Abstract
Purpose
To explore whether and how longitudinal medical records could be used as a source of reference in the early phases of signal detection and analysis of novel adverse drug reactions (ADRs) in a global pharmacovigilance database.
Methods
Drug and ADR combinations from the routine signal detection process of VigiBase® in 2011 were matched to combinations in The Health Improvement Network (THIN). The number and type of drugs and ADRs from the data sets were investigated. For unlabelled combinations, graphical display of longitudinal event patterns (chronographs) in THIN was inspected to determine if the pattern supported the VigiBase combination.
Results
Of 458 combinations in the VigiBase data set, 190 matched to corresponding combinations in THIN (after excluding drugs with less than 100 prescriptions in THIN). Eighteen percent of the VigiBase and 9% of the matched THIN combinations referred to new drugs reported with serious reactions. Of the 112 unlabelled combinations matched to THIN, 52 chronographs were inconclusive mainly because of lack of data; 34 lacked any outstanding pattern around the time of prescription; 24 had an elevation of events in the pre‐prescription period, hence weakened the suspicion of a drug relationship; two had an elevated pattern of events exclusively in the post‐prescription period that, after review of individual patient histories, did not support an association.
Conclusions
Longitudinal medical records were useful in understanding the clinical context around a drug and suspected ADR combination and the probability of a causal relationship. A drawback was the paucity of data for newly marketed drugs with serious reactions. © 2015 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: electronic medical records, temporal pattern discovery, adverse drug reactions, post‐marketing surveillance, signal detection and analysis, individual case safety reports, pharmacoepidemiology
Introduction
To evaluate the implications of suspected harm from a drug, as in safety signal analysis,1, 2 the fullest description of the clinical setting is essential. Too often, this information is incomplete in individual case safety reports (ICSRs) of suspected adverse drug reactions (ADRs).
The identification of signals in large collections of ICSRs often starts by selecting drug‐ADR combinations that are reported disproportionally more frequently than expected.3, 4, 5, 6, 7 Decisions need to be made whether the statistical signal should be subject to in‐depth investigation and whether a signal should be communicated. Sometimes decisions in this process must be based on dubious or very limited information being available.8 Randomized clinical trials, on which a drug's marketing approval is based, are not always publically available, and supportive published case reports might not yet exist.
Electronic medical record (EMR) databases contain clinical data—diagnoses, observations, laboratory results, treatments, and other useful information—collected longitudinally over substantial parts of a patient's life. Longitudinal data might help us understand the relative chronology of drug use and the relationships between clinical events. They present opportunities for further insight into drug safety problems and have been used primarily in confirmatory studies. Recently, several initiatives have investigated the feasibility of using EMRs in surveillance of ADRs as a complement to ICSRs.9, 10, 11, 12 In this study, we explored whether and how EMRs could be used as an additional source of reference in the early phases of signal detection and analysis.
Methods
The study is descriptive. Statistics of Disproportionate Reporting (SDRs) of drug‐ADR combinations (hereafter referred to as combinations) highlighted in routine signal detection of the WHO Global ICSR database, VigiBase®,13 were reviewed, and a temporal pattern discovery method14 on The Health Improvement Network (THIN) was used as a source of reference.
Data sources
The VigiBase triaged data set
The triaged data set in this study is based on VigiBase data from the third quarter of 2011. Drugs are defined with the WHO Drug Dictionary Enhanced™ (DDE) preferred base level and adverse reactions with the WHO‐Adverse Reaction Terminology (ART) preferred terms. At the time of study, VigiBase included 6.8 million ICSRs from 106 national pharmacovigilance (PV) centres within the WHO Programme for International Drug Monitoring, who transfers anonymous electronic reports to the Uppsala Monitoring Centre (UMC). The national PV centres and the UMC use VigiBase for signal detection and evaluation. The study at hand is based on routine signal detection by UMC at the time, using a triage algorithm to detect novel ADRs in VigiBase.4, 7 The triaged drug‐ADR combinations that became disproportionally reported for the first time and fulfil one of two additional criteria: new drug (first report with drug in VigiBase within most recent 5 years) with serious reactions (WHO‐ART critical terms) or an increase in the information component (IC) measure of disproportionality by at least 1 since the previous quarter.3, 6 The IC is computed as the logarithm of a shrunk observed‐to‐expected ratio, where the observed value is the number of reports with a specific drug and adverse reaction. The expected value is based on the total number of reports for the specific drug, the adverse reaction, and the complete database, respectively. If the IC is above 0, the combination is reported more frequently than overall in the database. SDRs are defined as combinations with a positive lower limit of the 95% credibility interval of the IC. A WHO‐ART critical term is a reaction that is possibly indicative of a serious disease state. The wording ‘serious reactions’ are used instead of WHO‐ART critical terms in this paper.
Each quarter, UMC staff manually review the triaged combinations and exclude those that are labelled according to the product information or unlikely to represent causal associations. The latter may be events likely related to the underlying disease or to co‐reported drugs. Further evaluations ensue, including ICSR causality assessment, after which a decision is made on whether the issue should be communicated as a signal.
THIN and vigiTrace
THIN is a UK EMR data resource, which in January 2011 covered more than 7.7 million individual patient records. The patients registered in THIN are broadly representative of the UK population.15
A software interface has been developed on THIN data to screen for temporal associations of medical events and drugs. The temporal pattern discovery method, that is, vigiTrace, has previously been described in detail.14 THIN prescription codes in the interface are grouped according to the WHO DDE preferred base level, that is, products containing the same substance are grouped and constituted the ‘drug’ in this study). Combination products in THIN have not yet been linked to the dictionary; therefore, these were excluded in this analysis. Medical events in THIN are registered with Read codes. Pre‐defined groupings of Read codes (‘Read group terms’) for 23 concept terms classified by Trifirò et al.16 were also accessible.
The interface displays a listing of medical events recorded after first prescription of drugs. A ‘first prescription’ denotes prescriptions without any record of the same substance 13 months prior to the prescription in a patient's record history. In the ‘chronographs’, the medical event pattern is graphically displayed over time around the first prescription of a specific drug. Individual patient history records can also be reviewed.
The strength of a temporal association is given for each drug and event combination. Observed‐to‐expected ratios in the 1 and 12 month post‐prescription periods are contrasted with the corresponding value in three pre‐prescription periods (prescription day; month prior to prescription; and in 13 to 36 months prior to prescription). The logarithm of the shrunk observed‐to‐expected ratio is listed for the combination and denoted IC∆, where the positive lower bound of the two‐sided 95% credibility interval is used to highlight temporal associations (IC∆025). The chronographs display monthly observed and expected rates, as well as IC values with the two‐sided 95% credibility interval, 3 years before and after first prescription of a drug. The observed count represents the number of patients with at least one occurrence of that medical event for that month, and the expected count is based on the proportion of patients in an external control group with that medical event registered in relation to first prescriptions of other medicines and on the number of patients on the drug of interest ‘at risk’ in the same time period.
Study process
Drugs in the VigiBase data set were matched to drugs in THIN via the WHO DDE preferred base. WHO‐ART preferred terms were manually mapped to correlating Read code terms. If similar Read codes were available, a Read group term (defined in the preceding texts) was used if relevant, and if not, the Read code with the highest frequency following the prescription was used.
VigiBase combinations with less than 100 first prescriptions in THIN (less likely to be informative) were excluded from the study and so were neonatal or pregnancy related events for lack of relevant control period. For pregnancy‐related events, the state of pregnancy is a strong time‐varying confounder that may induce systematic differences in self‐controlled analyses such as the one in vigiTrace.
In the chronograph evaluation, combinations that lacked statistical power in THIN were grouped separately. To detect a temporal association with a relative risk of 4 or more with at least 80% probability, the expected number of events in the 12 month post‐prescription period (with three pre‐prescription periods) needed to be at least 1.98, which constituted the cut‐off for the statistical power.
Analysis
The number and type of drugs and adverse reactions in the VigiBase data set, and that could be matched to THIN, were investigated. Drugs were grouped by the second‐level therapeutic subgroup Anatomical Therapeutic Chemical (ATC) classification and adverse reactions by the WHO‐ART System Organ Classes (SOCs). The triaged unlabelled VigiBase combinations that could be matched to THIN were screened to determine the existence of a temporal association, that is, if IC∆025 was positive. Three experts (RE, NN, KS) with clinical or statistical background inspected each chronograph and allocated them into groups. If an elevated pattern was noted exclusively after prescription, it was considered to support the VigiBase combination. If the pattern was elevated in the months before or on the day of prescription or if no elevation was noted in either pre‐prescription or post‐prescription periods (or in both), the pattern was considered to weaken the suspicion of a causal relationship.
Individual patient histories were assessed when there were 10 or less events within the 12 month post‐prescription period. The assessment focused on whether clinically related events occurred before the first prescription in question and whether the time between start of prescription and first record of the event was feasible.
Results
Nature of drugs and ADRs
After excluding 51 combination products and 13 drugs that had not been specified on a preferred base level, the VigiBase data set included 246 unique drugs. Figure 1 displays the number of unique drugs and combinations in VigiBase and for the matched THIN data set. Of the 246 drugs, antineoplastic agents were the most frequent. Of the drugs in VigiBase that were matched to THIN and included 100 or more first prescriptions (n = 119), diabetes agents were the most frequent. Those drugs in VigiBase and in the matched THIN data are displayed in Figure 2.
Figure 1.
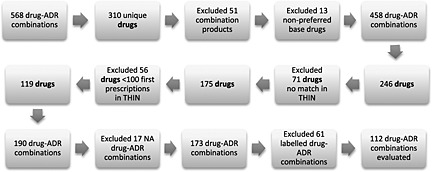
Flow chart displaying the number of included drugs and drug‐adverse drug reaction (ADR) combinations, NA = not applicable
Figure 2.
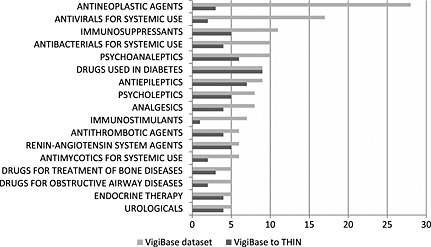
Number of substances grouped by the second‐level therapeutic subgroup ATC in the triaged VigiBase data set (246 drugs), the third quarter of 2011 (combination products excluded) and the number of substances matched to the THIN data set (119 drugs with at least 100 first prescriptions). The drug groups are sorted according to the most frequently represented substances (five or more) in the VigiBase data set
The VigiBase drugs included 458 drug‐ADR combinations, where the most frequent ADRs were general disorders, such as anaphylactic reactions and generalized oedema. For the 119 drugs matched to THIN, there were 190 combinations, of which the most frequent ADRs were related to metabolic and nutritional disorders, such as hypercalcaemia and diabetic coma. The most frequent ADRs by WHO‐ART SOC among the combinations in VigiBase and that could be matched to THIN are presented in Table 1.
Table 1.
The 10 most frequently represented adverse reactions by WHO‐ART System Organ Class for the 458 drug and adverse reaction combinations in VigiBase and the 190 matched pairs to THIN
| System Organ Class | No. of combinations VigiBase | No. of combinations (%) matched to THIN |
|---|---|---|
| Body as a whole—general disorders | 42 | 13 (31%) |
| Gastro‐intestinal system disorders | 42 | 15 (36%) |
| Neoplasm | 31 | 15 (48%) |
| Respiratory system disorders | 30 | 13 (43%) |
| Secondary terms | 29 | 11 (38%) |
| Metabolic and nutritional disorders | 28 | 16 (57%) |
| Central and peripheral nervous system disorders | 24 | 10 (42%) |
| Platelet, bleeding and clotting disorders | 22 | 8 (36%) |
| Musculo‐skeletal system disorders | 19 | 11 (58%) |
| Liver and biliary system disorders | 19 | 8 (42%) |
WHO‐ART = WHO‐Adverse Reaction Terminology.
Of the VigiBase combinations, 176/458 (38%) referred to serious reactions, and the corresponding number for the matched combinations in THIN was 67/190 (35%). Of the VigiBase combinations, 83/458 (18%) referred to new drugs with serious reactions, and the corresponding number for the matched combinations in THIN was 17/190 (9%).
Evaluation of the chronograph
For the chronograph evaluation, 15 of the 190 combinations that had been matched to THIN were excluded because of terms relating to birth defects (n = 6), pregnancy disorders (n = 6) and neonatal and infancy disorders (n = 3). Two additional combinations were excluded, as one concerned the term ‘posture abnormal’, which is too unspecific for evaluation, and the other represented five duplicates of the same case, resulting in only one evaluable VigiBase patient. Of the remaining 173 combinations, 61 were labelled ADRs.
Of the 112 unlabelled combinations, only one was temporally associated in THIN. The positive IC∆025 value (0.02) in the 12‐month post‐prescription period was for aluminium hydroxide and ‘[D]Rash and other nonspecific skin eruption’, classified as having an overall similar pre‐prescription and post‐prescription pattern when visually inspecting the chronograph.
In the graphical evaluation of the 112 combinations, 50 chronographs lacked statistical power and two had Read codes that were too unspecific making a quantitative evaluation inconclusive. For the remaining 60, two chronographs displayed an elevated pattern of medical events exclusively after prescription, 24 had an elevation of events before and/or on the day of prescription, and 34 referred to chronographs with no elevation in the pre‐prescription or post‐prescription periods. The characteristics of these 60 combinations are presented in Table 2. For 21/60 combinations, the chronographs referred to few events recorded in both the pre‐prescription and post‐prescription periods. Figure 3 displays the distribution of the classified chronograph patterns for the 112 combinations.
Table 2.
Characteristics of the 60 drug and adverse reaction combinations from VigiBase that had been matched to THIN with enough leverage to be subject to chronograph evaluation
| THIN characteristics | VigiBase characteristics | |||||
|---|---|---|---|---|---|---|
| Chronograph pattern | No. of combinations | Median and range of first prescriptions* | Median and range of combinations within 1/12 months | No. combinations with serious terms** / new drug*** with serious term** | First‐year drug**** | |
| 1 month | 12 months | |||||
| Elevated exclusively after prescription | 2 | n = 2213 and 82 593 | n = 1 and 1 | n = 5 and 3 | 0/0 | 2002/1968 |
| Elevated before prescription | 24 | M = 13 271 | M = 7.5 | M = 44 | 11/2 | M = 1997 |
| R = 103–884 258 | R = 0–521 | R = 0–3037 | R = 1968–2010 | |||
| No elevation before or after prescription | 34 | M = 12 186 | M = 2 | M = 9 | 12/0 | M = 1984 |
| R = 158–602 655 | R = 0–120 | R = 0–1170 | R = 1968–2007 | |||
M = median, R = range.
First prescriptions denote prescriptions without any record of the same substance 13 months prior to the prescription in a patient's record history.
Serious terms are defined by using the WHO‐Adverse Reaction Terminology critical terms, that is, a subset of terms that have been classified to be indicative of a serious disease state.
New drug is a drug entered in VigiBase for the first time during the past 5 years.
First year when drug was entered in VigiBase.
Figure 3.
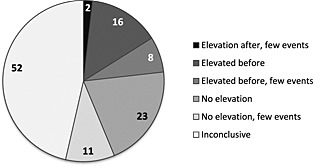
Distribution of the classified chronograph patterns for 112 unlabelled VigiBase drug and adverse reaction combinations matched to THIN data. ‘Elevation after’ = elevated records of event exclusively after prescription. ‘Elevated before’ = elevated records of event in the months before or on the day of prescription. ‘No elevation’ = no elevated records of event in either pre‐prescription or post‐prescription periods (or in both). The subgroups specified as ‘few events’ were based on few records of event displayed in the chronograph. ‘Inconclusive’ = 50 combinations without statistical power for an evaluation and two combinations with unspecific Read codes
The two chronographs with an elevation exclusively after prescription were fesoterodine and ‘Drug interaction’ (matched to Read code ‘Medication stopped‐interaction’) and carbamazepine and ‘Hepatic neoplasm benign’ (matched to ‘Primary malignant neoplasm of liver’). In THIN, medication stopped‐interaction was recorded for five cases within 5 months after more than 2200 fesoterodine prescriptions. There were no explicit records in these individual patient histories of which actual drug(s) had been stopped and suspected to interact, and no specific outcome was recorded in connection to the event for these cases. ‘Primary malignant neoplasm of liver’ was recorded for two cases, 2 and 43 days (respectively), after more than 82 000 carbamazepine prescriptions. Support for a possible causal association could not be found in either of these individual patient histories.
The chronographs with an elevation immediately before prescription most often referred to events related to the underlying disease (Table 3). Two of these combinations highlighted an elevated pattern before prescription that continued in the post‐prescription period, representing prednisolone and bronchiectasis (Figure 4) and levothyroxine and fibromyalgia (Figure 5).
Table 3.
Drug and event combinations with an elevated chronograph pattern before or on day of first prescription for matched VigiBase and THIN combinations (combinations with few events in the chronograph were excluded)
| Drug | Adverse reaction WHO‐ART preferred term | Medical event Read code | No. of reports in VigiBase** | No. of first prescriptions *** in THIN | First‐year drug**** |
|---|---|---|---|---|---|
| Alfuzosin | Bladder discomfort | Other bladder disorders | 5 | 23 714 | 1991 |
| Amisulpride | Dementia | Senile and presenile psychotic conditions | 8 | 7686 | 1989 |
| Avena sativa | Pruritus | Pruritus NOS | 7 | 40 641 | 2001 |
| Azathioprine | Bronchiectasis | Bronchiectasis | 5 | 18 856 | 1968 |
| Haloperidol | Gilles de la Tourette syndrome | Tics | 3 | 42 576 | 1968 |
| Ibandronic acid | Fracture pathological | Pathological fracture | 19 | 7412 | 1996 |
| Insulin aspart | Coma diabetic | Hypoglycaemic coma | 20 | 23 033 | 2000 |
| Levothyroxine | Fibromyalgia | Fibromyalgia | 29 | 189 757 | 1969 |
| Liraglutide | Diabetes mellitus aggravated | Type 2 diabetes mellitus | 8 | 1598 | 2009 |
| Magnesium hydroxide | Cholelithiasis | Cholelithiasis | 5 | 24 822 | 1972 |
| Magnesium hydroxide | Hypercalcaemia | Disorders of calcium metabolism | 5 | 24 822 | 1972 |
| Magnesium hydroxide | Hypocalcaemia | Disorders of calcium metabolism | 3 | 24 822 | 1972 |
| Melphalan | Spinal cord compression | Backache, unspecified | 3 | 123 | 1968 |
| Prednisolone | Bronchiectasis | Bronchiectasis | 14 | 884 258 | 1968 |
| Repaglinide | Hepatic failure | cg_Acute Liver Injury* | 7 | 2484 | 1999 |
| Sodium chloride | Incorrect technique in drug usage process | Medication error | 17 | 296 453 | 1973 |
WHO‐ART = WHO‐Adverse Reaction Terminology.
Grouping of Read codes.
The column displays the raw number of reports in VigiBase prior to any quality check or causality assessment.
First prescriptions denote prescriptions without any record of the same substance 13 months prior to the prescription in a patient's record history.
First year when drug was entered in VigiBase.
Figure 4.
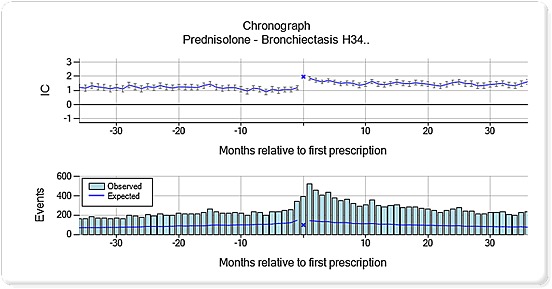
Chronograph for prednisolone and bronchiectasis (Read code H34). The top panel displays a shrunk logarithm of the observed‐to‐expected ratio (moderated towards the baseline value of one, when the observed or expected counts are low) denoted IC = information component. The bottom panel displays the underlying observed and expected counts
Figure 5.
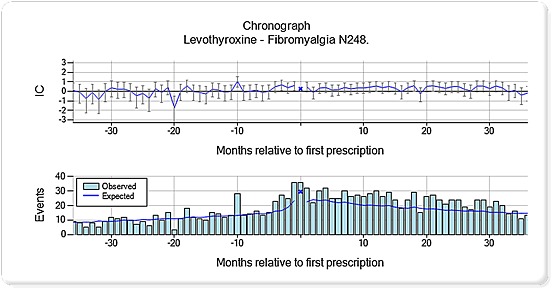
Chronograph for levothyroxine and fibromyalgia (Read code N248). The top panel displays a shrunk logarithm of the observed‐to‐expected ratio (moderated towards the baseline value of one, when the observed or expected counts are low) denoted IC = information component. The bottom panel displays the underlying observed and expected counts
Evaluating individual patient histories
Of the 112 combinations, 68 included at least one event recorded within the 12 month post‐prescription period in THIN, of which 35 combinations had 10 events or less and underwent review of individual patient histories. The combinations were assessed to be unrelated for the majority of the reviewed individual patient histories, commonly because of a noteworthy gap between prescription start and duration and the recorded onset of the event or because a similar event had been recorded prior to first prescription.
Discussion
Only around half of the unlabelled combinations matched to VigiBase were powered for analysis in THIN. Of these, a third included few events in the 12 month surveillance period, making a chronograph evaluation uncertain. Only in two chronographs was causation a possibility, but individual patient histories were not supportive. A majority of the informative chronographs had a pattern that weakened any suspicion of a causal association. Access to longitudinal data when assessing potential signals from ICSRs might be particularly relevant when considering possible biases and confounding.
The event pattern over time, before and after prescription, as displayed in the chronographs, provided complementary information to the focused description of an event as given in ICSRs. The chronograph patterns gave insight into specific problems and contributed with suggested explanations for why some events might have been reported as suspected ADRs in the first place. For bronchiectasis, an increased relative rate of event records was noted on the day of prescription, indicating that prednisolone was used to reduce air flow obstruction. Bronchiectasis was also associated with an elevated pattern in the post‐prescription period, which could be because of worsening of the underlying disease and/or a greater susceptibility to infection.17 Fibromyalgia was recorded commonly around the time of levothyroxine prescription and to a persistently higher extent compared with the background in the follow‐up period. This finding is interesting in the light of studies where thyroid autoimmunity seems to worsen fibromyalgia and may predispose to its development.18, 19 In Figure 5, fibromyalgia was increasingly recorded before patients were treated with thyroxine and remained higher than baseline after treatment suggesting that the fibromyalgia might be related to autoimmune phenomena rather than low thyroxine levels.
Triaged combinations require causality assessment of the individual reports before deciding if a signal should be communicated. In this study, the clinical assessment of individual patient histories in THIN helped determine the plausibility of a causal relationship for the two combinations with elevated patterns of events following prescription. Previously, it has been found that scrutinizing case details for appropriate coding and quality was important in the prospective Vaccine Safety Datalink project, where 9 of the 10 highlighted statistical signals were found to be spurious after in‐depth review.20 Patient records are not collected primarily for drug safety purposes, so information important for causality assessment might not be accessible in the EMR system, such as reasons for stopping a drug. For adequate evaluation of potential signals in EMRs, the original patient chart might need to be examined to confirm the coded records and retrieve supplementary information. A systematic approach to the review of individual patient histories needs to be developed to determine whether and what kind of pertinent information for causality assessment is in fact available in EMRs and how and when such an assessment should be performed relative to statistically highlighted signals.
A plausible temporal relationship between the start of a drug and the onset of an event is an important indicator in support of a possible causal relationship and is a major variable to aid detection of potential ADRs in the currently used method.14 However, the method could be improved to quantitatively include other aspects indicative of high likelihood of a causal relationship, similar to the recently proposed vigiRank method for ICSRs.21 More work could be done to highlight groups of patients having stopped a drug in connection to an event or having repeat prescriptions of a specific drug with the same event recorded after subsequent prescriptions, any of which might indicate drug‐related problems.
One third of the drugs in the VigiBase data set could not be identified in THIN. Few of the VigiBase combinations with new drugs and serious reactions could be matched to THIN. Some drugs might not have been launched in the UK, and others are used mostly in the hospital. The EMRs in our study were based on primary care data, and more matches might have been seen if hospital data had been used.
EMRs might have been shown more useful if a larger database had been used. With that said, only 1% of the drugs for over 19 000 000 individuals in the study of Coloma et al. were powered detect associations of rare ADRs, such as rhabdomyolysis.22 EMRs might be a more appropriate source to use in the detection and analysis of more commonly occurring events such as myocardial infarction, gastrointestinal bleeding22 and pneumonia.23.
None of the matched VigiBase and THIN combinations was communicated as signals. The high number of VigiBase combinations that seemed unrelated to the drug or related to the underlying disease could reflect the general experience of reviewing combination listings based solely on disproportionality measures. Method developments to capture potential signals with greater precision in VigiBase have been developed and are being implemented in routine use.21
If EMRs are to be used as a reference in routine signal detection and analysis, the terminology needs to be compatible with the terminology used in the pharmacovigilance database. In this study, much time was spent to manually match the WHO‐ART term to a Read code, and a perfect match was not always found. A closely related Read code could be found for many combinations, but for some matches, the term chosen was more or less specific than the WHO‐ART term.
Allocating chronograph patterns to the three defined groups by visual inspection was not clear cut, particularly when the graph included few events, which was the case in one third of the evaluated chronographs. However, the purpose of the classifications used in this study was only to present a rough sense of the patterns represented among the matched combinations.
The temporal pattern discovery method on EMRs was useful in understanding the clinical context around a drug and suspected ADR combination and the probability of a causal relationship, especially as it relates to confounding by underlying disease. A drawback was the paucity of data, especially for newly marketed drugs reported with serious reactions. Information from full hospital discharge summaries recorded in longitudinal primary care data may be useful in the future. As the assessment of individual patient histories was important to support or not support an association, a process for causality assessment of individual EMRs should be developed. More studies are needed to position the best use of EMRs in routine signal detection and analysis.
Conflict of Interest
The authors have no conflict of interest to declare relevant for this study.
Key Points.
Longitudinal electronic medical records provide useful clinical context for potential signals, which have been highlighted by individual case safety reports.
Only a small number of the potential signals detected in globally collected individual case safety reports could be evaluated in the electronic medical records because of paucity of data.
Lack of data for combinations on new drugs reported with serious reactions was a particular weakness.
Temporal pattern discovery on longitudinal medical records helped identification of drug and adverse reaction combinations from individual case safety reports that were most likely associated with the underlying disease.
Ethics Statement
The study protocol was approved by the scientific review committee (SRC). The SRC has been approved by the South‐East Multicentre Research Ethics Committee.
Acknowledgements
Thank you Marie Lindquist, Director at the Uppsala Monitoring Centre, for the idea to this study, Tomas Bergvall for your help with data extraction, and Niklas Norén (NN) for support in the evaluation of the chronographs. Jeanette Johansson was employed at the Uppsala Monitoring Centre until the end of February 2014 and her contributions to this paper were made during her employment.
Star, K. , Watson, S. , Sandberg, L. , Johansson, J. , and Edwards, I. R. (2015), Longitudinal medical records as a complement to routine drug safety signal analysis. Pharmacoepidemiol Drug Saf, 24, 486–494. doi: 10.1002/pds.3739.
An abstract based on parts of the content in this paper was presented at the 28th International Conference on Pharmacoepidemiology and Therapeutic Risk Management in 2012.
References
- 1. Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf 1994; 10: 93–102. [DOI] [PubMed] [Google Scholar]
- 2. Lindquist M, Edwards IR, Bate A, Fucik H, Nunes AM, Stahl M. From association to alert‐‐a revised approach to international signal analysis. Pharmacoepidemiol Drug Saf. 1999; 8(Suppl 1): S15–25. doi:10.1002/(SICI)1099-1557(199904)8:1+<S15::AID-PDS402>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3. Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 1998; 54: 315–321. [DOI] [PubMed] [Google Scholar]
- 4. Lindquist M. Use of triage strategies in the WHO signal‐detection process. Drug Saf 2007; 30: 635–637. [DOI] [PubMed] [Google Scholar]
- 5. Meyboom RH, Egberts AC, Edwards IR, Hekster YA, de Koning FH, Gribnau FW. Principles of signal detection in pharmacovigilance. Drug Saf 1997; 16: 355–365. [DOI] [PubMed] [Google Scholar]
- 6. Norén GN, Hopstadius J, Bate A. Shrinkage observed‐to‐expected ratios for robust and transparent large‐scale pattern discovery. Stat Methods Med Res 2013; 22: 57–69. doi:10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stahl M, Lindquist M, Edwards IR, Brown EG. Introducing triage logic as a new strategy for the detection of signals in the WHO Drug Monitoring Database. Pharmacoepidemiol Drug Saf 2004; 13: 355–363. doi:10.1002/pds.894. [DOI] [PubMed] [Google Scholar]
- 8. Hauben M, Bate A. Decision support methods for the detection of adverse events in post‐marketing data. Drug Discov Today 2009; 14: 343–357. doi:10.1016/j.drudis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 9. Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network‐‐improving the evidence of medical‐product safety. N Engl J Med 2009; 361: 645–647. doi:10.1056/NEJMp0905338. [DOI] [PubMed] [Google Scholar]
- 10. Stang PE, Ryan PB, Racoosin JA, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med 2010; 153: 600–606. doi:10.1059/0003-4819-153-9-201011020-00010. [DOI] [PubMed] [Google Scholar]
- 11. The European Medicines Agency (EMA) . The Pharmacoepidemiological Research on Outcomes of Therapeutics (PROTECT). Available at: http://www.imi‐protect.eu/wp3.shtml [25 Nov 2012].
- 12. Trifiro G, Fourrier‐Reglat A, Sturkenboom MC, Diaz Acedo C, Van Der Lei J. The EU‐ADR project: preliminary results and perspective. Stud Health Technol Inform 2009; 148: 43–49. [PubMed] [Google Scholar]
- 13. Lindquist M. Vigibase, the WHO Global ICSR Database System: Basic Facts. Drug Inf J 2008; 42: 409–419. [Google Scholar]
- 14. Norén GN, Hopstadius J, Bate A, Star K, Edwards IR. Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Discov 2010; 20: 361–387. [Google Scholar]
- 15. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011; 19: 251–255. [DOI] [PubMed] [Google Scholar]
- 16. Trifiro G, Pariente A, Coloma PM, et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmacoepidemiol Drug Saf 2009; 18: 1176–1184. doi:10.1002/pds.1836. [DOI] [PubMed] [Google Scholar]
- 17. Lasserson T, Holt K, Milan S, Greenstone M. Oral corticosteroids for bronchiectasis (stable and acute exacertations). Cochrane Database of Systematic Reviews 2001(4):CD002162. Epub 2001/11/01. Available at: http://www.bibliotecacochrane.com/pdf/CD002162.pdf [May 2011]. [DOI] [PMC free article] [PubMed]
- 18. Bazzichi L, Rossi A, Giuliano T, et al. Association between thyroid autoimmunity and fibromyalgic disease severity. Clin Rheumatol 2007; 26: 2115–2120. doi:10.1007/s10067-007-0636-8. [DOI] [PubMed] [Google Scholar]
- 19. Bazzichi L, Rossi A, Zirafa C, et al. Thyroid autoimmunity may represent a predisposition for the development of fibromyalgia? Rheumatol Int 2012; 32: 335–341. doi:10.1007/s00296-010-1620-1. [DOI] [PubMed] [Google Scholar]
- 20. Yih WK, Kulldorff M, Fireman BH, et al. Active surveillance for adverse events: the experience of the Vaccine Safety Datalink project. Pediatrics 2011; 127(Suppl 1): S54–S64. DOI: 10.1542/peds.2010‐1722I [DOI] [PubMed] [Google Scholar]
- 21. Caster O, Juhlin K, Watson S, Norén GN. Improved statistical signal detection in pharmacovigilance by combining multiple strength‐of‐evidence aspects in vigiRank. Drug Saf 2014; 37: 617–628. doi:10.1007/s40264-014-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coloma PM, Trifiro G, Schuemie MJ, et al. Electronic healthcare databases for active drug safety surveillance: is there enough leverage? Pharmacoepidemiol Drug Saf 2012; 21: 611–621. doi:10.1002/pds.3197. [DOI] [PubMed] [Google Scholar]
- 23. Star K, Bate A, Meyboom RH, Edwards IR. Pneumonia following antipsychotic prescriptions in electronic health records: a patient safety concern? Br J Gen Pract 2010; 60: e385–e394. doi:10.3399/bjgp10X532396. [DOI] [PMC free article] [PubMed] [Google Scholar]


