin–EDTA, pelleted and resuspended in medium. The cells were then stained with an equal volume of 0.2% (w/v) trypan blue solution and the number of viable cells was counted using a hemocytometer under an inverted microscope. All measurements were performed in triplicate.
DNA fragmentation analysis
Agarose gel electrophoresis was used to investigate DNA fragmentation in cells treated with P. crispum. MCF‐7 cells (2 × 106 mL−1) were grown in a 75 cm2 culture flask for 24 h. The cells were then treated with P. crispum dichloromethane extract (500 or 800 µg mL−1) for 24 or 48 h. After treatment, cells were lysed and the DNA was extracted using TRIzol® reagent according to the manufacturer's protocol. Extracted DNA was treated with RNase A (3 mg mL−1) at 37 °C for 1 h and proteinase K (200 µg mL−1) at 50 °C for 2 h. The purified DNA was stored at −20 °C until DNA electrophoresis. The experiment was performed in triplicate. Isolated DNA samples were subjected to electrophoresis in 1.8% (w/v) agarose gel (in TAE buffer) impregnated with 0.5 µg mL−1 ethidium bromide and run at 90 V for 50 min. The gel was observed under UV illumination and visualized using a gel documentation system (UVP, USA).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed by one‐way analysis of variance (ANOVA) with Tukey's multiple comparisons and Student's t‐test. A P‐value of <0.05 was considered statistically significant. Pearson's correlation coefficient was used to assess the correlation between TPC, FRAP and DPPH radical scavenging activity. Statistical Package for the Social Sciences (SPSS) version 18.0 (Chicago, IL, USA) and Microsoft Excel 2007 (Roselle, IL, USA) were used for the statistical and graphical evaluations.
RESULTS AND DISCUSSION
Total phenolic content
Phenolics are secondary metabolites that are ubiquitously present in plants. Positive correlations between antioxidant activities present in medicinal plants with their total phenolic content have been reported. In our study, the phenolic values of P. crispum extracts ranged from 9.63 ± 2.60 to 42.31 ± 0.50 mg GAE g−1 (Table 1). The dichloromethane extract displayed the highest phenolic content (P < 0.05) among the extracts. The nature of the extracting solvent is one of the most important factors in the extraction of antioxidants, thus explaining the various phenolic values from different extracts of P. crispum leaves and stems.
Table 1.
Phenolic content, ferric reducing antioxidant power and DPPH radical scavenging activity of Petroselinum crispum extracts
| P. crispum extract/positive controls | Total phenolic content (mg GAE g−1) | FRAP value (mmol g−1) | DPPH radical scavenging activity (µg mL−1) |
|---|---|---|---|
| Hexane | 20.17 ± 1.35b | 0.075 ± 0.002b | 4485.0 ± 78.0b |
| Dichloromethane | 42.31 ± 0.50d | 0.360 ± 0.009d | 3310.0 ± 80.5a |
| Ethyl acetate | 32.17 ± 2.24c | 0.139 ± 0.006c | 4712.0 ± 87.0c |
| Methanol | 24.77 ± 1.24b | 0.027 ± 0.004a | ND |
| Aqueous | 9.63 ± 2.60a | 0.014 ± 0.003a | ND |
| Positive control | |||
| Rutin | 649.93 ± 13.34 | 1.789 ± 0.214 | 42.7 ± 2.3 |
| Quercetin | 1275.62 ± 56.03 | 14.444 ± 0.934 | 22.2 ± 0.9 |
Results are presented as means ± SD (n = 3).
IC50 values are presented for the DPPH radical scavenging activity.
Values within the same column with different letters (a–d) are significantly different at P < 0.05 from the different extracts. ND, not detected.
A study by Luthria22 investigated the influence of particle size on phenolic compound extraction of P. crispum with ethanol:water, 50:50 (v/v), using a pressurized liquid extractor. They reported phenolic values ranging from 18.3 to 22.9 mg GAE g−1. In our study, we showed higher phenolic content in the dichloromethane (42.31 ± 0.50 mg GAE g−1), ethyl acetate (32.17 ± 2.24 mg GAE g−1) and methanolic (24.77 ± 1.24 mg GAE g−1) extracts of P. crispum compared to that reported by Luthria.22 The dichloromethane extract in our study also showed higher phenolic content compared to the P. crispum hydrodistilled extract (29.2 ± 0.44 mg GAE g−1) reported by Hinneburg et al. 23 A possible explanation for the different phenolic values between these studies and that reported in our study is that phenolic content in plants differ very much between cultivars of the same species and are influenced by genetic factors and environmental conditions.14 In addition, parameters such as solvent polarity, extraction procedures and conditions can influence the extraction of phenolic compounds from P. crispum.24 Our study showed that the dichloromethane solvent resulted in the highest extraction of phenolics from P. crispum leaves and stems.
Ferric reducing antioxidant power
The FRAP values of P. crispum extracts are presented in Table 1. Among the extracts tested, the dichloromethane extract of P. crispum exhibited the highest FRAP value (0.360 ± 0.009 mmol g−1, P < 0.05).
Pearson correlation analysis was performed to assess the relationship between phenolic content and ferric reducing activities of the leaf and stem extracts. A statistically significant positive correlation was identified between FRAP and phenolic content of P. crispum (r = 0.875, P < 0.01; Table 2). This indicates that phenolic compounds present in P. crispum contributed to their ferric reducing activities. The reductive ability of the extracts suggests their ability to donate electrons to reduce ferric tripyridyltriazine (Fe3+‐TPTZ) to the ferrous complex (Fe2+‐TPTZ). This implies that P. crispum extracts may provide antioxidative protection from free radicals in actual biological systems by donating electrons to radicals and blocking radical chain reactions from causing diseases related to chronic oxidative stress.25
Table 2.
Correlation analysis of total phenolic content with antioxidant activities of Petroselinum crispum extracts
TPC, total phenolic content; FRAP, ferric reducing antioxidant power; DPPH, 1,1‐diphenyl‐2‐picryl hydrazyl radical scavenging activity.
Correlation is significant at the 0.01 level.
DPPH radical scavenging activity
The dichloromethane extract of P. crispum showed the lowest IC50 value (3310.0 ± 80.5 µg mL−1, P < 0.05) compared to the other P. crispum extracts (Table 1). In a study by Zhang et al.,26 essential oil from P. crispum showed antioxidant activities in the β‐carotene bleaching assay (EC50 = 5.12 mg mL−1) and DPPH scavenging assay (EC50 = 80.21 mg mL−1). Hinneburg et al. 23 reported that the P. crispum hydrodistilled extract showed an IC50 value of 12.0 ± 0.10 mg mL−1 in the DPPH scavenging assay. In our study, the P. crispum extracts showed better DPPH radical scavenging activity (Table 1) compared to the two studies mentioned above.
A strong and significant positive correlation was seen between DPPH scavenging activity and phenolic content of P. crispum (r = 0.910, P < 0.01; Table 2). This shows that phenolic compounds of P. crispum could be responsible for the observed DPPH radical scavenging activity, since these compounds can readily donate hydrogen atoms to the radical. From the antioxidant study, it is observed that the dichloromethane extract displayed highest phenolic content and FRAP value while exhibiting best DPPH radical scavenging activity among the extracts of P. crispum leaves and stems.
Antiproliferative activity
Extracts of P. crispum (0–500 µg mL−1) were tested for their effect on the proliferation of MCF‐7, MDA‐MB‐231 and HT‐29 cells, using the MTT assay. Generally, extracts of P. crispum leaves and stems exhibited weak cytotoxic activity with percent inhibitions below 50% (Fig. 1). Among the five extracts analyzed, the dichloromethane extract exhibited the best antiproliferative activity. At the highest concentration tested (500 µg mL−1), the dichloromethane extract showed a percentage inhibition of 48.4% ± 1.8%, 25.5% ± 3.0% and 49.9% ± 1.0% on MCF‐7, MDA‐MB‐231 and HT‐29 cells, respectively (Fig. 1). The ethyl acetate, methanol and aqueous extracts showed less than 20% inhibition, even at 500 µg mL−1 of extract. The different cytotoxic effects of the various extracts in this study suggest the importance of using solvents of differing polarity in order to extract compounds with various polarities that contribute to different biological activities of the plant extract. Each extract of P. crispum behaved differently against the cell lines. The distinct effects of these extracts may be due to the phytodiversity or different mechanisms associated with the compounds present in the extracts and the various susceptibility levels of cell lines to the plant extracts.27, 28
Figure 1.
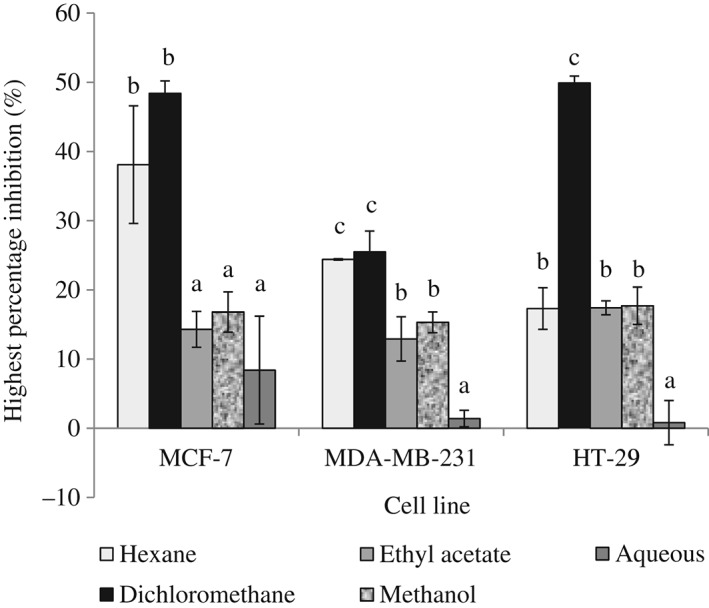
Anti‐proliferative activities of Petroselinum crispum extracts on cancer cell lines, MCF‐7, MDA‐MB‐231 and HT‐29. Results are presented as means ± SD (n = 3). Values within the same cell line with different letters (a–c) are significantly different at P < 0.05 from the different extracts. Extract concentration tested: 0–500 µg mL−1.
In a study by Yoshikawa et al.,29 the methanolic extract from the aerial parts of P. crispum (1 and 10 µg mL−1) was shown to have potent estrogenic activity and increased MCF‐7 cell proliferation. In our study, the methanol extract did not increase MCF‐7 cell proliferation but exhibited a very weak antiproliferative effect on MCF‐7 cells. Our study on P. crispum leaves and stems showed that the dichloromethane extract displayed best antioxidant and antiproliferative activities. Hence we selected the dichloromethane extract for further analysis.
DNA protective activity
Within living cells, reactive oxygen species are constantly being generated as normal by‐products of mitochondrial respiration. Uncontrolled levels of reactive oxygen species can cause severe damage to macromolecules, especially DNA, leading to degenerative diseases such as cancer.30 H2O2 is an oxidizing agent which produces reactive hydroxyl radicals that can induce strand breaks associated with DNA damage.20 The comet assay is a quick, simple and sensitive method for the evaluation of DNA damage, mainly single‐strand and double‐strand breaks in individual cells. The comet tail length is associated with DNA damage. Greater tail length signifies greater DNA damage.19
Cells pre‐treated with the dichloromethane extract of P. crispum at concentrations of 100–400 µg mL−1 showed a significant dose‐dependent increase in DNA protection (P < 0.05) compared to the control of H2O2 treatment alone (Table 3). At 400 µg mL−1 of extract pretreatment, DNA damage was reduced by 50.9% ± 6.6% compared to the control, indicating 50.9% DNA protection. The high phenolic content in the dichloromethane extract of P. crispum as shown in Table 1 may be responsible for the observed DNA protective effect. A study on spices (ginger, caraway, cumin, cardamom, star anise and fennel) has shown a strong positive correlation between DNA protection and phenols.20 Phenolics in P. crispum can lower H2O2 levels or hydroxyl radicals by increasing the levels of H2O2‐detoxifying enzymes in cells, thus preventing DNA damage.31 Studies have shown that supplementation of diets with fresh P. crispum leaves can increase antioxidant capacity of rat plasma,11 protect against mitochondrial oxidative damage in the mouse brain10 and decrease oxidative stress in humans.12 Our study shows that the P. crispum extract protected 3 T3‐L1 fibroblasts against H2O2‐induced DNA damage, suggesting that appropriate addition of the herb in the daily diet might reduce the effects of free radical‐induced carcinogenesis, hence affording some protection against cancer.
Table 3.
Protection from H2O2‐induced DNA damage in 3 T3‐L1 fibroblasts pre‐treated with Petroselinum crispum extract
| P. crispum dichloromethane extract (µg mL−1) | DNA protection (%) |
|---|---|
| 100 | 19.0 ± 6.1* |
| 200 | 23.1 ± 6.9* |
| 300 | 37.9 ± 7.8* |
| 400 | 50.9 ± 6.6* |
Results are presented as means ± SD (n = 3).
P < 0.05 compared to control (without extract treatment), as tested by Student's t‐test.
Inhibition of H2O2‐induced MCF‐7 cell migration using the scratch motility assay
Metastasis is the most characteristic aspect of malignant neoplasm and is the leading cause of the ineffectiveness of chemotherapeutic drugs and cancer deaths. The scratch motility assay tests the ability of P. crispum extracts to inhibit migration of cancer cells in the denuded area, thus indicating defense against metastasis.32 H2O2 was included in this experiment to induce the proliferation and migration of MCF‐7 cells. The concentration of H2O2 (1 µmol L−1) used in this assay has been previously tested in our laboratory and showed increased cell migration and proliferation.20
In this study, the scratch motility assay displayed the ability of P. crispum to suppress H2O2‐induced migration of MCF‐7 cells in a denuded area (Fig. 2). Treatment with P. crispum extract at 300 µg mL−1 resulted in the highest inhibition of MCF‐7 migration (41% ± 4%). At higher concentrations of P. crispum extract, the inhibitory effect on cell migration decreased; at the highest concentration tested, 600 µg mL−1, the inhibition of migration was the lowest (18% ± 7%). The dichloromethane extract of P. crispum inhibited the migration of MCF‐7 cells, but not in a directly proportional manner to the concentration of extract. It is interesting to note that the inhibition of proliferation induced by the extract was highest at the highest concentration of 500 µg mL−1. As cell migration is necessary for metastasis of cancer cells, inhibition of migration is an indication of protection against metastasis. Petroselinum crispum prevented migration of MCF‐7 cells, thus showing potential in preventing metastasis. The flavonoids present in P. crispum, apigenin and luteolin9 have been reported as chemopreventive agents of metastasis due to their ability to prevent tumor cell motility and invasion.33 Phenolics present in P. crispum might lower H2O2 levels or hydroxyl radicals by increasing the levels of H2O2‐detoxifying enzymes in cells such as glutathione peroxidase,31 thus preventing cancer cell proliferation and migration induced by H2O2. Antioxidants present in P. crispum can maintain H2O2 levels in cells within physiological levels and may be associated with the prevention of cancer cell proliferation and migration.
Figure 2.
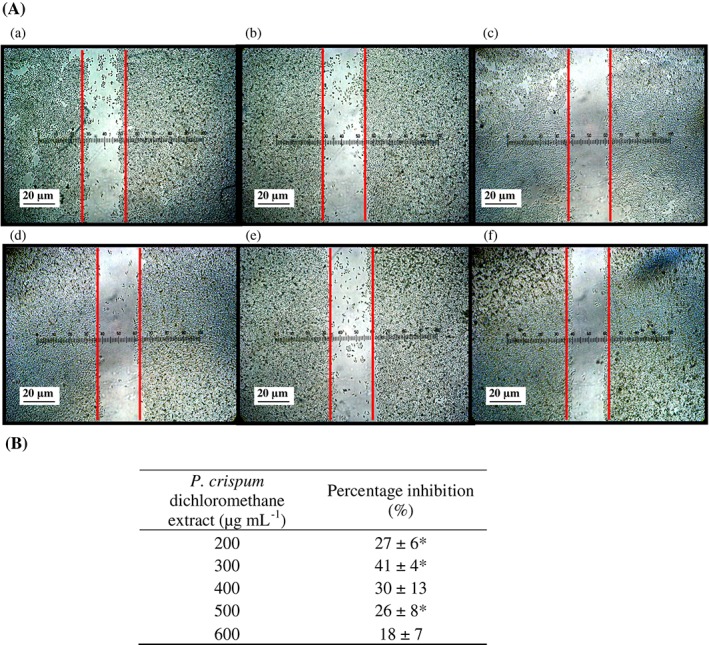
Effect of Petroselinum crispum dichloromethane extract on the inhibition of H2O2‐induced MCF‐7 cell migration in a denuded area using the scratch motility assay. (A) Photographs of cell migration (a) in untreated cells and (b) in cells treated with P. crispum dichloromethane extract at 200 µg mL−1, (c) 300 µg mL−1, (d) 400 µg mL−1, (e) 500 µg mL−1 and (f) 600 µg mL−1 after 24 h. (B) Percentage inhibition presented as means ± SD (n = 3). Asterisk represents * P < 0.05 compared to the control (without extract) as tested by Student's t‐test.
Trypan blue dye exclusion
Trypan blue is taken up by dead cells that have lost their membrane permeability barrier or dye exclusion capacity, while the intact plasma membrane of live cells excludes the dye.34 To assess the antiproliferative effect of P. crispum on MCF‐7 cells, trypan blue exclusion counts were conducted on cells treated with 0–800 µg mL−1 of dichloromethane extract for 48 h. A significant dose‐dependent decrease in live cell number (P < 0.05) was observed in cells treated with the dichloromethane extract of P. crispum compared to the control (Fig. 3). This is shown by the lower number of live cells counted as the concentration of dichloromethane extract treatment increased. Using the trypan blue exclusion assay, the percent of viable cells relative to untreated control was 55.04% ± 0.75% at 500 µg mL−1 of dichloromethane extract treatment, whereas at the highest concentration of extract treatment (800 µg mL−1) cell viability decreased to 30.10% ± 1.48%, indicating the antiproliferative activity of P. crispum dichloromethane extract on MCF‐7 cells. This antiproliferation profile by trypan blue exclusion assay further confirmed the inhibitory effect of P. crispum on MCF‐7 cell proliferation analyzed using the MTT assay (Fig. 1).
Figure 3.
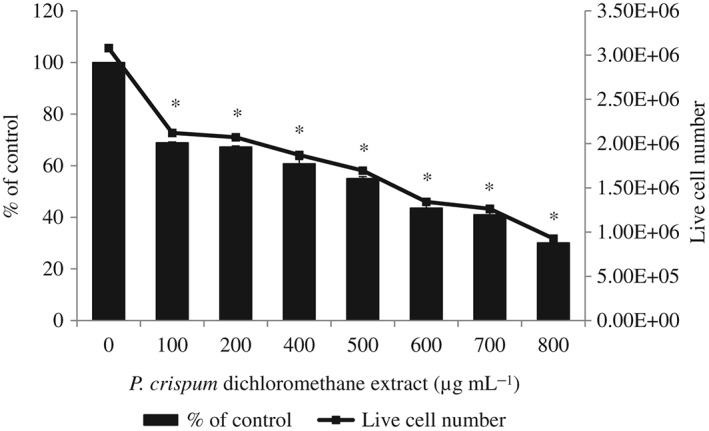
Trypan blue exclusion assay of MCF‐7 cells treated with Petroselinum crispum dichloromethane extract. The results are presented as the number of viable cells counted per well and the percent of viable cells relative to untreated control. The viability of untreated control cells was taken as 100%. Results are presented as means ± SD (n = 3). * P < 0.05 compared to the control (without extract) as tested by Student's t‐test.
DNA fragmentation analysis
DNA fragmentation is a hallmark of apoptosis. In agarose gel electrophoresis, apoptotic cells demonstrate a characteristic DNA ‘ladder’ pattern at ∼200 bp intervals, while necrotic cells are observed as a ‘smear’ of randomly degraded DNA.35 However, internucleosomal DNA fragmentation is not universal as it may not always occur during apoptosis.36
In this study, apoptotic DNA fragmentation was analyzed using agarose gel electrophoresis. DNA isolated from untreated control cells exhibited one clear band that pointed to the presence of living cells with intact DNA strand (Fig. 4). A typical DNA ladder pattern was not evident in MCF‐7 cells treated with dichloromethane extract of P. crispum (500 or 800 µg mL−1) for 24 or 48 h. Instead, a smear pattern of DNA fragmentation was observed in the extract‐treated cells, with more intense smearing seen in cells treated for 48 h, compared to 24 h. The results could indicate that P. crispum dichloromethane extract kills MCF‐7 cells by necrosis in a time‐dependent manner, where random DNA fragmentation occurs through the release of lysosomal DNases to form a ‘smear’ on agarose gels.37 Conversely, studies have reported that MCF‐7 cells can undergo apoptosis without showing DNA fragmentation due to lack of caspase‐3, which is responsible for this feature.38, 39 Thus, using the results from DNA fragmentation alone would not be definitive to accurately ascertain the mode of cell death (whether by apoptosis or necrosis, or both), in which P. crispum kills MCF‐7 cells. Further work will be needed for more in‐depth investigation into the mechanism of cell death induced by P. crispum.
Figure 4.
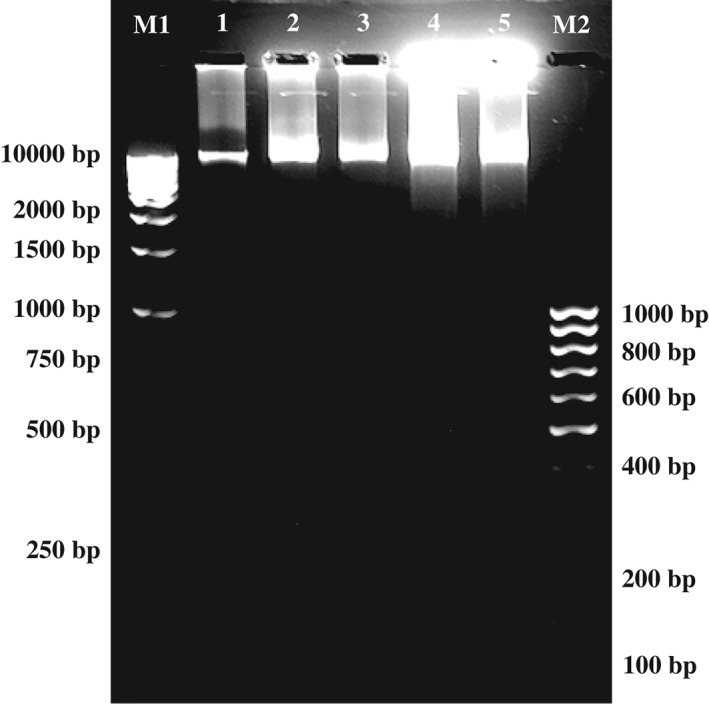
Electrophoresis of DNA extracted from MCF‐7 cells after treatment with Petroselinum crispum dichloromethane extract. M1: 1 kb DNA ladder; lane 1: DNA from cells of control; lane 2: DNA treated with 500 µg mL−1 extract for 24 h; lane 3: DNA treated with 800 µg mL−1 extract for 24 h; lane 4: DNA treated with 500 µg mL−1 extract for 48 h; lane 5: DNA treated with 800 µg mL−1 extract for 48 h; M2: 100 bp DNA ladder.
CONCLUSIONS
The dichloromethane extract of P. crispum leaves and stems showed antioxidant activities and also inhibition of proliferation and cell migration in MCF‐7 cells. The extract also protected against DNA damage induced by H2O2.
Regular addition of P. crispum in the daily diet as food or supplements can help strengthen the antioxidant systems of the body and reduce the effects of free radical‐induced carcinogenesis, cancer and subsequent metastasis caused by prolonged and excessive oxidative stress.
ACKNOWLEDGEMENTS
The study was supported by the University of Malaya Research University grants RG004/09AFR, PS250/2010B and RG341/11HTM.
The copyright line for this article was changed on 6 March 2015 after original online publication.
REFERENCES
- 1. Menglan S, Fading P, Zehui P, Watson MF, Cannon JFM, Holmes‐Smith I et al., Apiaceae (Umbelliferae). Flora of China 14:1–205 (2005). [Google Scholar]
- 2. Wong PYY and Kitts DD, Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem 97:505–515 (2006). [Google Scholar]
- 3. Bailey LH and Bailey EZ, Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada. Macmillan, New York: (1976). [Google Scholar]
- 4. Lopez M, Sanchez‐Mendoza I and Ochoa‐Alejo N, Comparative study of volatile components and fatty acids of plants and in vitro cultures of parsley (Petroselinum crispum (Mill) nym ex hill). J Agric Food Chem 47:3292–3296 (1999). [DOI] [PubMed] [Google Scholar]
- 5. Ezer N and Arisan OM, Folk medicines in Merzifon (Amasya, Turkey). Turk J Bot 30:223–230 (2006). [Google Scholar]
- 6. Adams M, Gmünder F and Hamburger M, Plants traditionally used in age related brain disorders: a survey of ethnobotanical literature. J Ethnopharmacol 113:363–381 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Soysal Y, Microwave drying characteristics of parsley. Biosyst Eng 89:167–173 (2004). [Google Scholar]
- 8. Sener G, Saçan Ö, Yanardag R and Ayanoglu‐Dülger G, Effects of parsley (Petroselinum crispum) on the aorta and heart of STZ induced diabetic rats. Plant Foods Hum Nutr 58:1–7 (2003).12859008 [Google Scholar]
- 9. Fejes S, Kery A, Blazovics A, Lugasi A, Lemberkovics E, Petri G et al., Investigation of the in vitro antioxidant effect of Petroselinum crispum (Mill.) Nym. ex AW Hill. Acta Pharm Hung 68:150–156 (1998). [PubMed] [Google Scholar]
- 10. Vora SR, Patil RB and Pillai MM, Protective effects of Petroselinum crispum (Mill) Nyman ex AW Hill leaf extract on d‐galactose‐induced oxidative stress in mouse brain. Indian J Exp Biol 47:338–342 (2009). [PubMed] [Google Scholar]
- 11. Hempel J, Pforte H, Raab B, Engst W, Böhm H and Jacobasch G, Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung 43:201–204 (1999). [DOI] [PubMed] [Google Scholar]
- 12. Nielsen S, Young J, Daneshvar B, Lauridsen S, Knuthsen P, Sandström B et al., Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr 81:447–455 (1999). [DOI] [PubMed] [Google Scholar]
- 13. Zheng GQ, Kenney PM, Zhang J and Lam LKT, Inhibition of benzo [a] pyrene‐induced tumorigenesis by myristicin, a volatile aroma constituent of parsley leaf oil. Carcinogenesis 13:1921–1923 (1992). [DOI] [PubMed] [Google Scholar]
- 14. Ramkissoon J, Mahomoodally M, Ahmed N and Subratty A, Antioxidant and anti‐glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med 6:561–569 (2013). [DOI] [PubMed] [Google Scholar]
- 15. Singleton V and Rossi JA, Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic 16:144–158 (1965). [Google Scholar]
- 16. Benzie IFF and Strain J, The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239:70–76 (1996). [DOI] [PubMed] [Google Scholar]
- 17. Cos P, Rajan P, Vedernikova I, Calomme M, Pieters L, Vlietinck AJ et al., In vitro antioxidant profile of phenolic acid derivatives. Free Radic Res 36:711–716 (2002). [DOI] [PubMed] [Google Scholar]
- 18. Mosmann T, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 (1983). [DOI] [PubMed] [Google Scholar]
- 19. Singh NP, McCoy MT, Tice RR and Schneider EL, A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191 (1988). [DOI] [PubMed] [Google Scholar]
- 20. Jayakumar R and Kanthimathi MS, Dietary spices protect against hydrogen peroxide‐induced DNA damage and inhibit nicotine‐induced cancer cell migration. Food Chem 134:1580–1584 (2012). [DOI] [PubMed] [Google Scholar]
- 21. Sato Y and Rifkin DB, Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol 107:1199–1205 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luthria DL, Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem 107:745–752 (2008). [Google Scholar]
- 23. Hinneburg I, Damien Dorman H and Hiltunen R, Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem 97:122–129 (2006). [Google Scholar]
- 24. Luthria DL, Mukhopadhyay S and Kwansa AL, A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J Sci Food Agric 86:1350–1358 (2006). [Google Scholar]
- 25. Dastmalchi K, Damien Dorman H, Koşar M and Hiltunen R, Chemical composition and in vitro antioxidant evaluation of a water‐soluble Moldavian balm (Dracocephalum moldavica L.) extract. LWT – Food Sci Technol 40:239–248 (2007). [Google Scholar]
- 26. Zhang H, Chen F, Wang X and Yao HY, Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res Int 39:833–839 (2006). [Google Scholar]
- 27. Chatelain K, Phippen S, McCabe J, Teeters CA, O'Malley S and Kingsley K, Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid Based Complement Alternat Med DOI:10.1093/ecam/nen047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ford J, Jiang M and Milner J, Cancer‐specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res 65:10457–10463 (2005). [DOI] [PubMed] [Google Scholar]
- 29. Yoshikawa M, Uemura T, Shimoda H, Kishi A, Kawahara Y and Matsuda H, Medicinal foodstuffs. XVIII. Phytoestrogens from the aerial part of Petroselinum crispum MILL. (Parsley) and structure of 6″‐acetylapiin and a new monoterpene glycoside, petroside. Chem Pharm Bull – Tokyo 48:1039–1044 (2000). [DOI] [PubMed] [Google Scholar]
- 30. Halliwell B, Antioxidants and human disease: a general introduction. Nutr Rev 55:44–49 (1997). [DOI] [PubMed] [Google Scholar]
- 31. Hashim M, Lincy S, Remya V, Teena M and Anila L, Effect of polyphenolic compounds from Coriandrum sativum on H2O2‐induced oxidative stress in human lymphocytes. Food Chem 92:653–660 (2005). [Google Scholar]
- 32. Hulkower KI and Herber RL, Cell migration and invasion assays as tools for drug discovery. Pharmaceutics 3:107–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weng CJ and Yen GC, Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti‐invasive and in vivo anti‐metastatic activities. Cancer Metastasis Rev 31: 323–351 (2012). [DOI] [PubMed] [Google Scholar]
- 34. Strober W, Trypan blue exclusion test of cell viability. Curr Protoc Immunol 21:A.3B.1–A.3B.2 (2001). [DOI] [PubMed] [Google Scholar]
- 35. Collins JA, Schandl CA, Young KK, Veseley J and Willingham MC, Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem 45:923–934 (1997). [DOI] [PubMed] [Google Scholar]
- 36. Vinatier D, Dufour P and Subtil D, Apoptosis: a programmed cell death involved in ovarian and uterine physiology. Eur J Obstet Gynaecol Reprod Biol 67:85–102 (1996). [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Sharov VG, Jiang N, Zaloga C, Sabbah HN and Chopp M, Ultrastructural and light microscope evidence of apoptosis after middle cerebral artery occlusion in the rat. Am J Pathol 146:1045–1051 (1995). [PMC free article] [PubMed] [Google Scholar]
- 38. Jänicke RU, Sprengart ML, Wati MR and Porter AG, Caspase‐3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360 (1998). [DOI] [PubMed] [Google Scholar]
- 39. Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE et al., Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12:3679–3684 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]


