Summary
This multicentre, randomized, phase II study was conducted to examine whether the addition of mogamulizumab, a humanized anti‐CC chemokine receptor 4 antibody, to mLSG15, a dose‐intensified chemotherapy, further increases efficacy without compromising safety of patients with newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma (ATL). Patients were assigned 1:1 to receive mLSG15 plus mogamulizumab or mLSG15 alone. The primary endpoint was the complete response rate (%CR); secondary endpoints included the overall response rate (ORR) and safety. The %CR and ORR in the mLSG15‐plus‐mogamulizumab arm (n = 29) were 52% [95% confidence interval (CI), 33–71%] and 86%, respectively; the corresponding values in the mLSG15 arm (n = 24) were 33% (95% CI, 16–55%) and 75%, respectively. Grade ≥ 3 treatment‐emergent adverse events, including anaemia, thrombocytopenia, lymphopenia, leucopenia and decreased appetite, were observed more frequently (≥10% difference) in the mLSG15‐plus‐mogamulizumab arm. Several adverse events, including skin disorders, cytomegalovirus infection, pyrexia, hyperglycaemia and interstitial lung disease, were observed only in the mLSG15‐plus‐mogamulizumab arm. Although the combination strategy showed a potentially less favourable safety profile, a higher %CR was achieved, providing the basis for further investigation of this novel treatment for newly diagnosed aggressive ATL. This study was registered at ClinicalTrials.gov, identifier: NCT01173887.
Keywords: adult T‐cell leukaemia‐lymphoma, CCR4, mogamulizumab, randomized phase II study, antibody therapy
Adult T‐cell leukaemia‐lymphoma (ATL) is an aggressive, peripheral T‐cell neoplasm caused by human T‐cell lymphotropic virus type I (Uchiyama et al, 1977; Matsuoka & Jeang, 2007), and is classified into four clinical subtypes: smouldering, chronic, lymphoma and acute (Shimoyama, 1991). Intensive chemotherapy has been recommended for patients with newly diagnosed acute lymphoma or with unfavourable chronic subtypes of ATL (i.e. aggressive ATL) (Tsukasaki et al, 2009). A phase III trial was performed in previously untreated patients with aggressive ATL to compare the effects of a dose‐intensified multidrug regimen, namely the modified LSG15 (mLSG15) regimen (VCAP‐AMP‐VECP: vincristine, cyclophosphamide, doxorubicin and prednisolone; doxorubicin, ranimustine and prednisolone; vindesine, etoposide, carboplatin and prednisolone) (Yamada et al, 2001) with the effects of CHOP‐14 (cyclophosphamide, doxorubicin, vincristine and prednisolone). The complete response rate (%CR) was higher in the mLSG15 arm (40%) than in the CHOP‐14 arm (25%; P = 0·020). The overall survival (OS) rates at 3 years were 24% and 13% in the mLSG15 and CHOP‐14 arms, respectively, with a significant difference (P = 0·028) observed between the two arms after adjustment for imbalances in baseline prognostic factors (Tsukasaki et al, 2007). However, the median survival time of 12·7 months in the mLSG15 arm (CHOP‐14 arm, 10·9 months) was lower than that observed for other haematological malignancies. Moreover, allogeneic haematopoietic cell transplantation (allo‐HCT) has been explored as a promising treatment for ATL, and it has been reported that allo‐HCT can potentially provide cures for 30–40% of transplant recipients. However, only few ATL patients benefit from transplantation, such as those who are younger, achieve sufficient disease control and have an appropriate stem cell source (Hishizawa et al, 2010; Ishida et al, 2012a).
Because CC chemokine receptor 4 (CCR4) is expressed on the surface of the tumour cells of most patients with ATL (Yoshie et al, 2002; Ishida et al, 2003), it has been postulated to represent a novel molecular target for immunotherapy for ATL. Therefore, a humanized anti‐CCR4 monoclonal antibody with a defucosylated Fc region, mogamulizumab (KW‐0761) was developed, and has been shown to markedly enhance antibody‐dependent cellular cytotoxicity (Shinkawa et al, 2003; Ishii et al, 2010). A phase I clinical study of mogamulizumab was performed in patients with relapsed CCR4‐positive peripheral T‐cell lymphoma (PTCL), including ATL (Yamamoto et al, 2010). This study showed good tolerability, predictable pharmacokinetics and preliminary evidence of the antitumour activity of mogamulizumab, and the recommended dose was determined to be 1·0 mg/kg (Yamamoto et al, 2010). In the subsequent phase II study, mogamulizumab monotherapy showed an overall response rate (ORR) of 50% in patients with relapsed ATL, with an acceptable toxicity profile (Ishida et al, 2012b). Accordingly, mogamulizumab was approved in Japan in 2012 for patients with CCR4‐positive relapsed/refractory ATL.
Herein, we report the results of a multicentre, randomized phase II study, the aim of which was to evaluate whether or not the addition of mogamulizumab to mLSG15 increases efficacy without compromising safety for patients with newly diagnosed aggressive ATL.
Patients and methods
Patients
Eligible patients included those newly diagnosed with CCR4‐positive aggressive ATL who were aged ≥20 years. CCR4 expression was determined by using immunohistochemistry or flow cytometry with a mouse anti‐CCR4 monoclonal antibody (KM2160) (Ishida et al, 2003; Yamamoto et al, 2010) and confirmed by a central review committee. All patients were required to have an Eastern Cooperative Oncology Group performance status of 0–2. Furthermore, the eligibility criteria included the following laboratory parameters: absolute neutrophil count ≥1·5 × 109/l, platelet count ≥100 × 109/l, haemoglobin level ≥80 g/l, aspartate aminotransferase level ≤2·5 × the upper limit of the normal range (ULN), alanine aminotransferase level ≤2·5 × ULN, total bilirubin level ≤2·0 mg/dl, serum creatinine level ≤ 1·3 mg/dl, and arterial partial oxygen pressure ≥65 mmHg or arterial blood oxygen saturation ≥93%. Patients were excluded if they had a severe infection, a history of organ transplantation, active concurrent cancer, central nervous system involvement, a bulky mass requiring emergent radiotherapy, or seropositivity for hepatitis B virus surface antigen, hepatitis C virus antibody or human immunodeficiency virus antibody.
Randomization and masking
Eligible patients were randomly assigned in a 1:1 ratio to the two treatment groups based on dynamic allocation and minimization (Pocock & Simon, 1975) by a central randomization centre (Bell Medical Solutions, Inc., Tokyo, Japan). For randomization, the first stratification factor was clinical subtype, and the second was age (<56 or ≥56 years). The study had an open‐label design.
Procedures
This was a multicentre, randomized, phase II study to compare the efficacy and safety of mLSG15 plus mogamulizumab with that of mLSG15 alone. Subjects assigned to the mLSG15‐plus‐mogamulizumab arm received eight intravenous 1·0 mg/kg mogamulizumab infusions during four mLSG15 cycles. Typically, mogamulizumab was administered the day before VCAP and VECP administration except for the first VCAP administration (Fig 1). When VCAP or VECP administration was delayed for any reason, mogamulizumab administration was delayed accordingly.
Figure 1.

Treatment protocol. The mLSG15 protocol consists of three chemotherapeutic regimens, namely VCAP, AMP and VECP. Subjects assigned to the mLSG15‐plus‐mogamulizumab arm received up to eight infusions of mogamulizumab during four cycles of mLSG15. Cytarabine, methotrexate and prednisolone were intrathecally injected before initiation of VCAP administration in cycles 2 and 4. VCAP: vincristine, cyclophosphamide, doxorubicin, and prednisolone; AMP: doxorubicin, ranimustine, and prednisolone; VECP: vindesine, etoposide, carboplatin, and prednisolone; IV, intravenous; PO, per os (oral administration); IT, intrathecal; VCR, vincristine; CPA, cyclophosphamide; ADM, doxorubicin; PSL, prednisolone; MCNU, ranimustine; VDS, vindesine; ETP, etoposide; CBDCA, carboplatin; Ara‐C, cytarabine; MTX, methotrexate. *Before cycles 2 and 4 (Days −2 to −1). †After VCAP in Cycle 1 (Days 2 to 5). ‡Preceding VECP in Cycles 1–4 (Days 12 to 14). §Preceding VCAP in Cycles 2–4 (Days −3 to −1).
The primary endpoint was %CR, and the secondary endpoints included ORR, %CR and response rate according to disease site; progression‐free survival (PFS); OS and safety. We estimated that 22 patients per arm would be required to achieve an 80% probability of detecting a higher %CR in the mLSG15‐plus‐mogamulizumab arm than in the mLSG15 arm, based on the selection design (Simon et al, 1985). We assumed that an increased %CR of 15% achieved upon adding mogamulizumab would imply clinical significance. This 15% increase in the %CR corresponded to the difference observed between mLSG15 and CHOP‐14, with a previous phase III study showing that the former treatment prolonged OS (Tsukasaki et al, 2007). Thus, if the true difference is 15%, there is an 80% chance of selecting the right treatment when one chooses the treatment with the higher CR rate. Objective responses were assessed after the second and fourth chemotherapy cycles in each arm by an independent efficacy assessment committee according to the modified response criteria for ATL (Tsukasaki et al, 2009). Adverse events (AEs) were graded according to the National Cancer Institute's Common Terminology Criteria for AEs version 4·0 (http://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02_2009-09-15_QuickReference_8.5x11.pdf), and were summarized according to the Medical Dictionary for Regulatory Activities System Organ Class and preferred terms. The presence of human anti‐mogamulizumab antibodies in plasma was also determined. Blood samples were collected from patients who had received at least one dose of mogamulizumab at time points determined by the protocol for the pharmacokinetic analysis. The maximum drug concentration (C max) and trough drug concentration (C trough) for each mogamulizumab administration were calculated. We also investigated the distributions of blood T‐cell subsets (CD4/CD25/CCR4‐positive cells and CD4/CD25/FOXP3‐positive cells) during and after treatment in each arm.
Statistical analysis
Survival estimates were calculated by using the Kaplan–Meier method. PFS was defined as the time from the day of starting the protocol treatment to progression, relapse, or death from any cause. OS was measured from the day of starting the protocol treatment to death from any cause. The numbers of T‐cell subsets in the two arms were compared by employing the Wilcoxon signed‐rank test for each sampling point at a significance level of 0·05.
Study oversight
The study was sponsored by Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan. The academic investigators and the sponsor were jointly responsible for the study design. The protocol was approved by the institutional review boards at each participating site and all patients provided written informed consent before enrolment, in accordance with the Declaration of Helsinki.
Results
Patients
Between August 2010 and September 2011, 54 patients with newly diagnosed aggressive ATL were enrolled at 18 institutions. Of these 54 patients, 29 in the mLSG15‐plus‐mogamulizumab arm and 24 in the mLSG15 arm received treatment according to our study protocol. One patient assigned to the mLSG15 arm was withdrawn from the study, owing to the patient's treatment having to be deferred due to abnormal laboratory values that met the protocol criteria, and the patient was unable to wait for the protocol treatment due to deterioration of his/her general condition. The demographics and characteristics of the remaining 53 patients are summarized in Table 1. Fifteen patients in the mLSG15‐plus‐mogamulizumab arm did not complete the planned treatment; of these, seven dropped out because of AEs, including infectious diseases; four dropped out because of progressive disease (PD); and the remaining four dropped out for different reasons, including withdrawal of consent and start of an alternative treatment. Thirteen patients in the mLSG15 arm did not complete the planned treatment; among these, four had AEs, four had PD, and the remaining five dropped out for other reasons (Fig 2).
Table 1.
Demographics and clinical characteristics
| mLSG15 + mogamulizumab (n = 29) | mLSG15 (n = 24)a | |
|---|---|---|
| ATL subtype | ||
| Acute | 20 (69%) | 17 (71%) |
| Lymphoma | 6 (21%) | 7 (29%) |
| Chronicb | 3 (10%) | 0 (0%) |
| Age, years | ||
| Median | 61 | 64 |
| Range | 49–81 | 37–74 |
| <56 | 11 (38%) | 6 (25%) |
| ≥56 | 18 (62%) | 18 (75%) |
| Sex | ||
| Male | 12 (41%) | 16 (67%) |
| Female | 17 (59%) | 8 (33%) |
| ECOG PS | ||
| 0 | 16 (55%) | 13 (54%) |
| 1 | 10 (35%) | 9 (38%) |
| 2 | 3 (10%) | 2 (8%) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status.
25 patients were randomized; 24 were treated.
Chronic type with poor prognostic factors.
Figure 2.
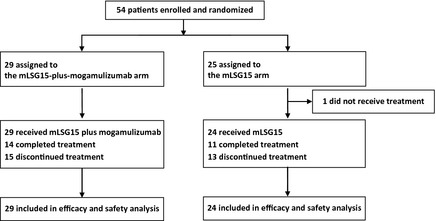
CONSORT diagram. Patients with newly diagnosed CC chemokine receptor 4 ‐positive aggressive adult T‐cell leukaemia‐lymphoma were assigned in a 1:1 ratio to receive treatment with mLSG15 plus mogamulizumab or mLSG15 alone. One patient assigned to the mLSG15 arm was withdrawn from the study, owing to the patient's treatment having to be deferred due to abnormal laboratory values that met the protocol criteria, and the patient was unable to wait for the protocol treatment due to deterioration of their general condition.
Efficacy
Of the 29 and 24 patients evaluable for efficacy in the mLSG15‐plus‐mogamulizumab and the mLSG15 arms, 25 patients [ORR, 86%; 95% confidence interval (CI), 68–96%] and 18 patients (ORR, 75%; 95% CI, 53–90%), respectively, had objective responses. The %CR, including unconfirmed CR, was higher in the mLSG15‐plus‐mogamulizumab arm (52%; 95% CI, 33–71%) than in the mLSG15 arm (33%; 95% CI, 16–55%), with a between‐group difference of 18·4% (95% CI, −8·9 to 43·8%; Table 2). The %CR according to the disease site in the mLSG15‐plus‐mogamulizumab and mLSG15 arms were 100% (14/14) and 43% (3/7) for blood, 92% (24/26) and 73% (16/22) for nodal and extranodal lesions and 50% (4/8) and 60% (3/5) for skin lesions, respectively. The response rate according to the disease site in the mLSG15‐plus‐mogamulizumab and mLSG15 arms were 100% (14/14) and 100% (7/7) for blood, 92% (24/26) and 77% (17/22) for nodal and extranodal lesions and 75% (6/8) and 80% (4/5) for skin lesions, respectively. The median PFS in the mLSG15‐plus‐mogamulizumab and mLSG15 arms were 8·5 months and 6·3 months, respectively (Fig 3A). The median OS was not reached in either arm (Fig 3B).
Table 2.
Response to treatment
| mLSG15 + mogamulizumab (n = 29) | mLSG15 (n = 24) | |
|---|---|---|
| CR | 9 | 5 |
| CRu | 6 | 3 |
| PR | 10 | 10 |
| CR + CRu | 15 | 8 |
| % CR (95% CI) | 52% (33–71) | 33% (16–55) |
| Between‐group difference (95% CI) | 18·4% (−8·9 to 43·8) | |
| CR + CRu + PR | 25 | 18 |
| ORR (95% CI) | 86% (68–96) | 75% (53–90) |
CR, complete response; CRu, uncertified complete response; PR, partial response; %CR, complete response rate; CI, confidence interval; ORR, overall response rate.
Figure 3.
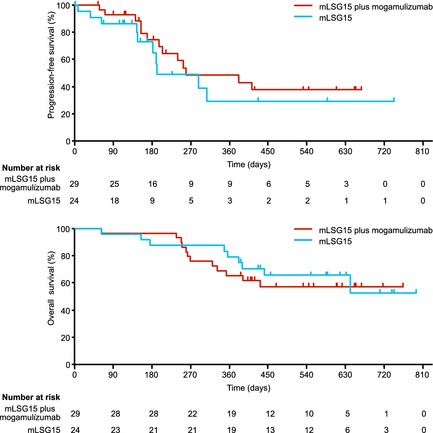
Progression‐free survival and overall survival. (A) Kaplan–Meier curve of estimated progression‐free survival (median, 8∙5 months and 6∙3 months in the mLSG15‐plus‐mogamulizumab and mLSG15 arms, respectively). (B) Kaplan–Meier curve of estimated overall survival (median, not achieved in either arm). The median follow‐up periods in the mLSG15‐plus‐mogamulizumab and mLSG15 arms were 413 days (range, 63–764 days) and 502 days (range, 62–794 days), respectively.
AEs
The treatment‐emergent AEs (TEAEs) of ≥grade 3 that occurred in at least two patients are listed in Table 3. The most common TEAEs of any grade in the mLSG15‐plus‐mogamulizumab arm were neutropenia (100%), thrombocytopenia (100%), leucopenia (100%), lymphopenia (97%), anaemia (97%) and febrile neutropenia (90%). The corresponding percentages in the mLSG15 arm were 96%, 96%, 92%, 96%, 92% and 88%, respectively. The following TEAEs of grade ≥ 3 were more frequently observed (≥10% difference) in the mLSG15‐plus‐mogamulizumab arm than in the mLSG15 arm: anaemia (97% vs. 79%), thrombocytopenia (90% vs. 71%), lymphopenia (97% vs. 75%), leucopenia (100% vs. 88%) and decreased appetite (28% vs. 13%). Papular rash (21%), hyperglycaemia (14%), pyrexia (14%), interstitial lung disease (10%), erythematous rash (7%), cytomegalovirus infection (7%) cytomegaloviral pneumonia (7%) and oxygen saturation decreased (7%) occurred only in the mLSG15‐plus‐mogamulizumab arm.
Table 3.
Treatment‐emergent adverse events in the mLSG15‐plus‐mogamulizumab (n = 29) and mLSG15 (n = 24) arms
| All grades | ≥Grade3 | |||
|---|---|---|---|---|
| mLSG15 + mogamulizumab n = 29 | mLSG15 n = 24 | mLSG15 + mogamulizumab n = 29 | mLSG15 n = 24 | |
| Blood and lymphatic system disorders | 29 (100%) | 22 (92%) | 29 (100%) | 22 (92%) |
| Anaemia | 28 (97%) | 22 (92%) | 28 (97%) | 19 (79%) |
| Febrile neutropenia | 26 (90%) | 21 (88%) | 26 (90%) | 21 (88%) |
| Gastrointestinal disorders | 29 (100%) | 23 (96%) | 7 (24%) | 7 (29%) |
| Stomatitis | 16 (55%) | 13 (54%) | 4 (14%) | 4 (17%) |
| General disorders and administration site conditions | 29 (100%) | 21 (88%) | 6 (21%) | 0 (0%) |
| Pyrexia | 24 (83%) | 15 (63%) | 4 (14%) | 0 (0%) |
| Infections and infestations | 19 (66%) | 16 (67%) | 10 (34%) | 7 (29%) |
| Bacteraemia | 4 (14%) | 3 (13%) | 3 (10%) | 3 (13%) |
| Pneumonia | 4 (14%) | 2 (8%) | 3 (10%) | 1 (4%) |
| Cytomegalovirus infection | 4 (14%) | 0 (0%) | 2 (7%) | 0 (0%) |
| Cytomegaloviral pneumonia | 2 (7%) | 0 (0%) | 2 (7%) | 0 (0%) |
| Investigations | 29 (100%) | 24 (100%) | 29 (100%) | 24 (100%) |
| Neutropenia | 29 (100%) | 23 (96%) | 29 (100%) | 22 (92%) |
| Thrombocytopenia | 29 (100%) | 23 (96%) | 26 (90%) | 17 (71%) |
| Lymphopenia | 28 (97%) | 23 (96%) | 28 (97%) | 18 (75%) |
| Leucopenia | 29 (100%) | 22 (92%) | 29 (100%) | 21 (88%) |
| Albuminaemia | 12 (41%) | 11 (46%) | 2 (7%) | 1 (4%) |
| Alanine transaminase increased | 12 (41%) | 10 (42%) | 2 (7%) | 2 (8%) |
| Aspartate transaminase increased | 9 (31%) | 8 (33%) | 2 (7%) | 1 (4%) |
| Potassium decreased | 9 (31%) | 6 (25%) | 3 (10%) | 1 (4%) |
| Sodium decreased | 8 (28%) | 7 (29%) | 4 (14%) | 2 (8%) |
| Phosphorus decreased | 8 (28%) | 3 (13%) | 3 (10%) | 1 (4%) |
| Blood pressure increased | 7 (24%) | 2 (8%) | 5 (17%) | 2 (8%) |
| Oxygen saturation decreased | 4 (14%) | 1 (4%) | 2 (7%) | 0 (0%) |
| Metabolism and nutrition disorders | 27 (93%) | 19 (79%) | 14 (48%) | 6 (25%) |
| Decreased appetite | 23 (79%) | 15 (63%) | 8 (28%) | 3 (13%) |
| Hyperglycaemia | 13 (45%) | 7 (29%) | 4 (14%) | 0 (0%) |
| Hyponatraemia | 4 (14%) | 3 (13%) | 2 (7%) | 2 (8%) |
| Hypophosphataemia | 4 (14%) | 3 (13%) | 4 (14%) | 2 (8%) |
| Hypokalaemia | 5 (17%) | 1 (4%) | 2 (7%) | 1 (4%) |
| Respiratory, thoracic and mediastinal disorders | 21 (72%) | 9 (38%) | 4 (14%) | 1 (4%) |
| Interstitial lung disease | 3 (10%) | 0 (0%) | 3 (10%) | 0 (0%) |
| Skin and subcutaneous tissue disorders | 29 (100%) | 20 (83%) | 15 (52%) | 1 (4%) |
| Papular rash | 12 (41%) | 0 (0%) | 6 (21%) | 0 (0%) |
| Erythematous rash | 8 (28%) | 0 (0%) | 2 (7%) | 0 (0%) |
Twenty serious AEs (SAEs) were reported in 12 patients in the mLSG15‐plus‐mogamulizumab arm. These included pneumonia in two patients, cytomegalovirus infection in two, interstitial lung disease in two, and the following events occurred in one patient each: febrile neutropenia, septic shock, cytomegaloviral pneumonia, pneumonitis, generalized erythema, viral encephalitis, oral disorder, bacteraemia, infection, exfoliative rash, ileus, cholecystitis, haemorrhagic cystitis and disease progression. The patient with septic shock did not recover and ultimately died. Another patient with haemorrhagic cystitis, which was suspected to be due to a viral infection, showed disease progression and died during the follow‐up period due to the haemorrhagic cystitis as an SAE. The remaining 17 SAEs in the mLSG15‐plus‐mogamulizumab arm all improved or resolved.
Eleven SAEs were reported in nine patients in the mLSG15 arm. These included two patients with bacteraemia, and the following events in one patient each: infection, enterocolitis, pneumonia, soft tissue inflammation, myelodysplastic syndrome, ischaemic colitis, herpes zoster, neurogenic bladder and febrile neutropenia. The outcomes of all SAEs in the mLSG15 arm, with the exception of myelodysplastic syndrome, improved or resolved. There were no deaths during the treatment or follow‐up period in the mLSG15 arm.
Pharmacokinetics and immunogenicity
Of the 29 patients enrolled in the mLSG15‐plus‐mogamulizumab arm, 16 (55%) completed the eight doses of mogamulizumab. The C max (at the end of the eighth infusion) and C trough (14 days after the eighth infusion) of mogamulizumab were 22·8 ± 4·6 and 94 ± 3·8 μg/ml (mean ± SD), respectively. None of the patients developed detectable levels of anti‐mogamulizumab antibodies.
T‐cell subset analysis
The numbers of circulating CD4/CD25/CCR4‐positive cells in the blood immediately before VCAP therapy for cycle three in the mLSG15‐plus‐mogamulizumab arm (mean, 0·0246 × 109/l; median, 0·015 × 109/l; range, 0·004–0·094 × 109/l) were significantly lower than those in the mLSG15 arm (mean, 0·4693 × 109/l; median, 0·234 × 109/l; range, 0·077–3·991 × 109/l) (P < 0·001). The corresponding numbers of these cells 28 days after VECP therapy (Cycle 4) in the mLSG15‐plus‐mogamulizumab arm (0·0173 × 109/l; 0·0095 × 109/l; 0·001–0·133 × 109/l) were significantly lower than those in the mLSG15 arm (0·1478 × 109/l; 0·133 × 109/l; 0·059–0·368 × 109/l) (P < 0·001) (Fig 4A). Similarly, the numbers of CD4/CD25/FOXP3‐positive cells in the blood immediately before VCAP therapy (Cycle 3) in the mLSG15‐plus‐mogamulizumab arm (0·0085 × 109/l; 0·004 × 109/l; 0–0·048 × 109/l) were significantly lower than those in the mLSG15 arm (0·2432 × 109/l; 0·074 × 109/l; 0·018–2·77 × 109/l) (P < 0·001), and the numbers of these cells 28 days after VECP therapy (Cycle 4) in the mLSG15‐plus‐mogamulizumab arm (0·0054 × 109/l; 0·003 × 109/l; 0–0·037 × 109/l) were significantly lower than those in the mLSG15 arm (0·0684 × 109/l; 0·0435 × 109/l; 0·016–0·25 × 109/l) (P < 0·001, Fig 4B).
Figure 4.
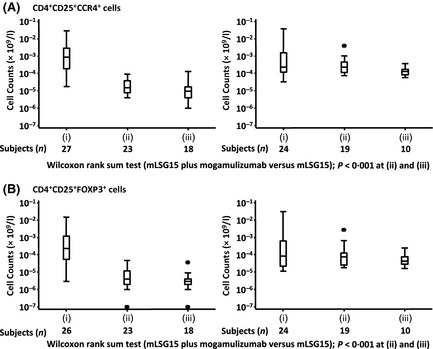
T‐cell subset analysis. Blood samples were taken (i) immediately before the initiation of treatment, (ii) immediately before VCAP therapy for cycle three, and (iii) 28 days after VECP therapy for cycle four. The numbers of CD4/CD25/CC chemokine receptor 4 (CCR4)‐positive cells (A) and CD4/CD25/FOXP3‐positive cells (B) are shown as box and whisker plots indicating the minimum, lower, median, upper quartile, and maximum values. The number of samples used for analysis at each point is indicated below the graph. The differences of each point [(ii) & (iii)] between the mLSG15‐plus‐mogamulizumab and mLSG15 arms are indicated as p‐values (Wilcoxon signed‐rank test) below the graphs. CCR4 was detected by using a monoclonal antibody (clone 1G1), with its binding to CCR4 being unaffected by the presence of mogamulizumab. VCAP: vincristine, cyclophosphamide, doxorubicin, and prednisolone; VECP: vindesine, etoposide, carboplatin, and prednisolone.
Discussion
This study showed that the %CR in patients who received mLSG15 plus mogamulizumab was higher than that obtained in those treated with mLSG15 alone (52% vs. 33%; difference, 18·4%). The increase in the %CR with the addition of mogamulizumab observed in this study surpassed the predicted, targeted, clinically significant 15% increase in patients with ATL. Importantly, the %CR in patients with lesions in the blood compartment was higher in the combination arm, leading to the increase in overall %CR. This finding was consistent with that observed in previous studies, in which ATL lesions in the blood were found to be more sensitive to mogamulizumab monotherapy than ATL lesions at other disease sites (Yamamoto et al, 2010; Ishida et al, 2012b).
Infections were more frequent in the combination arm. In particular, cytomegalovirus infection was observed in 14% of patients in the combination arm, whereas it was not observed in the chemotherapy alone arm. Furthermore, cytomegalovirus‐related SAEs occurred in three patients in the combination arm. Cytomegalovirus reactivation is observed in approximately 60% of patients with ATL during systemic chemotherapy (Ogata et al, 2011). Our study suggests that the addition of mogamulizumab to systemic chemotherapy might further increase the incidence of cytomegalovirus infection; therefore, careful monitoring for cytomegalovirus infection and appropriate use of antiviral therapy are recommended when systemic chemotherapy in combination with mogamulizumab is administered to patients with ATL.
In our previous study of mogamulizumab monotherapy for patients with relapsed ATL, skin rashes, including Stevens–Johnson syndrome, were the most frequently observed AEs (63%) (Ishida et al, 2012b, 2013). In the present study, as expected, AEs involving skin and subcutaneous tissue disorders were more frequent in the combination arm than in the chemotherapy alone arm. Even though no severe skin‐related AEs, such as Stevens–Johnson syndrome or toxic epidermal necrolysis, occurred in the present study, special attention should be paid to these skin‐related AEs when mogamulizumab is administered to patients with ATL.
Adult T‐cell leukaemia‐lymphoma cells constitutively express CD25 (Waldmann et al, 1984), and the present study had an eligibility criterion of CCR4 positivity. Hence, most of the CD4/CD25/CCR4‐positive cells were considered ATL cells. Compared to the chemotherapy alone arm, the combination arm showed a significant reduction in the number of CD4/CD25/CCR4‐positive cells. This finding is consistent with the proposed antitumour mechanism of mogamulizumab, in that mogamulizumab kills CCR4‐expressing ATL cells by increasing antibody‐dependent cellular cytotoxicity (Shinkawa et al, 2003; Ishii et al, 2010; Yamamoto et al, 2010). In humans, CCR4 is expressed on CD45RO‐positive, CD45RA‐negative, FOXP3‐positive activated regulatory T (Treg) cells (Miyara et al, 2009; Ishida & Ueda, 2011; Sugiyama et al, 2013). In addition, ATL cells from a subset of patients express FOXP3 and function as Treg cells (Yano et al, 2007). Thus, the CD4/CD25/FOXP3‐positive cells included not only endogenous activated Treg cells, but also ATL cells, in some patients. Our study indicated that compared to the chemotherapy alone arm, the combination arm showed a significant reduction in the number of CD4/CD25/FOXP3‐positive cells, which is consistent with the findings from our previous study of mogamulizumab monotherapy. In general, decreasing the number of Treg cells is considered a promising strategy for boosting antitumour immunity in patients with cancer, because the numbers of these cells increase in the tumour microenvironment, and they may play an important role in the ability of the tumour to escape host immunity in several different types of cancer (Ishida & Ueda, 2011; Jacobs et al, 2012). On the other hand, because alterations in Treg cell frequencies and/or function may contribute to various autoimmune diseases (Michels‐van Amelsfort et al, 2011), immune‐related AEs, such as skin disorders, which were also observed in our study, should be carefully monitored.
The present study was conducted according to the premise that mLSG15 is the most recommended chemotherapeutic regimen for patients with newly diagnosed aggressive ATL. We found higher rates of treatment‐related toxicities with mLSG15 compared to what has been reported for CHOP‐14 (Tsukasaki et al, 2007). In the context of this scenario, this study suggests that a younger patient population, particularly those aged <56 years, will benefit from VCAP‐AMP‐VECP, while an older population consisting of those aged 56–69 years will not; there are no data regarding mLSG15 therapy for patients with ATL aged >69 years (Tsukasaki et al, 2007). In the present study, the median ages in the mLSG15‐plus‐mogamulizumab and mLSG15 arms were 61 years and 64 years, respectively; patients potentially benefiting from mLSG15 (<56 years) accounted for only 38% of the patients in the mLSG15‐plus‐mogamulizumab arm and 25% of those in the mLSG15 arm. Adult T‐cell leukaemia‐lymphoma generally occurs in older individuals, with a median age at diagnosis of approximately 66 years (Iwanaga et al, 2012); therefore, further investigations are needed to determine whether mLSG15 is indeed the most suitable systemic chemotherapeutic regimen when combined with mogamulizumab.
CCR4 is expressed on the surface of tumour cells of patients from a subgroup of PTCL other than ATL, which also has an unfavourable prognosis (Ishida et al, 2004; Nakagawa et al, 2009). We have already completed a multicentre phase II study of mogamulizumab monotherapy for patients with relapsed CCR4‐positive PTCL in Japan (Clinicaltrials.gov: NCT01192984) (Ogura et al, 2014). Furthermore, other clinical trials of mogamulizumab for PTCL (Clinicaltrials.gov: NCT01611142) or cutaneous T‐cell lymphoma (Clinicaltrials.gov: NCT01728805) are currently underway worldwide. Further studies are expected to allow the determination of the efficacy of combining mogamulizumab with chemotherapy or other novel molecular target therapies for PTCL subtypes other than ATL.
Although this study offers a novel treatment option for newly diagnosed aggressive ATL, some limitations should be discussed. First, this study was designed to set the %CR as a primary endpoint; as a result, this study does not have enough power or a long enough follow‐up period to detect PFS and OS differences between the two arms. Thus, although a tendency towards prolongation of PFS in the combination arm was observed in the present study, this was not confirmed. Second, the treatment after the study protocol, including allo‐HCT and mogamulizumab, varied among the patients. Because the use of mogamulizumab for relapsed/refractory ATL was approved in Japan during the study period, the patients, including those in the chemotherapy alone arm, may have a chance to receive this drug. Both of these factors may affect the OS (Chihara et al, 2013).
In conclusion, although mLSG15 plus mogamulizumab was found to be associated with a potentially less favourable safety profile, particularly for infectious and skin‐related events, the majority of the AEs were manageable. The %CR was higher with combination therapy. Accordingly, this combination treatment appears to be a better option for managing patients with newly diagnosed aggressive ATL. Further clinical studies are necessary to evaluate the survival parameters in patients treated with chemotherapy plus mogamulizumab and to determine a more suitable combination regimen.
Author contributions
T.I., K.U., K.Y., N.U., A.U., K.T., S.A. and R.U. contributed to the conception and design of the study; T.I., T.J., S.T., H.S., K.U., K.Y., N.U., Y.S., K.N., A.U., K.T., H.F., K. Ishitsuka, S.Y., N.T., Y.M., K. Imada and T.M. contributed to the acquisition of data; T.I., K.T., S.A., M.T. and R.U. analysed and interpreted the data; all authors drafted and reviewed the manuscript and approved the final version for submission.
Conflicts of interest
T.I. has received honoraria and travel grants from Kyowa Hakko Kirin, and research funding from Kyowa Hakko Kirin, Chugai, Bayer and Celgene, and has served on the speakers bureau for Kyowa Hakko Kirin. T.J. has received honoraria and travel grants from Kyowa Hakko Kirin. S.T. has received travel grants and research funding from Kyowa Hakko Kirin. H.S. has served on the speakers bureau for Kyowa Hakko Kirin. K.Y. has a consultancy/advisory role with Kyowa Hakko Kirin and Novartis, has received honoraria from Kyowa Hakko Kirin, Novartis, Takeda and Janssen, and has received research funding from Kyowa Hakko Kirin, Novartis, Pfizer and ARIAD. Y.S. has received honoraria and research funding from Kyowa Hakko Kirin. K.N. has received honoraria from Kyowa Hakko Kirin. A.U. has received honoraria and research funding from Kyowa Hakko Kirin. K.T. has received honoraria from Kyowa Hakko Kirin and research funding from Kyowa Hakko Kirin, Celgene, Eisai, Solasia Pharma and Mundipharma. S.Y. has received honoraria and research funding from Kyowa Hakko Kirin. Y.M. has received honoraria, travel grants and research funding from Kyowa Hakko Kirin. K. Imada has received research funding from Kyowa Hakko Kirin. S.A. is employed by Kyowa Hakko Kirin, and is a stock owner. M.T. has a consultancy/advisory role with Kyowa Hakko Kirin, and has received honoraria from Kyowa Hakko Kirin. R.U. has a consultancy/advisory role with Mundipharma, and has received honoraria, travel grants and research funding from Kyowa Hakko Kirin and Chugai, and has served on the speakers bureau for Kyowa Hakko Kirin. The remaining authors declare no competing financial interests.
Acknowledgements
We thank all the patients and their families who participated in this clinical trial. We also thank all the nurses, clinical research coordinators, review committees and medical experts who were involved in this study; a complete membership list appears in Appendix 1. We are also grateful to Masatoshi Sugiura, Noboru Takizawa, and Kouichi Kawamura (Kyowa Hakko Kirin) for their help with data management. This study was supported by Kyowa Hakko Kirin.
Appendix I.
List of the review committees and medical experts who participated in this trial:
Junji Suzumiya, Shimane University Hospital
Takashi Terauchi, Research Centre for Cancer Prevention and Screening National Cancer Centre
Ukihide Tateishi, Yokohama City University Graduate School of Medicine
Junichi Tsukada, University of Occupational and Environmental Health
Koichi Nakata, University of Occupational and Environmental Health
Shigeo Nakamura, Nagoya University Graduate School of Medicine
Hiroshi Inagaki, Nagoya City University Graduate School of Medical Sciences
Koichi Ohshima, Kurume University School of Medicine
Michinori Ogura, Nagoya Daini Red Cross Hospital
Tetsuo Nagatani, Hachioji Medical Centre of Tokyo Medical University
Akimichi Morita, Nagoya City University Graduate School of Medical Sciences
Kazunari Yamaguchi, Institute of Molecular Embryology and Genetics, Kumamoto University
Yasuaki Yamada, Nagasaki University Graduate School of Biomedical Sciences
Shuichi Hanada, National Hospital Organization Kagoshima Medical Centre.
References
- Chihara, D. , Ito, H. , Matsuda, T. , Katanoda, K. , Shibata, A. , Taniguchi, S. , Utsunomiya, A. , Sobue, T. & Matsuo, K. (2013) Association between decreasing trend in the mortality of adult T‐cell leukemia/lymphoma and allogeneic hematopoietic stem cell transplants in Japan: analysis of Japanese vital statistics and Japan Society for Hematopoietic Cell Transplantation (JSHCT). Blood Cancer Journal, 3, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishizawa, M. , Kanda, J. , Utsunomiya, A. , Taniguchi, S. , Eto, T. , Moriuchi, Y. , Tanosaki, R. , Kawano, F. , Miyazaki, Y. , Masuda, M. , Nagafuji, K. , Hara, M. , Takanashi, M. , Kai, S. , Atsuta, Y. , Suzuki, R. , Kawase, T. , Matsuo, K. , Nagamura‐Inoue, T. , Kato, S. , Sakamaki, H. , Morishima, Y. , Okamura, J. , Ichinohe, T. & Uchiyama, T. (2010) Transplantation of allogeneic hematopoietic stem cells for adult T‐cell leukemia: a nationwide retrospective study. Blood, 116, 1369–1376. [DOI] [PubMed] [Google Scholar]
- Ishida, T. & Ueda, R. (2011) Immunopathogenesis of lymphoma: focus on CCR4. Cancer Science, 102, 44–50. [DOI] [PubMed] [Google Scholar]
- Ishida, T. , Utsunomiya, A. , Iida, S. , Inagaki, H. , Takatsuka, Y. , Kusumoto, S. , Takeuchi, G. , Shimizu, S. , Ito, M. , Komatsu, H. , Wakita, A. , Eimoto, T. , Matsushima, K. & Ueda, R. (2003) Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clinical Cancer Research, 9, 3625–3634. [PubMed] [Google Scholar]
- Ishida, T. , Inagaki, H. , Utsunomiya, A. , Takatsuka, Y. , Komatsu, H. , Iida, S. , Takeuchi, G. , Eimoto, T. , Nakamura, S. & Ueda, R. (2004) CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T‐cell and NK‐cell lymphomas with special reference to clinicopathological significance for peripheral T‐cell lymphoma, unspecified. Clinical Cancer Research, 10, 5494–5500. [DOI] [PubMed] [Google Scholar]
- Ishida, T. , Hishizawa, M. , Kato, K. , Tanosaki, R. , Fukuda, T. , Taniguchi, S. , Eto, T. , Takatsuka, Y. , Miyazaki, Y. , Moriuchi, Y. , Hidaka, M. , Akashi, K. , Uike, N. , Sakamaki, H. , Morishima, Y. , Kato, K. , Suzuki, R. , Nishiyama, T. & Utsunomiya, A. (2012a) Allogeneic hematopoietic stem cell transplantation for adult T‐cell leukemia‐lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood, 120, 1734–1741. [DOI] [PubMed] [Google Scholar]
- Ishida, T. , Joh, T. , Uike, N. , Yamamoto, K. , Utsunomiya, A. , Yoshida, S. , Saburi, Y. , Miyamoto, T. , Takemoto, S. , Suzushima, H. , Tsukasaki, K. , Nosaka, K. , Fujiwara, H. , Ishitsuka, K. , Inagaki, H. , Ogura, M. , Akinaga, S. , Tomonaga, M. , Tobinai, K. & Ueda, R. (2012b) Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. Journal of Clinical Oncology, 30, 837–842. [DOI] [PubMed] [Google Scholar]
- Ishida, T. , Ito, A. , Sato, F. , Kusumoto, S. , Iida, S. , Inagaki, H. , Morita, A. , Akinaga, S. & Ueda, R. (2013) Stevens‐Johnson Syndrome associated with mogamulizumab treatment of adult T‐cell leukemia/lymphoma. Cancer Science, 104, 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, T. , Ishida, T. , Utsunomiya, A. , Inagaki, A. , Yano, H. , Komatsu, H. , Iida, S. , Imada, K. , Uchiyama, T. , Akinaga, S. , Shitara, K. & Ueda, R. (2010) Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clinical Cancer Research, 16, 1520–1531. [DOI] [PubMed] [Google Scholar]
- Iwanaga, M. , Watanabe, T. & Yamaguchi, K. (2012) Adult T‐cell leukemia: a review of epidemiological evidence. Frontiers in Microbiology, 3, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, J.F. , Nierkens, S. , Figdor, C.G. , de Vries, I.J. & Adema, G.J. (2012) Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncology, 13, e32–e42. [DOI] [PubMed] [Google Scholar]
- Matsuoka, M. & Jeang, K.T. (2007) Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Reviews Cancer, 7, 270–280. [DOI] [PubMed] [Google Scholar]
- Michels‐van Amelsfort, J.M. , Walter, G.J. & Taams, L.S. (2011) CD4+ CD25+ regulatory T cells in systemic sclerosis and other rheumatic diseases. Expert Review of Clinical Immunology, 7, 499–514. [DOI] [PubMed] [Google Scholar]
- Miyara, M. , Yoshioka, Y. , Kitoh, A. , Shima, T. , Wing, K. , Niwa, A. , Parizot, C. , Taflin, C. , Heike, T. , Valeyre, D. , Mathian, A. , Nakahata, T. , Yamaguchi, T. , Nomura, T. , Ono, M. , Amoura, Z. , Gorochov, G. & Sakaguchi, S. (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity, 30, 899–911. [DOI] [PubMed] [Google Scholar]
- Nakagawa, M. , Nakagawa‐Oshiro, A. , Karnan, S. , Tagawa, H. , Utsunomiya, A. , Nakamura, S. , Takeuchi, I. , Ohshima, K. & Seto, M. (2009) Array comparative genomic hybridization analysis of PTCL‐U reveals a distinct subgroup with genetic alterations similar to lymphoma‐type adult T‐cell leukemia/lymphoma. Clinical Cancer Research, 15, 30–38. [DOI] [PubMed] [Google Scholar]
- Ogata, M. , Satou, T. , Kawano, R. , Yoshikawa, T. , Ikewaki, J. , Kohno, K. , Ando, T. , Miyazaki, Y. , Ohtsuka, E. , Saburi, Y. , Kikuchi, H. , Saikawa, T. & Kadota, J. (2011) High incidence of cytomegalovirus, human herpesvirus‐6, and Epstein‐Barr virus reactivation in patients receiving cytotoxic chemotherapy for adult T cell leukemia. Journal of Medical Virology, 83, 702–709. [DOI] [PubMed] [Google Scholar]
- Ogura, M. , Ishida, T. , Hatake, K. , Taniwaki, M. , Ando, K. , Tobinai, K. , Fujimoto, K. , Yamamoto, K. , Miyamoto, T. , Uike, N. , Tanimoto, M. , Tsukasaki, K. , Ishizawa, K. , Suzumiya, J. , Inagaki, H. , Tamura, K. , Akinaga, S. , Tomonaga, M. & Ueda, R. (2014) Multicenter phase II study of mogamulizumab (KW‐0761), a defucosylated anti‐cc chemokine receptor 4 antibody, in patients with relapsed peripheral T‐cell lymphoma and cutaneous T‐cell lymphoma. Journal of Clinical Oncology, 32, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Pocock, S.J. & Simon, R. (1975) Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics, 31, 103–115. [PubMed] [Google Scholar]
- Shimoyama, M. (1991) Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984–87). British Journal of Haematology, 79, 428–437. [DOI] [PubMed] [Google Scholar]
- Shinkawa, T. , Nakamura, K. , Yamane, N. , Shoji‐Hosaka, E. , Kanda, Y. , Sakurada, M. , Uchida, K. , Anazawa, H. , Satoh, M. , Yamasaki, M. , Hanai, N. & Shitara, K. (2003) The absence of fucose but not the presence of galactose or bisecting N‐acetylglucosamine of human IgG1 complex‐type oligosaccharides shows the critical role of enhancing antibody‐dependent cellular cytotoxicity. Journal of Biological Chemistry, 278, 3466–3473. [DOI] [PubMed] [Google Scholar]
- Simon, R. , Wittes, R.E. & Ellenberg, S.S. (1985) Randomized phase II clinical trials. Cancer Treatment Reports, 69, 1375–1381. [PubMed] [Google Scholar]
- Sugiyama, D. , Nishikawa, H. , Maeda, Y. , Nishioka, M. , Tanemura, A. , Katayama, I. , Ezoe, S. , Kanakura, Y. , Sato, E. , Fukumori, Y. , Karbach, J. , Jäger, E. & Sakaguchi, S. (2013) Anti‐CCR4 mAb selectively depletes effector‐type FoxP3 CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences of the United States of America, 110, 17945–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukasaki, K. , Utsunomiya, A. , Fukuda, H. , Shibata, T. , Fukushima, T. , Takatsuka, Y. , Ikeda, S. , Masuda, M. , Nagoshi, H. , Ueda, R. , Tamura, K. , Sano, M. , Momita, S. , Yamaguchi, K. , Kawano, F. , Hanada, S. , Tobinai, K. , Shimoyama, M. , Hotta, T. & Tomonaga, M. (2007) VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. Journal of Clinical Oncology, 25, 5458–5464. [DOI] [PubMed] [Google Scholar]
- Tsukasaki, K. , Hermine, O. , Bazarbachi, A. , Ratner, L. , Ramos, J.C. , Harrington, W. Jr , O'Mahony, D. , Janik, J.E. , Bittencourt, A.L. , Taylor, G.P. , Yamaguchi, K. , Utsunomiya, A. , Tobinai, K. & Watanabe, T. (2009) Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia‐lymphoma: a proposal from an international consensus meeting. Journal of Clinical Oncology, 27, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, T. , Yodoi, J. , Sagawa, K. , Takatsuki, K. & Uchino, H. (1977) Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood, 50, 481–492. [PubMed] [Google Scholar]
- Waldmann, T.A. , Greene, W.C. , Sarin, P.S. , Saxinger, C. , Blayney, D.W. , Blattner, W.A. , Goldman, C.K. , Bongiovanni, K. , Sharrow, S. & Depper, J.M. (1984) Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti‐Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. The Journal of Clinical Investigation, 73, 1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, Y. , Tomonaga, M. , Fukuda, H. , Hanada, S. , Utsunomiya, A. , Tara, M. , Sano, M. , Ikeda, S. , Takatsuki, K. , Kozuru, M. , Araki, K. , Kawano, F. , Niimi, M. , Tobinai, K. , Hotta, T. & Shimoyama, M. (2001) A new G‐CSF‐supported combination chemotherapy, LSG15, for adult T‐cell leukaemia‐lymphoma: Japan Clinical Oncology Group Study 9303. British Journal of Haematology, 113, 375–382. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K. , Utsunomiya, A. , Tobinai, K. , Tsukasaki, K. , Uike, N. , Uozumi, K. , Yamaguchi, K. , Yamada, Y. , Hanada, S. , Tamura, K. , Nakamura, S. , Inagaki, H. , Ohshima, K. , Kiyoi, H. , Ishida, T. , Matsushima, K. , Akinaga, S. , Ogura, M. , Tomonaga, M. & Ueda, R. (2010) Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. Journal of Clinical Oncology, 28, 1591–1598. [DOI] [PubMed] [Google Scholar]
- Yano, H. , Ishida, T. , Inagaki, A. , Ishii, T. , Kusumoto, S. , Komatsu, H. , Iida, S. , Utsunomiya, A. & Ueda, R. (2007) Regulatory T‐cell function of adult T‐cell leukemia/lymphoma cells. International Journal of Cancer, 120, 2052–2057. [DOI] [PubMed] [Google Scholar]
- Yoshie, O. , Fujisawa, R. , Nakayama, T. , Harasawa, H. , Tago, H. , Izawa, D. , Hieshima, K. , Tatsumi, Y. , Matsushima, K. , Hasegawa, H. , Kanamaru, A. , Kamihira, S. & Yamada, Y. (2002) Frequent expression of CCR4 in adult T‐cell leukemia and human T‐cell leukemia virus type 1‐transformed T cells. Blood, 99, 1505–1511. [DOI] [PubMed] [Google Scholar]


