Abstract
DIAMOND: multicenter, 24‐week, randomized trial investigating the effect of different once‐daily, prolonged‐release tacrolimus dosing regimens on renal function after de novo liver transplantation. Arm 1: prolonged‐release tacrolimus (initial dose 0.2mg/kg/day); Arm 2: prolonged‐release tacrolimus (0.15–0.175mg/kg/day) plus basiliximab; Arm 3: prolonged‐release tacrolimus (0.2mg/kg/day delayed until Day 5) plus basiliximab. All patients received MMF plus a bolus of corticosteroid (no maintenance steroids). Primary endpoint: eGFR (MDRD4) at Week 24. Secondary endpoints: composite efficacy failure, BCAR and AEs. Baseline characteristics were comparable. Tacrolimus trough levels were readily achieved posttransplant; initially lower in Arm 2 versus 1 with delayed initiation in Arm 3. eGFR (MDRD4) was higher in Arms 2 and 3 versus 1 (p = 0.001, p = 0.047). Kaplan–Meier estimates of composite efficacy failure‐free survival were 72.0%, 77.6%, 73.9% in Arms 1–3. BCAR incidence was significantly lower in Arm 2 versus 1 and 3 (p = 0.016, p = 0.039). AEs were comparable. Prolonged‐release tacrolimus (0.15–0.175mg/kg/day) immediately posttransplant plus basiliximab and MMF (without maintenance corticosteroids) was associated with lower tacrolimus exposure, and significantly reduced renal function impairment and BCAR incidence versus prolonged‐release tacrolimus (0.2mg/kg/day) administered immediately posttransplant. Delayed higher‐dose prolonged‐release tacrolimus initiation significantly reduced renal function impairment compared with immediate posttransplant administration, but BCAR incidence was comparable.
Keywords: calcineurin inhibitor, clinical research/practice, clinical trial, glomerular filtration rate (GFR), immunosuppression/immune modulation, immunosuppressant, liver allograft function/dysfunction, liver transplantation/hepatology, liver transplantation: split, tacrolimus
Short abstract
The DIAMOND study demonstrates that an initial lower dose or a delayed higher dose of prolonged‐release tacrolimus significantly reduces renal function impairment versus a higher initial dose of prolonged‐release tacrolimus administered immediately posttransplant, over 24 weeks of treatment in de novo liver transplant recipients.
Abbreviations
- AE
adverse event
- ANCOVA
analysis of covariance
- AR
acute rejection
- BCAR
biopsy‐confirmed acute rejection
- BD
twice daily
- CI
confidence interval
- CKD‐EPI
Chronic Kidney Disease Epidemiology Collaboration
- DIAMOND
ADVAGRAF™ studIed in combinAtion with MycOphenolate mofetil aND basiliximab in liver transplantation
- EQ5D
EuroQoL 5‐dimensions questionnaire
- FAS
full‐analysis set
- HIV
human immunodeficiency virus
- HR‐QoL
health‐related quality of life
- IV
intravenous
- MELD
Model for End‐stage Liver Disease
- mITT
modified intent‐to‐treat population
- MMF
mycophenolate mofetil
- NODM
new‐onset diabetes mellitus
- PPS
per‐protocol set
- SAF
safety‐analysis set
- SD
standard deviation
- TEAE
treatment‐emergent adverse event
Introduction
Tacrolimus is accepted as the mainstay of immunosuppression in liver transplantation. Results from a previous study suggested that delayed introduction of reduced‐dose, immediate‐release tacrolimus (BD) coupled with interleukin‐2 receptor blockade, mycophenolate mofetil (MMF) and corticosteroids was associated with significantly reduced impairment of renal function and incidence of acute rejection (AR) versus tacrolimus BD at a higher dose (without delay) plus corticosteroids in liver transplant recipients 1.
The DIAMOND (ADVAGRAF™ studIed in combinAtion with MycOphenolate mofetil aND basiliximab in liver transplantation) study investigated renal function with once‐daily, prolonged‐release tacrolimus (Advagraf)‐based immunosuppression in de novo liver transplant recipients. It was designed to determine whether regimens with delayed introduction of prolonged‐release tacrolimus until Day 5 or a reduced initial dose of prolonged‐release tacrolimus improved renal function versus prolonged‐release tacrolimus given at an initial dose of 0.2 mg/kg/day immediately posttransplant.
Materials and Methods
Study design (clinical trials.gov; NCT01011205)
DIAMOND was a multicenter, 24‐week, randomized, open‐label, parallel‐group, Phase IIIb study conducted at 72 sites in 23 countries between September 2009 and January 2013. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation guidelines and applicable laws and regulations. An independent ethics committee from each study center granted approval before initiation. Written informed consent was obtained from all participants.
Eligible patients were 18 years or older and underwent primary orthotopic or split liver transplantation. Patients were excluded from the study if they received a multi‐organ transplant, an ABO incompatible graft, or a graft from a non‐heart beating donor. Patients were also excluded if they had received a previous transplant, or were receiving ongoing systemic corticosteroids. There were no restrictions on donor age, cold ischemia time or renal function.
Randomization and masking
The randomization sequence was prepared by Astellas Pharma Europe Ltd, UK and coordinated centrally using an interactive voice response system to randomize eligible patients to one of three treatment arms (1:1:1). Treatment allocation was stratified according to study center and hepatitis C virus status of the recipient.
Procedure
According to the randomization schedule, patients received prolonged‐release tacrolimus (Advagraf; Astellas Pharma Europe Ltd, UK) at an initial dose of 0.2 mg/kg/day on Day 1 posttransplant (Arm 1), prolonged‐release tacrolimus at an initial dose of 0.15–0.175 mg/kg/day on Day 1 posttransplant (Arm 2), or prolonged‐release tacrolimus at an initial dose of 0.2 mg/kg/day on Day 5 posttransplant (Arm 3; Figure 1). For Arms 1 and 2, the first dose of prolonged‐release tacrolimus was administered in the morning post‐liver transplant or within 18 h of skin closure. Subsequent oral doses of prolonged‐release tacrolimus were allowed in the morning after Day 1. For all arms, prolonged‐release tacrolimus was administered orally in one dose/day and adjusted based on clinical efficacy and tolerability, taking into account the recommended trough concentrations. Measurements of tacrolimus whole blood trough levels were not centralized. Tacrolimus whole blood trough levels were monitored using microparticle enzyme immunoassay (IMX®), chemiluminescent microparticle immunoassay (Abbott Diagnostics), enzyme‐multiplied immunoassay, antibody‐conjugated magnetic immunoassay (Siemens Diagnostics), or high‐performance liquid chromatography tandem mass spectrometry, according to local practice. Target tacrolimus trough levels were 5–15 ng/mL until Day 42, from Days 43–168 were 5–12 ng/mL in Arms 1 and 3, and 4–12 ng/mL in Arm 2. For patients in Arm 2, if at Day 43 a patient had not received treatment for an acute rejection (AR) episode and the last trough level recorded was ≥ 5 ng/mL, their tacrolimus dose was reduced by 20–25%.
Figure 1.
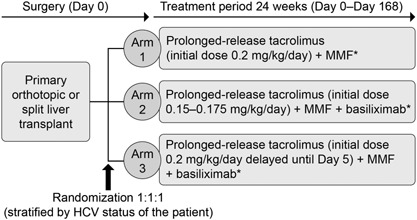
DIAMOND study design. Multicenter, randomized, open‐label, parallel‐group comparative Phase IIIb study. *0 mg–1,000 mg IV bolus corticosteroid (pre‐, intra‐, or post‐operatively) on Day 0. Arm 2 only: if the patient had not received treatment for an acute rejection episode and the last recorded trough level recorded was ≥ 5 ng/mL at Day 43, then the dose was reduced by 20–25%. HCV, hepatitis C virus; IV, intravenous; MMF, mycophenolate mofetil.
In Arms 2 and 3 only, basiliximab was administered as a single dose of 20 mg during the transplant procedure once hemostasis had been achieved, or immediately posttransplant on Day 0. A second dose of basiliximab (20 mg) was administered on Day 4. All patients received MMF (1 g), administered intravenously (IV) within 12 h following skin closure, then at 1 g BD until Day 14 (Days 3–5 by IV, Days 6–14 orally). Patients received a maintenance dose of MMF (0.5 g BD orally) thereafter. In all arms, an optional single dose of corticosteroids was administered as an IV bolus of ≤ 1,000 mg on Day 0 (pre‐, intra‐ or post‐operatively), according to the investigator's preference. No maintenance corticosteroids were administered.
Primary efficacy variable
The primary endpoint was renal function estimated by GFR (MDRD4) at Week 24. Arms 2 and 3 were tested for non‐inferiority of mean eGFR versus Arm 1.
Secondary efficacy variables
Secondary endpoints included eGFR (Chronic Kidney Disease‐Epidemiology Collaboration [CKD‐EPI] and cystatin C), GFR (iohexol clearance) and creatinine clearance (CrCl, Cockcroft–Gault) at Week 24. Other secondary endpoints included the incidence of composite efficacy failure, defined as graft loss (re‐transplantation or death) or biopsy‐confirmed AR (BCAR); incidence of graft and patient survival, AR and BCAR. A subanalysis on the effect of the Model for End‐stage Liver Disease (MELD) score at baseline (< 25 or ≥ 25) on eGFR (MDRD4) at Week 24 was performed. MELD scores were calculated retrospectively. Health‐related quality of life (HR‐QoL) was measured at baseline (maximum of 10 days posttransplant) and at Week 24 using the EuroQoL 5‐dimensions (EQ5D) questionnaire completed by the patient.
Safety
Adverse events (AEs) and laboratory parameters were monitored throughout the study. Safety assessments included treatment‐emergent AEs (TEAEs), fatal TEAEs, and AEs by gender. Comedications were classified into therapeutic groups, such as antihyperlipidemic and antihypertensive medications; medications that could be prescribed for > 1 indication were included in these groups. Diabetes mellitus was defined as elevated fasting blood glucose levels of > 7 mmol/L 2 on ≥ 2 occasions or by the administration of long‐term antidiabetic treatment.
Statistical analyses
A planned sample size of 900 patients (300/group) provided 80% power to detect a difference of ≥ 10% between groups, assuming a 10% exclusion rate from the per‐protocol set (PPS; all randomized patients who received ≥ 1 dose of study drug, were transplanted, and did not have a major protocol violation). If non‐inferiority was demonstrated, then 900 patients were considered sufficient to test superiority for renal function.
To prove non‐inferiority, a 10% difference in mean eGFR (MDRD4), which corresponds to a non‐inferiority margin of 6 mL/min/1.73m2, was considered to be within the range in which a meaningful clinical difference between regimens would become apparent (based on N = 900, with a 10% dropout rate to give 269 evaluable patients/arm; 80% power to detect a difference). Non‐inferiority was demonstrated if the lower limit of the 95% confidence interval (CI) for the difference in renal function was above –10% for Arms 2 and 3 versus Arm 1 at Week 24 (PPS). The Bonferroni–Holm method was applied for multiple comparisons. If non‐inferiority was satisfied, superiority was tested using the full‐analysis set (FAS; all randomized transplanted patients who received ≥ 1 dose of study drug).
Primary analysis of efficacy data was undertaken on the PPS and the FAS, using least‐square (LS) means. Mean (SD) eGFR (MDRD4) in Arms 2 and 3 were compared for non‐inferiority with Arm 1 at Week 24, using analysis of covariance (ANCOVA) with Dunnett's adjustment for multiplicity. CIs were displayed two‐sided at a 95% level. Secondary analyses of renal function were analyzed using the FAS at Week 24. Composite efficacy failure, graft and patient survival, AR, and BCAR were analyzed by Kaplan–Meier procedures using the modified intent‐to‐treat (mITT) population. The mITT and safety‐analysis set (SAF) were defined as all patients who were transplanted. The primary and secondary endpoints were calculated using the adjusted means. The subanalysis of eGFR (MDRD4) at Week 24 stratified by baseline MELD score was calculated using unadjusted means (FAS). Safety analyses were performed on the SAF. For all comparisons, p < 0.05 was considered statistically significant.
Results
Patient and donor demographics
Overall, 857 patients were transplanted and included in the SAF (Figure 2). The FAS consisted of 844 patients, 615 (72.9%) of whom completed the study. The main reason for discontinuation from the FAS in all arms was AEs. Patient demographics and baseline characteristics were similar between arms (Table 1).
Figure 2.
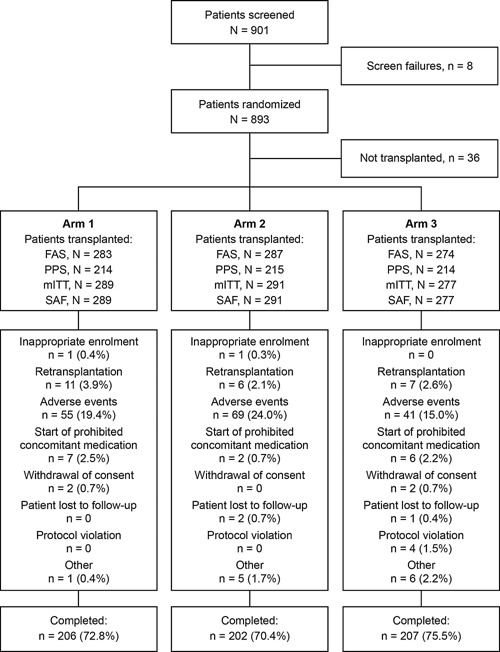
Patient disposition and reasons for discontinuation. Arm 1: Prolonged‐release tacrolimus (initial dose 0.2 mg/kg/day) + MMF; Arm 2: Prolonged‐release tacrolimus (initial dose 0.15–0.175 mg/kg/day) + MMF + basiliximab; Arm 3: Prolonged‐release tacrolimus (initial dose 0.2 mg/kg/day delayed until Day 5) + MMF + basiliximab. Four patients in Arm 3 had protocol violations (FAS): interruption of study medication > 7 consecutive days (two patients), SAE (one patient), and received mycophenolic acid (one patient); AEs were given as the primary reason for discontinuation by the study investigators. The types of AEs leading to discontinuation were not reported, as patients may have had multiple AEs at the time of discontinuation. AE, adverse event; FAS, full‐analysis set; mITT, modified intent to treat; MMF, mycophenolate mofetil; PPS, per‐protocol set; SAF, safety‐analysis set; SAE, serious adverse event.
Table 1.
Patient and donor characteristics at baseline (FAS)
| Parameter | Arm 1 (n = 283) | Arm 2 (n = 287) | Arm 3 (n = 274) |
|---|---|---|---|
| Patient characteristics | |||
| Age, years* | 54.3 (9.1) | 54.0 (9.7) | 53.7 (10.6) |
| Gender, n (%) | |||
| Male | 201 (71.3) | 203 (70.7) | 190 (69.3) |
| Female | 81 (28.7) | 84 (29.3) | 84 (30.7) |
| Not recorded | 1 | 0 | 0 |
| Race, n (%) | |||
| Caucasian | 272 (96.5) | 269 (93.7) | 257 (93.8) |
| Black/African | 1 (0.4) | 7 (2.4) | 7 (2.6) |
| Asian | 1 (0.4) | 2 (0.7) | 2 (0.7) |
| Other | 8 (2.8) | 9 (3.1) | 8 (2.9) |
| Not recorded | 1 | 0 | 0 |
| Weight, kg† | 77.0 (16.6) | 78.0 (17.0) | 78.1 (16.2) |
| BMI, kg/m2 ‡ | 26.2 (4.9) | 26.5 (5.4) | 26.7 (4.7) |
| Viral status at baseline, n (%) | |||
| HIV‐negative | 278 (98.6) | 285 (99.3) | 267 (97.4) |
| HIV unknown | 4 (1.4) | 2 (0.7) | 7 (2.6) |
| HIV not recorded | 1 | 0 | 0 |
| HCV‐positive | 88 (31.2) | 77 (26.8) | 77 (28.1) |
| HCV‐negative | 192 (68.1) | 208 (72.5) | 196 (71.5) |
| HCV unknown | 2 (0.7) | 2 (0.7) | 1 (0.4) |
| HCV not recorded | 1 | 0 | 0 |
| Primary diagnosis for transplantation, n (%) | |||
| Cirrhosis | 209 (73.9) | 224 (78.0) | 197 (71.9) |
| Hepatocellular carcinoma | 88 (31.1) | 79 (27.5) | 84 (30.7) |
| Budd–Chiari syndrome | 2 (0.7) | 2 (0.7) | 1 (0.4) |
| Metabolic disease | 5 (1.8) | 4 (1.4) | 11 (4.0) |
| Sclerosing cholangitis | 15 (5.3) | 21 (7.3) | 20 (7.3) |
| Other | 28 (9.9) | 18 (6.3) | 18 (6.6) |
| eGFR (MDRD4)§, mL/min/1.73m2 | 90.6 (39.1) | 89.3 (40.7) | 89.9 (34.6) |
| Number of patients on dialysis pre‐transplant | 6 | 9 | 3 |
| MELD scoreǁ | |||
| < 25 | 14.2 (5.3) | 14.6 (4.9) | 14.0 (4.9) |
| ≥ 25 | 31.4 (5.3) | 31.4 (4.7) | 29.6 (3.9) |
| Cold ischemia time¶, hours | 6.7 (3.1) | 6.7 (2.5) | 6.6 (2.7) |
| Donor characteristics | |||
| Age, years** | 51.2 (17.5) | 50.5 (18.5) | 51.3 (18.0) |
| Gender, n (%) | |||
| Male | 158 (56.0) | 168 (58.5) | 144 (52.6) |
| Female | 124 (44.0) | 119 (41.5) | 130 (47.4) |
| Not recorded | 1 | 0 | 0 |
| Race, n (%) | |||
| Caucasian | 159 (56.4) | 161 (56.1) | 152 (55.5) |
| Black/African | 3 (1.1) | 0 | 1 (0.4) |
| Asian | 1 (0.4) | 4 (1.4) | 0 |
| Other | 119 (42.2) | 122 (42.5) | 121 (44.2) |
| Not recorded | 1 | 0 | 0 |
| Type of donor, n (%) | |||
| Living, related | 5 (1.8) | 5 (1.7) | 6 (2.2) |
| Living, nonrelated | 2 (0.7) | 1 (0.3) | 2 (0.7) |
| Deceased | 275 (97.5) | 281 (97.9) | 266 (97.1) |
| Not recorded | 1 | 0 | 0 |
Results are shown as mean (SD) unless otherwise indicated. Patients with missing baseline data were excluded from these analyses for Arms 1–3, respectively: *n = 282, n = 287, n = 274; †n = 281, n = 286, n = 270; ‡n = 280, n = 285, n = 269; §n = 281, n = 285 and n = 271; ǁMELD was calculated retrospectively; MELD < 25: n = 239, n = 238 and n = 217; MELD ≥ 25: n = 29, n = 35, n = 35; ¶n = 238, n = 243, n = 228; **n = 282, n = 287, n = 273.
eGFR, estimated glomerular filtration rate; FAS, full‐analysis set; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MELD, Model of End‐stage Liver Disease; MDRD4, Modification of Diet in Renal Disease‐4; SD, standard deviation.
Dosing and exposure
Consistent with the study protocol, mean prolonged‐release tacrolimus dose was lower in Arm 2 versus Arm 1 in the immediate posttransplant period but similar by Day 6 (Supplementary Table S1). Prolonged‐release tacrolimus was delayed until Day 5 in Arm 3; between Days 5 and 21 mean doses were higher in Arm 3 versus Arms 1 and 2. By Day 28, doses were generally comparable in all arms (Supplementary Table S1). Mean (SD) tacrolimus dose in Arm 2 was 0.125 (0.073) mg/kg at Day 35, 0.121 (0.073) mg/kg at Day 49, and 0.096 (0.061) mg/kg at Day 168, indicating that dose reduction did not occur for the large majority of patients, even though this was specified in the study protocol. Mean (SD) doses of prolonged‐release tacrolimus at Day 168 in Arms 1 and 3 were 0.090 (0.059) and 0.095 (0.061) mg/kg, respectively.
Tacrolimus trough levels were readily achieved immediately posttransplant (Figure 3a). Mean tacrolimus trough levels in Arm 2 were lower versus Arm 1 for the first 2 weeks posttransplant and remained marginally lower until Day 28. Three patients in Arm 3 received prolonged‐release tacrolimus before Day 5, resulting in a mean tacrolimus trough level of 0.57 ng/mL at Day 5. By Day 8, 3 days after prolonged‐release tacrolimus initiation, mean (SD) tacrolimus trough levels for Arms 1 and 3 were generally comparable. By Day 28, trough levels were comparable for Arms 1 and 2, and comparable for Arm 3 by Day 35 (Figure 3a). Trough levels remained similar between arms for the remainder of the study and were 8.14 (3.168) ng/mL, 8.24 (2.894) ng/mL, and 8.33 (3.149) ng/mL in Arms 1–3, respectively at Day 168 (Figure 3b). Similar patterns were observed for median tacrolimus dose and exposure (Supplementary Table S2). In all arms, the majority of patients had tacrolimus trough levels of 5–15 ng/mL throughout the study (Supplementary Table S3).
Figure 3.
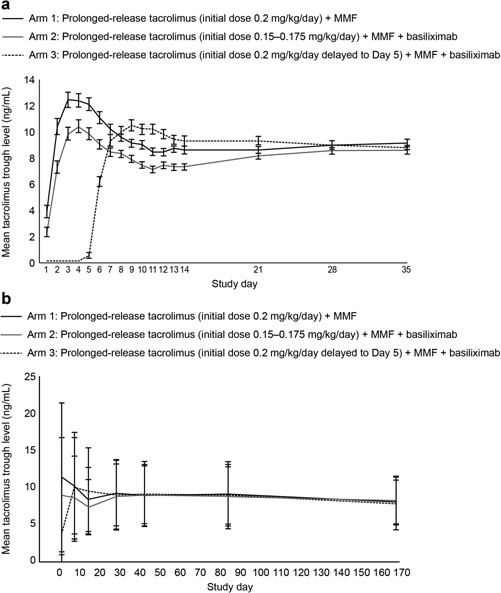
Mean tacrolimus trough levels (a) in the first 35 days posttransplant and (b) throughout the study period, stratified by treatment arm over 24 weeks of treatment (FAS). Error bars represent standard error of mean. In Arm 3, three patients received prolonged‐release tacrolimus before Day 5. FAS, full‐analysis set; MMF, mycophenolate mofetil.
Mean doses of MMF (Arms 1–3) and basiliximab (Arms 2 and 3) were comparable between arms throughout the study. Mean and median doses of corticosteroids were comparable peri‐operatively, but fewer patients received corticosteroids for AR in Arm 2 versus Arms 1 and 3 (9.4% vs. 14.8% and 14.2%). Mean (SD) cumulative dose of corticosteroids was 1,373 (1,566) mg, 1,177 (1,235) mg, and 1,407 (1,610) mg for Arms 1–3. In total, 10.6%, 12.2%, and 10.6% of patients in each arm did not receive corticosteroids.
Primary efficacy endpoint (FAS)
Non‐inferiority was established for eGFR (MDRD4) at Week 24 for Arms 2 and 3 versus Arm 1 (PPS); hence data analyses for superiority testing are presented. Renal function at Week 24, estimated by GFR (MDRD4), was significantly higher in Arms 2 and 3 versus 1 (76.4 and 73.3 vs. 67.4 mL/min/1.73m2; Arm 2 vs. 1: p = 0.001, Arm 2–Arm 1, 95% CI: 3.3, 14.8; Arm 3 vs. 1: p = 0.047, Arm 3–Arm 1, 95% CI: 0.1, 11.8; Table 2). eGFR (MDRD4) was also comparable between Arm 2 versus 3 (p = 0.230, Arm 2–Arm 3, 95% CI: –2.0, 8.3; Table 2, Figure 4a).
Table 2.
Treatment differences in primary and secondary efficacy endpoints at Week 24
| Parameters | Arm 1 | Arm 2 | Arm 3 | p‐value | ||
|---|---|---|---|---|---|---|
| Arm 1 vs Arm 2 | Arm 1 vs Arm 3 | Arm 2 vs Arm 3 | ||||
| Primary efficacy endpoint Renal function*, LS mean in mL/min/1.73m2 | ||||||
| eGFR (MDRD4) | 67.4 | 76.4 | 73.3 | 0.001 | 0.047 | 0.230 |
| Secondary efficacy endpoint Renal function*, LS mean (n) in mL/min/1.73m2 | ||||||
| CrCl (Cockcroft–Gault) | 67.2 (278) | 76.7 (283) | 72.9 (269) | < 0.001 | 0.071 | 0.152 |
| eGFR (CKD‐EPI) | 65.7 (278) | 73.5 (283) | 71.0 (269) | 0.002 | 0.046 | 0.282 |
| eGFR (cystatin C) | 60.5 (209) | 64.8 (204) | 64.9 (193) | 0.268 | 0.256 | 0.972 |
| GFR (iohexol clearance) | 56.5 (173) | 56.9 (183) | 52.6 (172) | 0.945 | 0.445 | 0.401 |
| Composite efficacy failure‐free survival†, n (%) | 210 (72.0) | 227 (77.6) | 206 (73.9) | 0.065 | 0.726 | 0.161 |
| Graft survival†, n (%) | 251 (86.5) | 256 (87.7) | 246 (88.6) | 0.642 | 0.479 | 0.793 |
| Patient survival†, n (%) | 259 (89.3) | 260 (89.1) | 251 (90.4) | 0.948 | 0.668 | 0.617 |
| Acute rejection†, n (%) | ||||||
| Patients with AR | 52 (18.0) | 36 (12.4) | 51 (18.4) | 0.025 | 0.890 | 0.019 |
| Rejection episode | ||||||
| 0 | 235 (81.3) | 252 (86.6) | 223 (80.5) | – | – | – |
| 1 | 46 (15.9) | 34 (11.7) | 47 (17.0) | – | – | – |
| 2 | 8 (2.8) | 4 (1.4) | 7 (2.5) | – | – | – |
| 3 | 0 (0.0) | 1 (0.3) | 0 (0.0) | – | – | – |
| BCAR†, n (%) | ||||||
| Patients with BCAR | 46 (17.9%) | 30 (12.1%) | 42 (16.8%) | 0.016 | 0.782 | 0.039 |
*Analyses using FAS: n = 283, n = 287, n = 274 for Arms 1–3, respectively. †Analyses using mITT: n = 289, n = 291, n = 277 for Arms 1–3.
Composite efficacy failure, graft and patient survival, acute rejection and BCAR were analyzed by Kaplan–Meier analysis. AR, acute rejection; BCAR, biopsy‐confirmed acute rejection; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; FAS, full‐analysis set; GFR, glomerular filtration rate; LS, least‐square; MDRD4, Modification of Diet in Renal Disease‐4; mITT, modified intent to treat.
Figure 4.
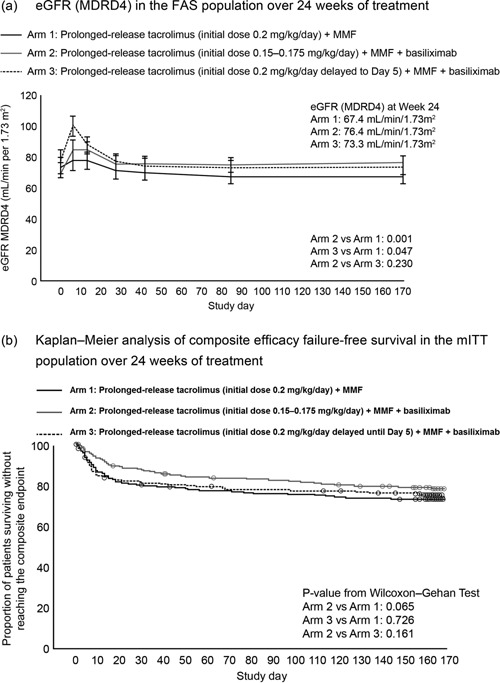
(a) eGFR (MDRD4) in the FAS population, and (b) Kaplan–Meier analysis of composite efficacy failure‐free survival in the mITT population, over 24 weeks of treatment. Data in Figure 4a are represented as least‐square means and error bars represent standard error of the mean. eGFR, estimated glomerular filtration rate; FAS, full‐analysis set; MDRD4, Modification of Diet in Renal Disease‐4; mITT, modified intent to treat; MMF, mycophenolate mofetil.
Secondary efficacy endpoints
Renal function (FAS)
As per the primary endpoint, eGFR (CKD‐EPI) was significantly higher in Arm 2 and 3 versus Arm 1. CrCl (Cockcroft–Gault) was significantly higher in Arm 2 versus 1 but not in Arm 3 versus 1 (Table 2). No significant differences were observed between arms for estimated (cystatin C) or measured (iohexol clearance) renal function (Table 2).
Owing to technical constraints, iohexol clearance and cystatin C measurements were performed on a relatively small number of patients. It is possible that these measurements were conducted in a subset of patients with better renal function, giving rise to selection bias. In order to remove such biases, a subanalysis evaluating eGFR (MDRD4) was performed in the subset of patients who were assessed for eGFR (MDRD4), cystatin C, and iohexol clearance. A total of 472 patients (55.9%) had all three GFR assessments: 159 (56.2%) in Arm 1, 163 (56.8%) in Arm 2, and 150 (54.7%) in Arm 3. The LS means of eGFR (MDRD4) were 80.7, 86.4, and 83.5 mL/min/1.73m2 for Arms 1–3, respectively (Arm 2 vs. 1: p = 0.080, Arm 3 vs. 1: p = 0.555, Arm 2 vs. 3: p = 0.539). These results are in line with eGFR (MDRD4) results obtained for the FAS, and therefore suggest that the potential for selection biases was low.
Other secondary endpoints (mITT and SAF)
Kaplan–Meier estimates of composite efficacy failure‐free survival at Week 24 are presented in Table 2 and Figure 4b. The incidence of graft and patient survival at Week 24 was comparable between arms (Table 2). In total, 44 (5.1%) patients died during the study; an additional 45 patients died between early withdrawal and the 24‐week follow‐up. The most common causes of death were infection (4.2%, 4.1%, and 4.3% for Arms 1–3) and cardiac events (1.0%, 3.1%, and 0.4%). Kaplan–Meier estimates of mortality at Weeks 4 and 12 were comparable for all arms (Week 4: 4.9%, 4.5%, and 4.7%; Week 12: 8.2%, 8.4%, and 7.3% for Arms 1–3). There were no differences in the overall incidence of mortality (p = 0.067) or the main cause of death (infections: 4.6% vs. 3.2%) between gender.
The incidence of AR and BCAR was low across arms, with more patients in Arm 2 having significantly higher rejection‐free survival versus Arms 1 and 3 at Week 24 (Table 2). The majority of BCAR episodes were mild or moderate in severity. Fewer patients in Arm 2 required corticosteroid treatment for AR versus Arms 1 and 3 (9.3% vs. 14.5% and 14.1%). Less than 2% of patients experienced steroid‐resistant BCAR in each arm.
Subanalysis by baseline MELD
A total of 99 patients in the FAS had a MELD score ≥ 25 at baseline (n = 29, 35, and 35 in Arms 1–3). For patients with a MELD score ≥ 25 at baseline (calculated retrospectively), the mean (SD) change in eGFR at Week 24 was –13.7 (39.1), +3.5 (56.9), and −4.4 (39.9) mL/min/1.73m2 in Arms 1–3, respectively (Arm 2 vs. 1: p = 0.157, Arm 3 vs. 1: p = 0.443). For patients with a MELD score < 25 at baseline (n = 239, 238, and 217 in Arms 1–3), the mean (SD) change in eGFR at Week 24 was significantly lower in Arms 2 and 3 versus 1 (–24.1 (40.2) and –26.2 (39.2) vs. –33.6 (39.2) mL/min/1.73m2; Arm 2 vs. 1: p = 0.009, Arm 3 vs. 1: p = 0.048); while it was comparable in Arm 2 versus 3 (p = 0.574).
Health‐related quality of life (mITT)
HR‐QoL was similar across all arms throughout the study, with a large proportion of patients having EQ5D index summary scores between 0.5 and 1. The improvements from baseline to Week 24 in EQ5D summary index scores were similar between arms at Week 24 (0.163, 0.156, and 0.176 in Arms 1–3). Categorical responses to the items of the EQ5D questionnaire were also comparable between arms.
Safety outcomes (SAF)
The incidence of TEAEs was similar between arms (Table 3). The incidence of diabetes mellitus posttransplant was low throughout the study and no major neurological disorders were reported (Table 3). Cardiovascular disorders occurred in 22.5%, 23.0%, and 20.2% of patients in Arms 1–3.
Table 3.
Incidence of most common treatment‐emergent adverse events, laboratory parameters, and comedications of interest in each treatment arm (SAF)
| Adverse event | Arm 1 (n = 289) | Arm 2 (n = 291) | Arm 3 (n = 277) |
|---|---|---|---|
| Overall | 259 (89.6) | 258 (88.7) | 244 (88.1) |
| Blood and lymphatic system disorders | |||
| Anemia | 97 (33.6) | 84 (28.9) | 87 (31.4) |
| Leukopenia | 50 (17.3) | 49 (16.8) | 52 (18.8) |
| Thrombocytopenia | 50 (17.3) | 50 (17.2) | 44 (15.9) |
| Gastrointestinal disorders | |||
| Diarrhea | 88 (30.5) | 84 (28.9) | 84 (30.3) |
| Nausea | 60 (20.8) | 42 (14.4) | 47 (17.0) |
| Abdominal pain | 33 (11.4) | 38 (13.1) | 34 (12.3) |
| Constipation | 26 (9.0) | 32 (11.0) | 46 (16.6) |
| Vomiting | 38 (13.1) | 31 (10.7) | 31 (11.2) |
| Metabolism and nutrition disorders | |||
| Hyperglycemia | 49 (17.0) | 54 (18.6) | 37 (13.4) |
| Hyperkalemia | 41 (14.2) | 42 (14.4) | 29 (10.5) |
| Hypokalemia | 24 (8.3) | 30 (10.3) | 25 (9.0) |
| Renal and urinary disorders | |||
| Renal failure | 75 (26.0) | 61 (21.0) | 55 (19.9) |
| Acute renal failure | 29 (10.0) | 26 (8.9) | 23 (8.3) |
| Proteinuria | 4 (1.4) | 4 (1.4) | 1 (0.4) |
| General disorders and administration site disorders | |||
| Pyrexia | 44 (15.2) | 35 (12.0) | 45 (16.2) |
| Peripheral edema | 32 (11.1) | 35 (12.0) | 32 (11.6) |
| Respiratory, thoracic and mediastinal disorders | |||
| Pleural effusion | 75 (26.0) | 55 (18.9) | 54 (19.5) |
| Immune system disorders | |||
| Liver transplant rejection | 56 (19.4) | 38 (13.1) | 53 (19.1) |
| Hepatobiliary disorders | |||
| Cholestasis | 38 (13.1) | 44 (15.1) | 44 (15.9) |
| Psychiatric disorders | |||
| Insomnia | 28 (9.7) | 36 (12.4) | 35 (12.6) |
| Musculoskeletal and connective tissue disorders | |||
| Back pain | 32 (11.1) | 28 (9.6) | 21 (7.6) |
| Adverse events of special interest | |||
| Diabetes mellitus* | 11 (3.8) | 13 (4.5) | 11 (4.0) |
| Neurological disorders | 94 (32.5) | 103 (35.4) | 89 (32.1) |
| Tremor | 30 (10.4) | 29 (10.0) | 30 (10.8) |
| Vascular disorders | 83 (28.7) | 96 (33.0) | 94 (33.9) |
| Hypertension | 62 (21.5) | 74 (25.4) | 74 (26.7) |
| Hypotension | 30 (10.4) | 34 (11.7) | 26 (9.4) |
| Laboratory parameters | |||
| Total cholesterol, mmol/L | 4.038 | 4.011 | 4.174 |
| HDL, mmol/L | 1.063 | 1.047 | 1.140 |
| LDL, mmol/L | 2.368 | 2.378 | 2.466 |
| Triglycerides, mmol/L | 1.537 | 1.438 | 1.469 |
| Comedications of interest† | |||
| Antihyperlipidemic medications | 20 (6.9) | 24 (8.2) | 26 (9.4) |
| Antihypertensive medications | 98 (33.9) | 98 (33.7) | 88 (31.8) |
Results are reported in ≥ 10% of patients and shown as n (%); SAF, safety‐analysis set. *Diabetes mellitus was defined as elevated fasting blood glucose levels of > 7 mmol/L 2 on two or more occasions or by the administration of long‐term antidiabetic treatment. †Medications that could be prescribed for more than one indication are included.
Discussion
Results from the DIAMOND study showed that once‐daily, prolonged‐release tacrolimus plus MMF (with and without basiliximab) and a single bolus of corticosteroid is efficacious and has a manageable tolerability profile in de novo liver transplant recipients over 24 weeks of treatment. Prolonged‐release tacrolimus initiated at a dose of 0.15–0.175 mg/kg/day immediately posttransplant, with a subsequent lower tacrolimus exposure over the first month, was associated with a significant reduction in impairment of renal function and a significantly lower incidence of BCAR versus a higher initial dose, prolonged‐release tacrolimus‐based regimen administered immediately posttransplant (Arm 1). Delaying the initiation of a higher dose prolonged‐release tacrolimus‐based regimen (Arm 3) also significantly reduced renal function impairment versus immediate posttransplant administration; however, the incidence of BCAR was comparable between the two arms. The incidence of mortality was also comparable between arms, and there were no significant differences in mortality between genders. In a previous study, female liver transplant recipients were reported to have higher mortality with prolonged‐release tacrolimus than males, although this may have been a chance finding as no explanation for this gender imbalance was apparent 3.
Mean target tacrolimus trough levels were achieved early after prolonged‐release tacrolimus initiation (within 2 days) in all arms, indicating that prolonged‐release tacrolimus is readily absorbed early after administration. Although the study protocol specified a dose reduction in Arm 2 at Day 43, this did not occur in the majority of patients. This may indicate that physicians are reluctant to reduce the dose of tacrolimus in liver transplant recipients who are considered stable on their current immunosuppressive regimen.
In this study, using an initial lower dose of prolonged‐release tacrolimus or delaying initiation of a higher dose of prolonged‐release tacrolimus (both resulting in lower tacrolimus exposure), without maintenance steroids, significantly improved renal function as estimated by GFR (MDRD4), versus a prolonged‐release tacrolimus‐based regimen given at a higher initial dose immediately posttransplant. A similar pattern was also observed when renal function was measured by Cockcroft–Gault and CKD‐EPI methods. For renal function estimated by cystatin C and measured by iohexol clearance, there was no statistical difference between arms at Week 24. Renal function estimated by cystatin C was conducted at the end of the study, and may have been overlooked when evaluating patients who had withdrawn from treatment prematurely. In addition, the iohexol clearance test is a renal function test that is generally not routinely employed in liver transplant centers and was unfortunately omitted in a significant number of patients in this study. Nevertheless, a subanalysis in which eGFR (MDRD4) was performed in the subset of patients in whom renal function was assessed by eGFR (MDRD4), cystatin C, and iohexol clearance, showed a pattern consistent with that of the FAS population. It is well documented that all prediction equations for measuring renal function have their own limitations. In the absence of an absolute measure without bias, due to its sensitivity and ease of use eGFR (MDRD4) may remain the choice for measuring renal function in these populations, while additional analyses can be of benefit to assess the sensitivity of the data.
The observation that renal function was improved in Arms 2 and 3 versus 1 in the DIAMOND study suggests that tacrolimus exposure (or delayed exposure) early posttransplant is a critical factor for preserving renal function over the longer term. This may be counterintuitive given that tacrolimus levels towards the end of the study were comparable between arms, and had been so for approximately 5 months. One would expect that the benefit of prolonged‐release tacrolimus dose reduction or delayed introduction of high‐dose, prolonged‐release tacrolimus in terms of acute nephrotoxic relief would not translate into better renal function at Week 24. It is possible, therefore, that the early posttransplant period represents a critical time whereby reducing or delaying tacrolimus exposure may be beneficial.
The incidence of patients free from composite efficacy failure at Week 24 was comparable between arms. The incidence of AR and BCAR were comparable to previously reported clinical trials with tacrolimus 3, 4 and episodes were mainly mild or moderate in severity. Although it would be reasonable to expect that reducing the dose of immunosuppression may lead to an increase in the incidence of AR, especially in regimens without maintenance corticosteroids, Arm 2 was associated with a significantly lower incidence of AR and BCAR versus Arms 1 and 3. As basiliximab also plays a role in preventing AR, the addition of basiliximab to lower dose prolonged‐release tacrolimus (Arm 2) may help to explain the significant reduction in AR and BCAR versus Arm 1, where prolonged‐release tacrolimus was administered without basiliximab. However, basiliximab was also administered in Arm 3. These data suggest that administering prolonged‐release tacrolimus immediately posttransplant is important in preventing AR. The addition of basiliximab also confers a clinical benefit in terms of AR, providing patients are also receiving prolonged‐release tacrolimus.
Other studies have shown that a reduced dose of corticosteroids or their complete withdrawal following liver transplantation does not affect the incidence of AR 5, and may provide some benefit in reducing steroid‐related AEs. Although maintenance corticosteroids were not utilized in the current study, rates of AR and BCAR were low, as was the reported incidence of diabetes. This suggests that a single bolus of corticosteroids is sufficient as part of a prolonged‐release tacrolimus plus MMF‐based regimen to provide effective immunosuppression following liver transplantation.
The tolerability profiles and incidence of AEs were comparable between arms and no new safety signals were observed. Of particular interest was the low reported incidence of diabetes mellitus throughout this study. In the ReSpECT study, where patients received tacrolimus BD at different dosages or delayed until Day 5, the incidence of diabetes mellitus was higher in all arms compared with the current study 1. However, comparisons between these two trials should be performed with caution, given the differences in study designs and tacrolimus formulations. As corticosteroid use post‐liver transplantation has previously been associated with an increase in the incidence of diabetes 6, it is possible that the low incidence of diabetes was due to the use of the maintenance corticosteroid‐free protocols employed.
In addition to the usual limitations of an open‐label study and the short study duration, a high number of patients enrolled in the DIAMOND study had eGFR > 60 mL/min/1.73m2 at baseline, which may not be reflective of the overall European liver transplant population. Interestingly, there were still differences in renal function observed between arms even in populations with favorable pre‐transplant renal function. The subanalysis by MELD score at baseline showed that the mean change in eGFR (MDRD4) over the 24‐week period was significantly less in Arms 2 and 3 versus 1 in patients with MELD score < 25 at baseline. MELD scores for the subanalysis were, however, calculated retrospectively and were lower than expected for the patient population in this study. This may have been due to the under‐reporting of pre‐transplant dialysis status in the study centers.
Results from this study indicate that prolonged‐release tacrolimus plus MMF‐based immunosuppression, with a single bolus of corticosteroid, is efficacious with a manageable tolerability profile in de novo liver transplant patients. Prolonged‐release tacrolimus at a dose of 0.15–0.175 mg/kg/day, and subsequent lower exposure, initiated immediately post‐liver transplant was associated with reduced impairment of renal function and a significantly lower incidence of BCAR versus a higher initial dose prolonged‐release tacrolimus‐based regimen administered immediately posttransplant. Delaying the initiation of a higher dose prolonged‐release tacrolimus‐based regimen also significantly reduced renal function impairment compared with immediate posttransplant administration; however, the incidence of BCAR was comparable between arms. As these data were powered to assess superiority, this observation has the potential to markedly influence current clinical practice. Results from this study indicate that early tacrolimus exposure, in the immediate posttransplant period, may be critical in maintaining renal function over the long term. Avoiding the use of maintenance steroids in prolonged‐release tacrolimus plus MMF‐based regimens had no apparent detrimental effect on the incidence of acute rejection, and may have contributed to a low reported rate of NODM after transplantation.
Participating Centers
Argentina: R Mastai; Austria: J Pratschke; Belarus: O Rummo; Belgium: A De Roover, C Moreno, J Pirenne, X Rogiers; Brazil: I Ferreira Boin; Canada: V Bain, M Laryea, D Marleau, P Marotta, U Steinbrecher; Colombia: G Mejίa; Czech Republic: P Trunečka; Finland: H Isoniemi; France: Y Calmus, C Ducerf, D Durand, C Duvoux, D Eyraud, J Gugenheim, J Hardwigsen, M Neau‐Cransac, GP Pageaux, L Rostaing, E Salame, D Samuel, C Vanlemmens, P Wolf; Germany: M Bartels, WO Bechstein, T Becker, M Heise, J Klempnauer, T Lorf, V Müller, S Nadalin, P Neuhaus, H Schlitt, U Settmacher, HH Wolters; Hungary: RM Langer; Ireland: A McCormick; Italy: U Baccarani, F Calise, U Cillo, M Colledan, O Cuomo, AD Pinna, G Tisone, U Valente; Mexico: M Vilatoba; Poland: A Chmura, M Krawczyk; Romania: I Popescu; Russian Federation: AV Zhao, SV Gautier; South Africa: R Britz; Spain: R Barcena, R Charco, V Cuervas‐Mons, J Fabregat, E Moreno, M Navasa, A Otero, T Serrano; Sweden: S Friman; Switzerland: PA Clavien; United Kingdom: M Attia, N Heaton, P Muiesan.
Disclosures
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. P Trunečka has served on advisory boards for Astellas, received consultancy fees and honoraria from Astellas and Pfizer, and has participated in clinical trials sponsored by Astellas; J Klempnauer has received support from Astellas, BMS, Genzyme, Novartis, and Roche, and has participated in clinical trials sponsored by Astellas; WO Bechstein has received honoraria and served on advisory boards for Astellas, and has participated in clinical trials sponsored by Astellas; S Friman has received honoraria from Astellas, BMS and Roche, and has participated in clinical trials sponsored by Astellas; H Isoniemi has received consulting fees from Astellas, has served on advisory boards for Astellas and Roche, and has participated in clinical trials sponsored by Astellas; M Brown, and N Undre are employees of Astellas; J Pirenne, A Zhao, L Rostaing, U Settmacher, C Mönch, and G Tisone have participated in studies sponsored by Astellas. This study was sponsored and funded by Astellas Pharma Europe Limited.
Supporting information
Additional supporting information may be found in the online version of this article.
Table S1: Mean prolonged‐release tacrolimus doses and trough levels stratified by treatment arm (FAS).
Table S2: Median prolonged‐release tacrolimus doses and trough levels stratified by treatment arm over 24 weeks of treatment (FAS).
Table S3: Proportion of patients within various tacrolimus trough level ranges at each time point (FAS).
Acknowledgments
The authors would like to thank Graham Wetherill and Gbenga Kazeem from Astellas Pharma Europe Ltd for their statistical support. The authors would also like to thank Nina Kennard and Yvonne Fok from iS Health for their editorial support in the preparation of this manuscript. Editorial support was funded by Astellas Pharma Europe Ltd.
†DIAMOND: ADVAGRAF™ studIed in combinAtion with MycOphenolate mofetil aND basiliximab in liver transplantation
References
- 1. Neuberger JM, Mamelok RD, Neuhaus P, et al. Delayed introduction of reduced‐dose tacrolimus, and renal function in liver transplantation: The “ReSpECT” study. Am J Transplant 2009; 9: 327–336. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Part 1: Definition, diagnosis and classification of diabetes mellitus and its complications. WHO/NCD/NCS/99.2 1999; Available at: http://whqlibdoc.who.int/hq/1999/who_ncd_ncs_99.2.pdf. Assessed November 2014.
- 3. Trunečka P, Boillot O, Seehofer D, et al. Once‐daily prolonged‐release tacrolimus (ADVAGRAF) versus twice‐daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant 2010; 10: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 4. Boudjema K, Camus C, Saliba F, et al. Reduced‐dose tacrolimus with mycophenolate mofetil vs standard‐dose tacrolimus in liver transplantation: A randomized study. Am J Transplant 2011; 11: 965–976. [DOI] [PubMed] [Google Scholar]
- 5. Wu LW, Guo ZY, Tai Q, et al. Steroid elimination within 24 hours after orthotopic liver transplantation: Effectiveness and tolerability. Hepatobiliary Pancreat Dis Int 2012; 11: 137–142. [DOI] [PubMed] [Google Scholar]
- 6. Bodziak KA, Hricik DE. New‐onset diabetes mellitus after solid organ transplantation. Transpl Int 2009; 22: 519–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Table S1: Mean prolonged‐release tacrolimus doses and trough levels stratified by treatment arm (FAS).
Table S2: Median prolonged‐release tacrolimus doses and trough levels stratified by treatment arm over 24 weeks of treatment (FAS).
Table S3: Proportion of patients within various tacrolimus trough level ranges at each time point (FAS).


