Abstract
We describe a new preservation modality combining machine perfusion (MP) at subnormothermic conditions (21°C) with a new hemoglobin‐based oxygen carrier (HBOC) solution. MP (n = 6) was compared to cold static preservation (CSP; n = 6) in porcine orthotopic liver transplants after 9 h of cold ischemia and 5‐day follow‐up. Recipients' peripheral blood, serial liver biopsies, preservation solutions and bile specimens were collected before, during and after liver preservation. Clinical laboratorial and histological analyses were performed in addition to mitochondrial functional assays, transcriptomic, metabolomic and inflammatory mediator analyses. Compared with CSP, MP animals had: (1) significantly higher survival (100% vs. 33%; p < 0.05); (2) superior graft function (p < 0.05); (3) eight times higher hepatic O2 delivery than O2 consumption (0.78 mL O2/g/h vs. 0.096 mL O2/g/h) during MP; and (4) significantly greater bile production (MP = 378.5 ± 179.7; CS = 151.6 ± 116.85). MP down‐regulated interferon (IFN)‐α and IFN‐γ in liver tissue. MP allografts cleared lactate, produced urea, sustained gluconeogenesis and produced hydrophilic bile after reperfusion. Enhanced oxygenation under subnormothermic conditions triggers regenerative and cell protective responses resulting in improved allograft function. MP at 21°C with the HBOC solution significantly improves liver preservation compared to CSP.
Keywords: animal models: porcine, basic (laboratory) research/science, donors and donation: donation after circulatory death (DCD), ischemia reperfusion injury (IRI), liver transplantation/hepatology, metabolomics, organ perfusion and preservation, pathology/histopathology, regenerative medicine
Short abstract
The authors compare machine perfusion with a cell‐free oxygen carrier solution at 21°C with cold storage preservation in a porcine liver transplant model and demonstrate the significant impact of effective ex vivo oxygenation for liver allograft function over a 5‐day period.
Abbreviations
- BA
bile acid
- BCAA
branched‐chain amino acid
- CSP
cold static preservation
- DCDs
donors after cardiac death
- ECDs
expanded criteria donors
- GCDC
glycochenodeoxycholate
- HA
hepatic artery
- HBOC
hemoglobin‐based oxygen carrier
- IR
ischemia reperfusion
- LT
liver transplantation
- MP
machine perfusion
- OCS
oxygen carrier solution
- PCA
principal component analysis
- PRS
postreperfusion syndrome
- PTO2
tissue oxygen tension
- PV
portal vein
- RBCs
red blood cells
Introduction
Liver transplantation (LT) remains the ultimate therapy for end‐stage liver disease and irreparable acute liver failure. Despite small increments in the number of deceased donors, LT remains severely limited by the number of suitable donor livers. Expanded criteria donors (ECDs) have increased significantly over time, while donors after cardiac death (DCDs) have risen even further 1. However, liver utilization remains suboptimal, with organ discard rates in the United States ranging from 20% to 40% overall and >50% for DCDs 2.
The inexorable growth of DCD offers has been offset by limitations imposed by cold static preservation (CSP). Organ storage under hypothermic (4°C) and anoxic conditions results in progressive decay of organ quality, which exponentially increases the risk of using ECD livers in patients with the highest Model of End‐Stage Liver Disease scores 3. Because of its potential negative impact on organ quality and function, CSP has downstream implications on recipient morbidity and mortality and is directly related to hospital length‐of‐stay, quality of life and cost 4. Enhanced machine perfusion (MP) devices and innovative preservation solutions are at the forefront of a revolution poised to eliminate the major limitations imposed by CSP. The first clinical application of MP in livers was performed with a system providing continuous pressures through both the portal vein (PV) and hepatic artery (HA) vascular beds using a nonoxygen carrier solution (non‐OCS) under anoxic and hypothermic conditions for a short time 5. Subsequent clinical applications for MP employed a single roller pump device utilizing human red blood cells (RBCs) as the OCS under normothermic conditions 6. A third application was recently implemented by providing a brief period of hypothermic oxygenation, wherein continuous pressures were provided through the PV system only under subnormothermic conditions using a non‐OCS 7.
Herein, we report the first application of a new preservation modality wherein liver allografts are fully oxygenated under dual pressures under subnormothermic conditions (21°C) with a new hemoglobin‐based oxygen carrier (HBOC) solution specifically developed for ex vivo utilization.
Methods
Two groups of six animals (Landrace pigs, 60 kg) underwent orthotopic LT after a period of 9 h of cold ischemia time (CIT) and were followed for 5 days. The protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conducted according to the NIH Guide for the Care and Use of Laboratory Animals. The allografts were recovered from animals under general anesthesia, procured according to standard surgical techniques and flushed simultaneously through the HA and PV with University of Wisconsin solution (total of 5 L) at 4°C immediately after cross clamp and exsanguination. A passive veno‐venous bypass circuit was established during the anhepatic phase in the recipient's operation to assure stable hemodynamic conditions. The preservation solutions were rinsed from the allografts with a 1 L flush of cold lactated Ringer's solution immediately prior to implantation in both groups. All recipients received solumedrol (1 g) intraoperatively and tacrolimus (0.3 mg/kg) in the postoperative period.
The CSP allografts were preserved under standard hypothermic conditions with continuous temperature monitoring. The MP livers had both arterial and PV cannulas inserted before placement into the device. Baseline and postprocurement biopsies were performed in both groups. All allografts were weighed before and after preservation. Liver biopsies and perfusate samples were collected every 3 h. Bile and blood samples were obtained daily. All surviving animals underwent end‐study necropsy after being euthanized on the fifth postoperative days (Table 1). Two Jackson–Pratt drains for ascites collection and one biliary drain for bile collection were placed and exteriorized prior to the completion of the operation.
Table 1.
Study sample collection schedule
| Test | Type | Frequency | Analysis |
|---|---|---|---|
| Clinical | Hemodynamics, ABG profile, methemoglobin, inputs and outputs, bile production, ascites, urine output, overall impression (mobility, ability to eat, mental status) | Hourly in the 1st 24 h and daily afterward | Medical team and veterinarians |
| Laboratorial (recipient's peripheral blood) | Total bilirubin, AST, ALT, GGTP, BUN, creatinine, CBC, electrolytes, Prograf levels, PLT, PT, INR, ABGs, albumin | Every 12 h in the 1st 24 h and daily afterward | University of Pittsburgh labs |
| Tissue studies | Histopathology | BL, PR, 3, 6, 9 h, PR, N | Blind analysis performed by staff pathologists |
| EM | BL, PR, N | ||
| Microarray | BL, PR, N | Affymetrix Chips | |
| Oximetry | BL, 3, 6, 9 h, PR | Oxford Optronics probes | |
| Mitochondrial function | BL, 3, 6, 9 h | Oxygraph chamber | |
| Cytokine profile | BL, 3, 6, 9 h | Luminex | |
| Metabolomics | BL, 3, 6, 9 h | Metabolon | |
| Perfusate | ABGs, electrolytes, AST, LDH, methemoglobin | BL, 3, 6, 9 h | University of Pittsburgh labs |
| Cytokine profile | BL, 3, 6, 9 h | Luminex | |
| Metabolomics | BL, 3, 6, 9 h | Metabolon | |
| Bile | Volume, metabolomics | PR, daily for 5 days, N | Metabolon |
BL, baseline, N, necropsy, PR, postreperfusion.
Prospective sample collection schedule conducted throughout the study. Clinical data were reported by the clinicians and veterinarians directly involved in animal care. Peripheral blood samples were obtained from a previously placed central line (Broviac catheter) and processed by the University's clinical labs. Tissue studies were performed in liver biopsies' specimens and additional tissues (e.g. spleen, kidney, pancreas, lung, heart, lymph nodes) collected during the end‐study necropsy. Perfusate samples were obtained from both groups. Extensive arterial blood gases' analysis was performed in the MP group. Bile samples were collected prospectively from a pediatric feeding tube placed into the common bile duct and exteriorized (external biliary drainage) after liver implantation. Additional analyses (e.g. microarray, cytokine and metabolomics) were performed on liver tissue, perfusate and bile.
Machine perfusion
An MP system (Liver Device) from Organ Assist (Groningen, the Netherlands) was utilized 8. The device was primed with 4 L of perfusate to achieve an oxygen saturation SaO2 > 95% and paO2 > 400 mmHg within the target temperature (21°C) prior to liver allograft placement in the perfusion chamber. A closed perfusion circuit was established through the dual infusion ports (pulsatile HA flow at 60 bpm and continuous PV flow). The perfusate was oxygenated continuously (FiO2 = 60% @ 800 mL/min, O2 delivery = 14.1 mL O2/min) through microporous oxygenators. The common bile duct was cannulated for bile collection. The liver was kept fully immersed in the preservation solution in an upside down position, allowing free gravity drainage of the supra‐hepatic veins into the inferior vena cava cuff. The following perfusate parameters were assessed every 15 min for 1 h and hourly thereafter: pH, pCO2, pO2, FiO2, HCO3, K+, Na+, Ca2+, lactate, aspartate transaminase (AST) and lactate dehydrogenase. MP was conducted under low flows (PV flow = 259 ± 40 mL/min; HA flow = 91 ± 36 mL/min), stable temperature (21°C) and low pressures (PV = 3.5 ± 0.5 mmHg, HA = 18 ± 2 mmHg) for an average time of 7 h and 28 min (448.66 ± 9.14 min).
HBOC solution
A new, cell‐free HBOC solution was developed by combining a stable second‐generation bovine‐derived hemoglobin compound 9 (Hemopure™; OPK Biotech, Cambridge, MA) with a hetastarch‐based colloid (Belzer Machine Perfusion Solution, Preservation Solutions, Inc., Elkhorn, WI). The HBOC component had an initial hemoglobin concentration of 13 g/dL, a half‐life of 20 h and a clearance of 0.12 L/h. This solution can bind up to 1.36 mL O2 per gram of hemoglobin when fully saturated 10, 11. The combined solution (Vir1; Virtech Bio LLC, Wilmington, DE) had a final hemoglobin concentration of 3.5 g/dL and the following characteristics: pH 7.62, osmolality = 296 mOsm/kg, COP = 59.1 mmHg, Na+ = 105 mmol/L, K+ = 17.3 mmol/L, Cl− = 36 mmol/L and Ca++ = 0.24 mmol/L 12.
Histological analysis and ischemia reperfusion scoring system
Tissue samples for the assessment of hepatocellular injury were collected before, during and after preservation, postreperfusion and at end‐study necropsy. All liver samples were fixed in 10% buffered formalin, embedded in paraffin, sectioned (5 µm) and stained with hematoxylin and eosin for histological analyses. The severity of liver ischemia reperfusion (IR) injuries was blindly graded by transplant pathologists initially using the International Banff Criteria 13. A modified Suzuki's criterion 14 was subsequently applied to quantify the IR injuries and correlate the clinical and histopathological findings. Complete histological features were assessed both in the portal tracts and the hepatic lobules. The scored number was further weighted (none = 0; mild 1–25% = 1; moderate 25–50% = 2; and significant >50% = 3); and then re‐scored to reflect its contribution to the injury (grouped into four categories and subsequently expressed as mean ± standard deviation [SD]).
Analysis of inflammatory mediators
Tissue and perfusate assays of interferon (IFN)‐α, IFN‐γ, IL‐10, IL‐12/IL‐23 p40, IL‐1β, IL‐4, IL‐6, IL‐8 and tumor necrosis factor (TNF)‐α were carried out using a Luminex™ beadset from Affymetrix (Santa Clara, CA). GM‐CSF, IL‐1α, IL‐1ra, IL‐2 and IL‐18 were measured using a Luminex™ beadset from Millipore (Merck KGaA, Darmsdadt, Germany) 15.
Transcriptomic profile‐microarrays
The microarray analysis (probe labeling, hybridization and scanning) of 20 000 genes was performed using Affymetrix GeneChip Porcine Genome Array 16.
Metabolomics analysis
A total of 223 biochemical compounds of known identity in liver perfusate and 377 biochemicals in the bile that differed significantly between groups were identified with t‐tests or Wilcoxon rank sum tests (nonnormal data) for pair‐wise comparisons 17. Overall process variability was determined by calculating the median relative SD. Principal component analysis (PCA) was used for data reduction; the original variables were replaced by independent linear combinations of the data whereby those linear combinations were ordered in terms of the percentage of variability encountered. Weights for the original variables reflected the degree to which a given variable contributes to a given principal component (i.e. to a given linear combination). PCA was subsequently performed to identify subsets of variables most strongly correlated with a given procedure 18.
Microbiological surveillance
Samples of preservation solutions from both groups were collected at baseline, 3, 6 and 9 h, homogenized and placed into sterile collection bottles for subsequent culture and sensitivity assays 19.
Additional details for above methods can be found in the online Supplemental Methods.
Statistical analysis
Continuous measures were expressed as the mean ± SD within each group (e.g. MP and CSP). Two group comparisons of continuous measures between MP and CSP were conducted using the two‐sample t‐test at a given time point and Wilcoxon rank‐sum test. For tests across multiple time points, a linear mixed model (with a random effect to account for multiple measurements per subject) was used to test for an overall group difference. Differences in survival were evaluated using the log‐rank test rather than the exact binomial test of final mortality counts. No adjustments were made for multiple comparisons as results were all in the same direction (with MP providing superior results) thus showing consistent evidence for the same conclusion. Time courses of inflammatory mediators were assessed across time and treatment groups using two‐way analysis of variance. p‐Values of less than 0.05 were considered statistically significant.
Results
Prereperfusion events
Baseline allograft weight increased in both groups but less so in the MP (MP = 63.37 ± 39.34 g and CSP = 107 ± 65.61 g, respectively).
The MP perfusate exhibited arterial oxygen saturations (SaO2) between 90% and 100% in the HA port, and venous saturations (SvO2) between 70% and 80% in the PV port. pO2a (HA) was kept between 450 and 550 mmHg and pO2v (PV) between 100 and 200 mmHg. The CO2 content was assessed continuously from both the arterial and venous ports. Venous pCO2 slightly increased during the first 30 min of MP (17.5 ± 10 mmHg), but subsequently decreased to <10 mmHg within the first hour (Figure 1). Oxygen delivery (0.78 mL O2/g/h) was eightfold higher than oxygen consumption (0.096 mL O2/g/h) in the MP group at 21°C. The MP allografts maintained a physiologic pH during the ex vivo stage, and did not require additional infusions as previously seen with the use of non‐OCSs with the same device. Furthermore, MP allografts were able to clear lactate while producing urea and bile (1 cc/h) (Figure 2). Methemoglobin values remained at normal levels both in the perfusate during MP and in the recipient's peripheral blood after allograft implantation (Figure 3).
Figure 1.
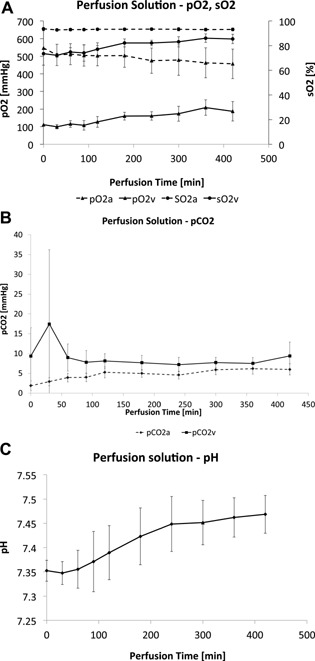
Arterial blood gases (ABGs) obtained from perfusate during machine perfusion protocol. (A) The y‐axes show oxygen pressures (pO2 mmHg) on the left and oxygen saturation (sO2%) on the right (mean ± SD). The x‐axes show the perfusion time in minutes (min). The HA pO2 is represented as pO2a. The PV pO2 is represented as pO2v. The HA saturation represented as sO2a. The PV saturation is represented as sO2v. The FiO2 was constant at 60%. (B) Carbon dioxide (CO2) pressures (mmHg) overtime (minutes) in the MP group (mean ± SD). (C) pH (mean ± SD) overtime (minutes) in the MP group.
Figure 2.
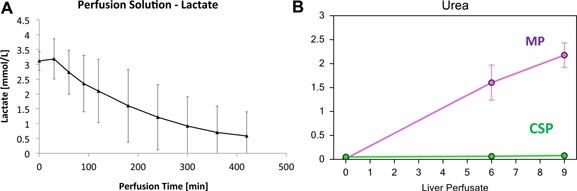
(A) Lactate concentration (mmol/L) values (mean ± SD) in the perfusate over time (minutes) during MP. (B) Urea concentration (mmol/L) values (mean ± SD) in the perfusate overtime (hours) during MP.
Figure 3.
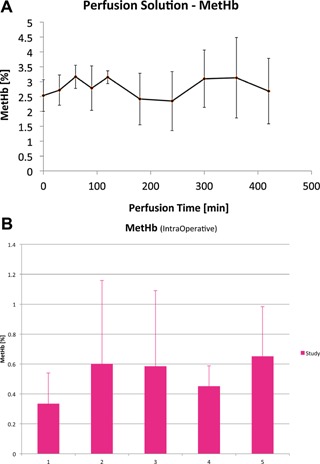
Methemoglobin levels during MP and after liver implantation. (A) Methemoglobin (MetHb) saturation (%) values (mean ± SD) in the perfusate over time (minutes) during MP. (B) MetHb saturation (%) values (mean ± SD) over time (hours) in the recipient's peripheral blood during liver transplantation in the MP group.
Liver tissue oxygenation (PTO2) was initially recorded at 80 mmHg during the donor (FiO2 = 100%) operation prior to organ removal and subsequently recorded as greater than 200 mmHg during MP. Mitochondrial function assessed by oxygraph chamber showed sustained ATP production and respiratory control ratio in both groups (n = 2). Reactive oxygen species (H2O2 nmol/min/mg) were lower in the MP group after reperfusion compared to CSP.
Recipient operation and postreperfusion events
Operative times were 22% shorter (p < 0.05) in the MP group (MP = 269.17 ± 20.27 min; CSP = 337.2 ± 14.22 min) due to the lack of significant postreperfusion syndrome (PRS) mainly characterized by vasodilatation, hypotension, acidosis, coagulopathy and oliguria. Intraoperative administration of intravenous fluids was significantly higher (58.5%, p < 0.05) in the CSP group due to a pronounced period of vasodilatation (central venous pressure = 4.5 ± 1.5 mmHg, hemoglobin = 12 ± 1 g/dL, mean arterial pressure = 70 ± 15 mmHg). Liver allograft reperfusion was achieved after the completion of the PV anastomosis. The warm ischemia times were similar in both groups (MP = 35.83 ± 3.18; CSP = 30 ± 7.4). Arterial reperfusion occurred within 60 min of PV reperfusion (MP = 39.6 ± 14; CSP = 50.6 ± 19.9). The MP group had no to mild signs of PRS after implantation, while the CSP group had moderate to severe signs. The CSP animals experiencing moderate to severe PRS developed PNF subsequently and died from liver allograft failure (intractable lactic acidosis followed by respiratory failure). Postoperative alanine transaminase (ALT) and AST values were significantly lower (p < 0.05) in the MP group (ALT: MP = 27 ± 8.2; CSP = 84 ± 31.7; and AST: MP = 199 ± 136.9; CSP = 935 ± 512.5). Massive centrilobular necrosis was detected at necropsy in the CSP animals. These findings are corroborated by similar studies showing high mortality in this porcine model when CIT was >9 h 20, 21, 22.
All surviving animals were euthanized on the fifth day; the MP group had a significantly higher survival rate (100% vs. 33%, p < 0.05) (Figure 4). MP animals had significantly better (p < 0.05) graft function than the CSP animals when all data analyses were combined (mixed model). MP allograft biopsies showed no to mild IR injuries compared to moderate to severe in the CSP group. Based on IR scores, the MP group recovered significantly (p < 0.05) better from the histological point of view when compared to the CSP group (Figure 5).
Figure 4.
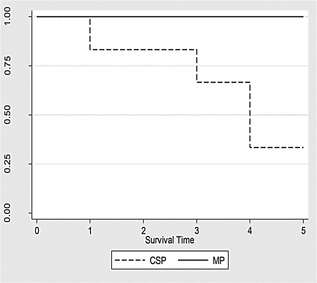
Survival curve for both groups CSP and MP during a 5‐day period. MP had a 100% survival and CSP had a 33% survival (p < 0.05). Early deaths on the CSP group were due to primary non‐function leading into irreversible liver allograft failure.
Figure 5.

Histological analysis of ischemia reperfusion (IR). IR scores (Suzuki modified) were determined by serial analysis of inflammatory changes within the portal tracts and the hepatic lobules. (A) Comparison of average IR scores for cold static preservation (CSP) and machine perfusion (MP). The IR scores at necropsy were significantly lower in the MP group (p < 0.05). (B) Histological images hematoxylin and eosin of the liver biopsies obtained on both groups (CSP at the top and MP at the bottom) during preservation, after reperfusion and at the end‐study necropsy.
Bile production (MP = 378.5 ± 179.7; CSP = 151.6 ± 116.85) was significantly higher (p < 0.05) during the first 24 h and throughout the study (5 days) in MP livers. The MP group had significantly (p < 0.05) higher urine output (MP = 3507.5 ± 840 mL; CSP = 840 ± 577.1 mL) and lower ascites production (MP = 1409.3 ± 1057 mL; CSP = 2666.2 ± 1599.7 mL) during the same time period.
Transmission electron microscopy showed integrity of hepatocyte mitochondria after reperfusion and at necropsy in the MP group (Figure 6). The CSP group displayed significant mitochondrial damage at reperfusion and necropsy, characterized by progressive membrane damage leading to the formation of mitochondrial permeability transition pores. Furthermore, MP AST perfusate levels were significantly lower at 3 (55.67 ± 21.51 vs. 149.2 ± 53.49), 6 (59.66 ± 25.81 vs. 157 ± 57.74) and 9 h (74.33 ± 29.21 vs. 186 ± 90.92) than those in the CSP livers (p < 0.05) (Figure 7).
Figure 6.
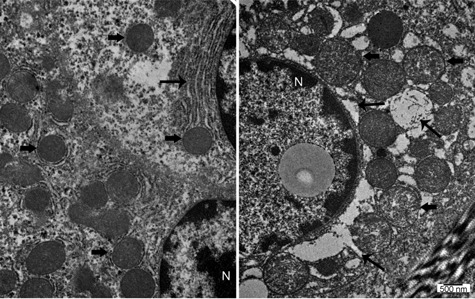
Transmission electron microscopy analysis. The thick arrows point to the mitochondria. Machine perfusion (MP) mitochondria in the left panel have normal features. Cold static preservation (CSP) mitochondria in the right panel are swollen, which is usually an indication of mitochondrial dysfunction. The thinner arrows point to the endoplasmic reticulum (ER). MP in the left panel has normal anatomical features. CSP in the right panel show signs of ER stress. The nuclei (N) are shown as they indicate that these cells are alive and not necrotic.
Figure 7.
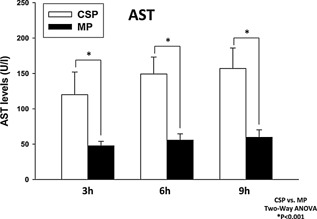
AST (U/I) levels in the perfusate of both groups (CSP white; MP black) during liver preservation. MP provided effective oxygenation and significantly lower (p < 0.05) scores of hepatocellular damage when compared to CSP over a period of 9 h.
Transcriptomic analysis
There was a striking increase in proliferation‐associated genes (Jun, Fos, ATP synthase F0 subunit 8, Apolipoprotein A‐II, Metallothionein isoform, Acyl coenzyme A synthetase, Syndecan 2, Collagen Alpha 2, Prothymosin alpha) in the MP group when compared to CSP. Many hepatocyte‐differentiation genes were also up‐regulated, including albumin, apolipoproteins and several cytochrome P450 members.
The 100 most affected genes in the MP group showed over‐expression of general metabolic, anti‐inflammatory and regenerative functions, as well as protective mechanisms against free radicals. MP also resulted in increased expression of several genes associated with entry of hepatocytes into the G1 cell cycle phase. Genes associated with hepatocyte differentiation were also up‐regulated. Enrichment by biological process networks following MP suggested a marked up‐regulation of genes related to metabolic processes (34%), cellular processes (25%) and cell communication (17%). Additional Biological Process Network Analysis (INGENUITY®; QIAGEN Silicon Valley, Redwood City, CA) 23 showed a significant (p < 0.05) difference in gene expression related to hepatic system development and function, cellular growth and proliferation, cellular development and cell cycle when MP was compared to CSP. Metabolic Network Analysis (INGENUITY®) showed significant up‐regulation of genes related to drug, amino acid, vitamin, mineral and carbohydrate metabolism in addition to free radical scavenging and energy production in the MP group. The MP group displayed significantly higher serum albumin levels (p < 0.05) in the immediate postoperative period, which appeared to be prompted by a significant up‐regulation of albumin‐related genes during MP. Thus, MP with full oxygenation enhanced signaling pathways for HGF, EGF, TGF‐β, ErbB and PI3K/AKT among others, and triggered proliferative and regenerative transcriptional pathways compared to CSP 24 (Figure 8).
Figure 8.
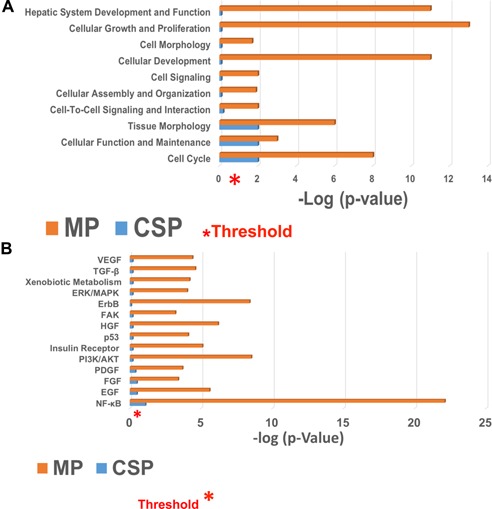
Comparison of biological processes and signaling pathways for machine perfusion (MP) and cold static preservation (CSP). (A) Biological process network analysis. Differential regulation of biological process networks during liver preservation was analyzed by comparing machine perfusion (MP) to cold storage preservation (CSP) in liver tissues after allograft reperfusion (both portal and arterial) using INGENUITY®. The biological processes that were found to be significantly affected are displayed along the y‐axis. The x‐axis displays the −log of p‐value (threshold) and was calculated using Fisher's exact test right‐tailed. The most significant up‐regulation was observed in genes associated with hepatic system development and cellular proliferation. (B) Signaling pathway analysis comparing machine perfusion (MP) and CSP after the full implantation of the liver allograft. Growth factor signaling pathways that are critical for liver function and growth were generated using INGENUITY®. The x‐axis displays the −log of p‐value (threshold) and was calculated by Fisher's exact test right‐tailed. It was observed that signaling pathways that are critical for liver growth were significantly up‐regulated in MP liver samples compared to the CSP group. NF‐κB is fully activated in hepatocyte regeneration and undergoes significant up‐regulation after partial hepatectomy.
Metabolomic analysis
Over 600 metabolites were analyzed when the two groups were initially compared. Ascorbate and threonate levels were significantly higher in the MP group at 6 and 9 h. Evidence of lipoxygenase activity differed by preservation method. 15‐HETE was specifically elevated in MP perfusate, whereas 12‐HETE was elevated in CSP across all time points. Signs of energy stress and purine nucleotide breakdown were noted in the CSP livers, as indicated by the significantly higher levels of adenosine monophosphate and hypoxanthine. Glucose, amino acid cycle and branched‐chain amino acid (BCAA) mobilization were markedly elevated in MP samples. Glucose levels were higher in the MP perfusate throughout the study. BCAA oxidation showed a strong differential increase in MP perfusate at 6 and 9 h as demonstrated by valine, isoleucine and leucine levels and their respective deamination products.
Five bile acid (BA) conjugates (e.g. taurocholate and glycochenodeoxycholate [GCDC]) were significantly higher in CSP perfusate during preservation. Similarly, taurine and its immediate precursor, hypotaurine, were relatively abundant in CSP perfusate but significantly lower in the MP group. Immediately following transplantation, the bile sterol precursors lathosterol and cholesterol were similar in both groups; however, lathosterol levels became significantly higher in the bile of the MP group, showing an early recovering in biliary phospholipid secretion. The same pathway was seen with campesterol levels, which fell significantly in the CSP when compared to MP group (Figure 9). CSP livers secreted a relatively higher bile salt/phospholipid ratio after transplant when compared to MP.
Figure 9.
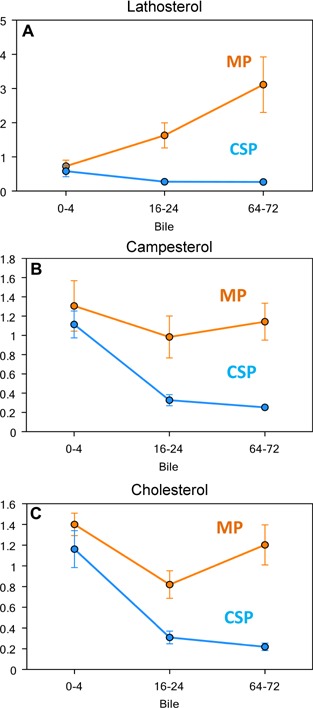
Comparison of lipid concentration in bile of MP and CSP groups performed by metabolomics' analysis (Metabolon®, Durham, NC). The MP is brown and the CSP is blue. Bile samples were sequentially obtained through a catheter previously placed in the common bile duct (external biliary drainage). (A) Lathosterol concentration (µM) performed by gas chromatography/mass spectrometry (GC/MS) in the recipient's bile for the first 72 h after liver transplantation. (B) Campesterol concentration (µM) performed by GC/MS in the recipient's bile for the first 72 h after liver transplantation. (C) Cholesterol concentration (µM) performed by GC/MS in the recipient's bile for the first 72 h after liver transplantation.
MP‐treated livers did not release GCDC during perfusion; however, its secretion resumed almost immediately following reperfusion. BA secretion was decreased in both groups immediately after reperfusion, but was restored subsequently in MP livers within the first 24 h. Furthermore, the levels of a few sulfated BAs (e.g. glycocholenate sulfate) were significantly elevated in the CSP group.
The insulin resistance biomarker alpha‐hydroxybutyrate was initially low in both groups but its levels in CSP bile increased during subsequent follow up. Insulin resistance is likely a sign of impaired functional recovery following transplant 25. Signs of oxidative and inflammatory stress differed with a greater level of hydrogen peroxide quenching by ascorbate in MP perfusate and greater pro‐inflammatory 12‐lipoxygenase product 12‐HETE in CSP perfusate 26.
MP livers experienced a sustainable level of metabolic activity at 21°C, which resulted in a more seamless resumption of hepatic functions, such as regulating the supply of glucose, lipids and amino acids for catabolic and anabolic metabolism after transplantation. MP bile samples displayed significantly higher (p < 0.05) levels of cholesterol, lathosterol and campesterol, in the initial postoperative period revealing a sustained ability to extract lipophilic nutrients from the gut.
PCA was carried out on the metabolomic profile of the perfusates of both groups (Figure 10) to elucidate principal drivers of the metabolic changes across the three time points. This analysis suggested that the principal dynamic characteristics in MP livers were carbohydrate metabolism (ribulose, ribose, glycolate) and antioxidant defenses (oxidized homo‐glutathione). In CSP livers, PCA suggested the characteristics were predominantly due to ethanolamine, suggesting a role for fatty acid metabolism 27.
Figure 10.
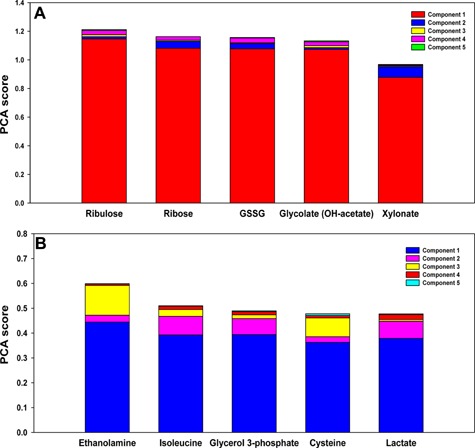
Principal component analysis (PCA) of perfusate metabolomic profile. (A) PCA suggests importance of carbohydrate metabolism and antioxidant defenses in MP livers. PCA was carried out on the metabolomic profile of perfusate at three time points (3, 6 and 9 h). Variables are ordered by the sum of their contribution to all components, with contributions to individual components represented by different colored sections of the bars. In MP livers, variables representing carbohydrate metabolism (ribulose, ribose, glycolate) and antioxidant defenses (oxidized homo‐glutathione–GSSG) are principal drivers of metabolic changes. (B) PCA suggests importance of fatty acid metabolism in CSP livers. PCA was carried out on the metabolomic profile of perfusate at three time points (3, 6 and 9 h). Variables are ordered by the sum of their contribution to all components, with contributions to individual components represented by different colored sections of the bars. In CSP livers, PCA showed ethanolamine to be the principal driver of metabolic changes, suggesting a role for fatty acid metabolism.
Inflammatory mediator analyses
MP was associated with down‐regulation of both type I (IFN‐α) and type II (IFN‐γ) interferons, consistent with prior studies that showed elevated inflammation and apoptosis subsequent to IR due to the IRF‐1 pathway in CSP 28, 29. MP was associated with down‐regulated TNF‐α levels in liver tissue when compared to CSP. This detrimental TNF‐α activation pathway seen in the CSP group was further corroborated by higher levels of additional Kupffer cell mediators IL‐1β and IL‐12/IL‐23 p40 found on liver tissues 30, 31 (Figure 11).
Figure 11.
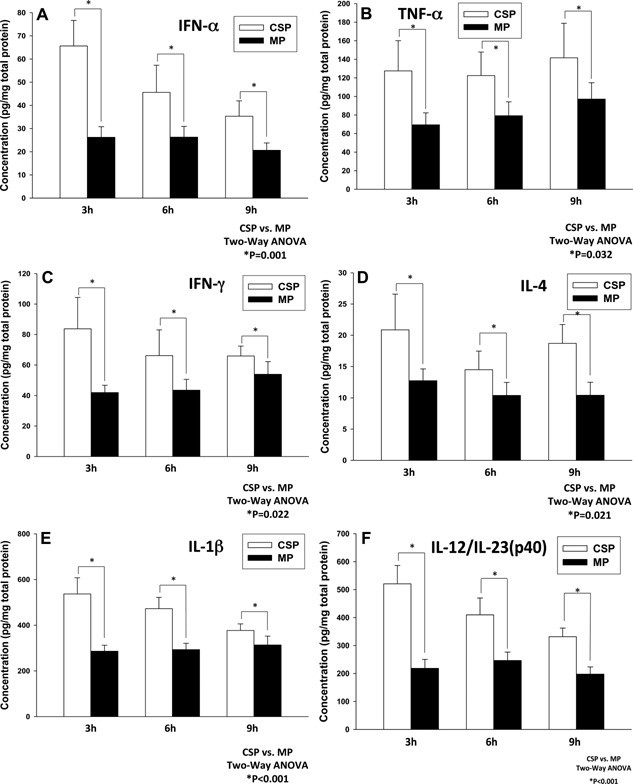
Comparison of the cytokine profile for machine perfusion (MP) and cold static preservation (CSP) groups obtained at 3, 6 and 9 h during liver preservation. MP suppresses TNF‐α driven pro‐inflammatory cytokines secreted by T cells and NKT (IFN‐γ), Kupffer cells (IL‐1b) and hepatocytes (IL‐12/IL‐23) when compared to CSP. (A) MP suppresses IFN‐α secretion. (B) MP suppresses TNF‐α secretion. (C) MP suppresses IFN‐γ secretion. (D) MP suppresses IL‐4 secretion. (E) MP suppresses IL‐1β secretion. (F) MP suppresses IL‐12/IL‐23 secretion.
Microbiological surveillance
There were no microorganisms isolated from any samples from either group.
Discussion
LT volumes have been relatively stagnant for the past several years in the United States. As indications for the procedure continue to expand, the volume of available livers is decreasing, resulting in an unacceptable number of deaths on the waiting list 32. Therefore, it is imperative that we improve the viability and usability of currently available but discarded organs.
The combination of an HBOC with hetastarch‐based colloids has been previously shown to be effective in sustaining adequate oxygenation and flow through the microcirculation while minimizing adverse effects seen with HBOC alone when utilized in vivo under physiologic conditions 33. This dilutional environment (hemoglobin = 3.5 g/dL) at lower temperatures was able to sustain low methemoglobin levels while providing reliable oxygen delivery and effective CO2 removal. Additional advantages of this solution include no risk of hemolytic reactions, low viscosity, unlimited availability and long storage life. Furthermore, the HBOC solution is universally compatible and carries an insignificant risk of disease transmission 34, 35.
The high mortality observed in the CSP animals was expected based on previous reports 19, 20, 21, 22 from swine models utilizing CIT > 9 h. Volume infusion and vasopressors were required to sustain hemodynamic stability after reperfusion. There were no technical complications in either experimental group. An additional CSP control group (n = 6) was performed using HTK, Custodiol® (Essential Pharmaceuticals, Ewing, NJ), and the results were similar to the University of Wisconsin animals (mortality = 67% in 5 days; data not shown).
Previous studies outlining the use of RBCs in extra‐corporeal devices have shown unavoidable hemolysis followed by the progressive deterioration of cell integrity documented on both roller and centrifugal pumps. Hemolysis remains a biological limitation for the prolonged utilization of RBCs in MP systems 36.
Among the options available for OCSs, HBOCs have shown superiority compared to perfluoro carbon and other non‐hemoglobin based products over the last two decades 37. The solution described herein is a second‐generation HBOC, in which bovine hemoglobin molecules are successfully extracted, purified and chemically cross‐linked (polymerized) for stability. We have tested another non‐HBOC solution (Lifor; Lifeblood Medical, Adelphia, NJ) 38, 39, 40 with the same device at 21°C in three additional animals. Massive centrilobular necrosis was observed after 9 h on MP, in spite of bile production and acceptable pH values during preservation (data not shown). Additional experiments were also conducted with the same MP device utilizing BMPS (Preservation Solutions, Elkhorn, WI) as the perfusate at 12°C (data not shown). The recipients developed moderate to severe PRS leading to allograft failure and subsequent death after 9 h of MP.
The debate over the role of pulsatile pressures in MP continues 41. We believe pulsatile pressure is important to assure effective perfusion through the high‐resistance microvasculature within the arterial‐biliary plexus as well as sustain sinusoidal system patency in the absence of the transient negative pressures supplied by diaphragmatic excursions in our physiological in vivo environment.
Adequate liver function has historically been associated with sustainable bile production, and these experiments reinforce this principle 42. MP allografts were able to produce up to 1 cc/h of bile despite being kept at 21°C for an extended time period. Sequential bile analysis showed lower cholesterol and lipid amounts within the bile of the CSP allografts, which translates to a deficiency in bile conjugation after implantation 43.
This new MP/HBOC system has a unique ability to provide effective oxygenation to the liver tissue. Perfused livers appear to sustain lower oxygen extraction under subnormothermic conditions 44, which reinforces the discrepancy between oxygen delivery and oxygen consumption witnessed in our ex vivo system with low hemoglobin levels (3.5 g/dL). MP animals experienced a markedly uncomplicated course compared with the CSP group. The initial bile production seen during the MP stage further increased during implantation as the allografts were rewarmed. The MP group showed signs of a prompt recovery from mild IR injuries in addition to an anatomically intact biliary system.
Our comprehensive data analysis combining transcriptomic, metabolomic and protein‐level inflammatory mediators highlights the notable benefits of MP when compared to CSP. Gene expression was clearly driven toward reanimation and resuscitation pathways while most gene expression related to metabolic, cellular and cell communication processes experienced significant up‐regulation 45 compared to the anoxic and hypothermic conditions imposed by CSP.
From the clinical standpoint, the recipients of liver allografts preserved by this combination of MP with an HBOC solution experienced significant benefits when modulation in gene expression 46 and inflammatory pathways were translated into graft function. A clear drive toward the induction of liver regeneration was detected by transcriptomic analysis. Inflammatory mediator analysis revealed a down‐regulation of TNF‐α in the MP group. Metabolomics' analysis revealed sustained metabolic activity (e.g. gluconeogenesis, albumin secretion, BCAA secretion, urea production and reactive oxygen species scavenging) in the MP group. Bile analysis over a 5‐day period suggested that hydrophilic bile was being secreted by the MP group in contrast to signs of hydrophobic bile documented in the CSP group. In spite of these encouraging findings, we acknowledge that this was a small study, and further work is required to fully explore the mechanisms initially outlined by transcriptomics and metabolomics' analysis.
The sustained increased in BCAA in the perfusate seen in our porcine model has been previously described in healthy fasted rats under MP while being adequately oxygenated 47.
The detergent action of hydrophobic bile salts (e.g. GCDC) plays a significant role in biliary epithelial cell membrane damage and seems to promote apoptosis 48. Conversely, hydrophilic bile salts can protect cholangiocytes from hydrophobic bile salt‐induced injury 49. When analyzing BA synthesis and subsequent bile secretion, MP livers secreted hydrophilic bile (e.g. significantly lower levels of taurodeoxycholate), which is by itself an important protective factor for biliary epithelial cells after reperfusion compared to the hydrophobic bile (e.g. significantly higher levels of glycocholenate sulfate) secreted in CSP.
In conclusion, MP at 21°C with an HBOC solution achieved a 100% survival rate with excellent graft function in these pre‐clinical large animal experiments. The modified HBOC solution provided consistent oxygen delivery to the tissues and was not shown to cause additional oxidative stress damage after reperfusion. Porcine liver allografts under MP produced urea, cleared lactate, secreted bile and showed no signs of secondary infections while being sustained at a low metabolic rate at 21°C over a 7:30 h period. MP livers appeared to secrete protective hydrophilic bile after reperfusion while showing no signs of subsequent epithelial biliary cell damage. MP with a cell‐free OCS can be safely and effectively conducted at 21°C under dual pressures with low flows. This approach has shown significant advantages compared to the current standard‐of‐care (CSP) and might allow the expansion of this technology into the recovery and amelioration of grafts obtained from ECDs.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Supplemental Methods
Acknowledgments
The authors gratefully acknowledge the outstanding contributions of Douglas Landsittel, PhD, Professor of Medicine, Biostatistics and Clinical Translational Science and David Geller, MD, Professor of Surgery in providing invaluable expertise and guidance in the final edition of the manuscript. We also appreciated the key role played by Tanya Minter and Donna Kocan in the final edition of the manuscript. This study was fully funded by a charitable fund kindly provided by Mr. and Mrs. Garcia de Souza. YV, RZ, DB and DS received partial funding from NIH grant UO1‐DK072146.
References
- 1. Wertheim JA, Petrowsky H, Saab S, Kupiec‐Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant 2011; 11: 1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orma ES, Barritt S IV, Wheeler SB, Hayashi PH. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transplant 2013; 19: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley DP, Fernandez LA, Leverson G, et al. Donation after cardiac death: The University of Wisconsin experience with liver transplantation. Ann Surg 2005; 242: 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—An analysis of the national registry. J Hepatol 2011; 55: 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant 2010; 10: 372–381. [DOI] [PubMed] [Google Scholar]
- 6. Vogel T, Brockmann JG, Coussios CC, Friend PJ. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant Rev 2012; 26: 156–162. [DOI] [PubMed] [Google Scholar]
- 7. Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol 2013; 4: 765–772. [DOI] [PubMed] [Google Scholar]
- 8. van der Plaats A, 't Hart NA, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Hypothermic machine preservation in liver transplantation revisited: Concepts and criteria in the new millennium. Ann Biomed Eng 2004; 32: 623–631. [DOI] [PubMed] [Google Scholar]
- 9. Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC‐201 as an alternative to blood transfusion: Efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma 2008; 64: 1484–1497. [DOI] [PubMed] [Google Scholar]
- 10. Jahr JS, Walker V, Manoochehri K. Blood substitutes as pharmacotherapies in clinical practice. Curr Opin Anesthesiol 2007; 20: 325–330. [DOI] [PubMed] [Google Scholar]
- 11. McNeil JD, Propper B, Walker J, et al. A bovine hemoglobin‐based oxygen carrier as a pump prime for cardiopulmonary bypass; reduced system lactic acidosis and improved cerebral oxygen metabolism during low flow in a porcine model. J Thorac Cardiovasc Surg 2011; 142: 411–417. [DOI] [PubMed] [Google Scholar]
- 12. Fontes PA, Marsh JW, Lopez RC, et al. Machine perfusion with a new oxygen‐carrier solution: The future of liver preservation [abstract]. Hepatology 2012; 56: 1524A. [Google Scholar]
- 13. Banff schema for grading liver allograft rejection: An international consensus document. Hepatology 1997; 3: 658–663. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki S, Toledo‐Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury: Modulating effects of FK506 and cyclosporine. Transplantation 1993; 55: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 15. Barclay D, Zamora R, Torres A, Namas R, Steed D, Vodovotz Y. A simple, rapid and convenient Luminex TM‐compatible method of tissue isolation. J Clin Lab Anal 2008; 22: 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vekemans K, Balligand E, Liu Q, et al. Differential gene expression profile of porcine livers subjected to warm ischemia alone. Transplant Proc 2011; 43: 3460–3464. [DOI] [PubMed] [Google Scholar]
- 17. Krumsieck J, Suhre K, Evans AM, et al. Mining the unknown: A systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet 2012; 8: e1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mi Q, Constantine G, Ziraldo C, et al. A dynamic view of trauma/hemorrhage‐induced inflammation in mice: Principal drivers and networks. PLoS ONE 2011; 6: 19424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakelin SJ, Casey J, Robertson A, et al. The incidence and importance of bacterial contaminants of cadaveric renal perfusion fluid. Transplant Int 2005; 17: 680–686. [DOI] [PubMed] [Google Scholar]
- 20. Ambiru S, Uryuhara K, Talpe S, et al. Improved survival of orthotopic liver allograft in swine by addition of trophic factors to UW solution. Transplantation 2004; 77: 302–319. [DOI] [PubMed] [Google Scholar]
- 21. Minor T, Koetting M, Koetting M, et al. Hypothermic reconditioning by gaseous oxygen improves survival after liver transplantation in the pig. AJT 2011; 11: 2627–2634. [DOI] [PubMed] [Google Scholar]
- 22. Fondevila C, Hessheimer AJ, Flores E, et al. Step‐by‐step guide for simplified model of porcine orthtotopic liver transplant. J Surg Res 2011; 167: e39–e45. [DOI] [PubMed] [Google Scholar]
- 23. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mi H, Muruganujan A, Thomas PD. Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 2013; D1: D377–D386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex‐vivo. Metab Eng 2009; 11: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Harrison‐Shostak DC, Wang XF, Nieminen AL, Lemastters JJ, Herman B. Role of phospholipid catabolism in hypoxic and ischemic injury. Adv Lipobiol 1997; 2: 167–194. [Google Scholar]
- 27. Namas R, Lagoa C, Barclay D, et al. Hemoadsorption reprograms inflammation in experimental Gram‐negative septic peritonitis: Insights from in vivo and in silico studies. Mol Med 2012; 18: 1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ueki S, Dhupar R, Cardinal J, et al. Critical role of interferon regulatory factor‐1 in murine liver transplant ischemia reperfusion injury. Hepatology 2010; 51: 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim KH, Dhupar R, Ueki S, et al. Donor graft interferon regulatory factor‐1 gene transfer worsens liver transplant ischemia/reperfusion injury. Surgery 2009; 146: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/Reperfusion injury in liver surgery and transplantation: Pathophysiology. HPB Surg 2012; doi: 10.1155/2012/176723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abu‐Amara M, Yang SY, Tappuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: Processes and inflammatory networks—A review. Liver Transplant 2010; 16: 1016–1032. [DOI] [PubMed] [Google Scholar]
- 32. Hayashi PH, Axelrod DA, Galanko J, Salvalaggio PR, Schnitzler M. Regional differences in deceased donor liver transplantation and their implications for organ utilization and allocation. Clin Transplant 2011; 25: 156–163. [DOI] [PubMed] [Google Scholar]
- 33. Plock J, Contaldo C, Sakai H, et al. Is hemoglobin in hemoglobin vesicles infused for isovolemichemodilution necessary to improve oxygenation in critically ischemic hamster skin? Am J Physiol 2005; 289: H2624–H2631. [DOI] [PubMed] [Google Scholar]
- 34. Patel MJ, Webb EJ, Shelbourn TE, et al. Absence of immunogenicity of diaspirin cross‐linked hemoglobin in humans. Blood 1998; 91: 710–716. [PubMed] [Google Scholar]
- 35. Abe H, Ikebuchi K, Hirayama J, et al. Virus inactivation in hemoglobin solution by heat treatment. Artif Cells Blood Substit Immobil Biotechnol 2001; 29: 381–388. [DOI] [PubMed] [Google Scholar]
- 36. Sakota D, Sakamoto R, Sobajima H, et al. Mechanical damage of red blood cells by rotary blood pumps: Selective destruction of aged red blood cells and subhemolytic trauma. Artif Organs 2008; 32: 785–791. [DOI] [PubMed] [Google Scholar]
- 37. Kim HW, Greenburg AG. Artificial oxygen carriers as red blood cell substitutes: A selected review and current status. Artif Organs 2004; 28: 813–828. [DOI] [PubMed] [Google Scholar]
- 38. Regner KR, Nilakantan V, Ryan RP, et al. Protective effect of Lifor solution in experimental renal ischemia‐reperfusion injury. J Surg Res 2010; 164: e291–e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stowe DF, Camara AK, Heisner JS, Aldakkak M, Harder DR. Ten‐hour preservation of guinea pig isolated hearts perfused at low flow with air‐saturated Lifor solution at 26°C: Comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol 2007; 293: H895–H901. [DOI] [PubMed] [Google Scholar]
- 40. Olschewski P, Gass P, Ariyakhagorn V, et al. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology 2010; 60: 337–343. [DOI] [PubMed] [Google Scholar]
- 41. Gallinat A, Fox M, Luer B, Efferz P, Paul A, Minor T. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation 2013; 96: 538–542. [DOI] [PubMed] [Google Scholar]
- 42. Vajdová K, Smreková R, Kukan M, Lutterová M, Wsólová L. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia‐reperfusion of rat livers. Cryobiology 2000; 41: 145–152. [DOI] [PubMed] [Google Scholar]
- 43. Buis AI, Geuken E, Visser DS, et al. Altered bile composition after liver transplantation is associated with the development of non‐anastomotic biliary strictures. J Hepatol 2009; 50: 69–79. [DOI] [PubMed] [Google Scholar]
- 44. Izamis ML, Toloboom H, Uygun B, Berthiaume F, Yarmush M, Uygun K. Resuscitation of ischemic donor livers with normothermic machine perfusion: A metabolic flux analysis of treatment in rats. PLoS ONE 2013; 8: e69758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knudsen AR, Kannerup AS, Dich R, et al. Ischemic pre‐ and postconditioning has pronounced effects on gene expression profiles in rat livers after ischemia/reperfusion. Am J Physiol Gastrointest Liver Physiol 2012; 303: G482–G489. [DOI] [PubMed] [Google Scholar]
- 46. Conti A, Scala S, D'Agostino P, et al. Wide gene expression profiling of ischemia‐reperfusion injury in human liver transplantation. Liver Transplant 2007; 13: 99–113. [DOI] [PubMed] [Google Scholar]
- 47. Fischer MM, Kerly M. Amino acid metabolism in the perfused rat liver. J Physiol 1964; 174: 273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Komichi D, Tazuma S, Nishioka T, Hyogo H, Une M, Chayama K. Unique inhibition of bile salt‐induced apoptosis by lecithins and cytoprotective bile salts in immortalized mouse cholangiocytes. Dig Dis Sci 2003; 48: 2315–2322. [DOI] [PubMed] [Google Scholar]
- 49. Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology 2002; 36: 525–531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods


