Abstract
Vagus nerve stimulation (VNS) is effective in refractory epilepsy and depression and is being investigated in heart failure, headache, gastric motility disorders and asthma. The first VNS device required surgical implantation of electrodes and a stimulator. Adverse events (AEs) are generally associated with implantation or continuous on−off stimulation. Infection is the most serious implantation‐associated AE. Bradycardia and asystole have also been described during implantation, as has vocal cord paresis, which can last up to 6 months and depends on surgical skill and experience. The most frequent stimulation‐associated AEs include voice alteration, paresthesia, cough, headache, dyspnea, pharyngitis and pain, which may require a decrease in stimulation strength or intermittent or permanent device deactivation. Newer non‐invasive VNS delivery systems do not require surgery and permit patient‐administered stimulation on demand. These non‐invasive VNS systems improve the safety and tolerability of VNS, making it more accessible and facilitating further investigations across a wider range of uses.
Keywords: depression, epilepsy, headache, implantable, migraine, safety, transcutaneous, vagus nerve stimulation
Introduction
Vagus nerve stimulation (VNS) is a viable treatment option in refractory epilepsy and depression 1. Until recently, all VNS therapy required surgical implantation of electrodes (around the cervical vagus nerve) connected to a stimulating device implanted under the anterior chest wall 1, 2. Implantable VNS is safe and well tolerated 1, but adverse events (AEs) are associated with both the surgical procedure and the electrical stimulation itself 1, 3. Subsequently, non‐invasive VNS (nVNS) delivery options that eliminate the need for surgical implantation were developed. These alternative VNS delivery systems avoid surgery‐related AEs (e.g. infection, cardiac events) and may limit AEs related to the continuous on−off stimulation cycle of implantable devices, since nVNS devices can be adjusted to balance efficacy and tolerability 4, 5.
This review provides a summary of the efficacy, safety and tolerability of VNS delivery systems including both surgically implantable VNS and newer devices in development that deliver VNS non‐invasively.
Vagus nerve function and anatomic connections
The vagus (tenth cranial) nerve is a mixed parasympathetic nerve, containing both afferent and efferent sensory fibers. An estimated 80% of vagus nerve fibers are afferent and convey visceral, somatic and taste sensations (Fig. 1) 6, 7, 8, 9. The vagus nerve is subdivided into five groups: (1) special visceral afferents, (2) general visceral afferents, (3) general somatic afferents, (4) special visceral efferents and (5) general visceral efferents 10. Thorough reviews of vagus nerve anatomy and function have been discussed previously 11, 12. The vagus nerve connections allow it to modulate the function of higher brain centers, forming the basis for its potential use in many disorders.
Figure 1.
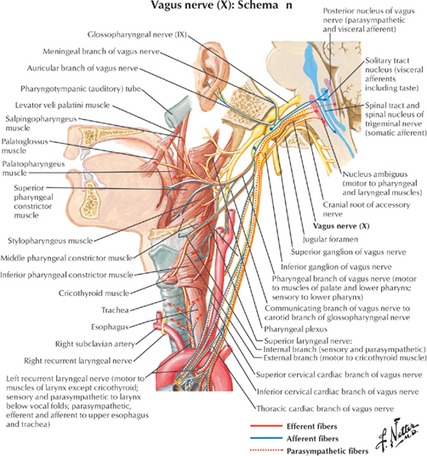
Vagus nerve innervation. (Reprinted with permission from Massey 9).
Effectiveness of implantable VNS
VNS Therapy system
Early animal studies supported VNS effectiveness in human epilepsy 13, 14, 15, 16. Clinical studies of the implantable VNS Therapy system (Fig. 2a; Cyberonics, Inc., Houston, TX, USA) in refractory epilepsy demonstrated 50% seizure reduction in 24.5%–46.6% of patients 2, 17, 18. The VNS Therapy system was approved for the treatment of medically refractory epilepsy in Europe in 1994 and in the USA and Canada in 1997. As of August 2014, over 100 000 VNS devices were implanted in more than 75 000 patients worldwide 19.
Figure 2.
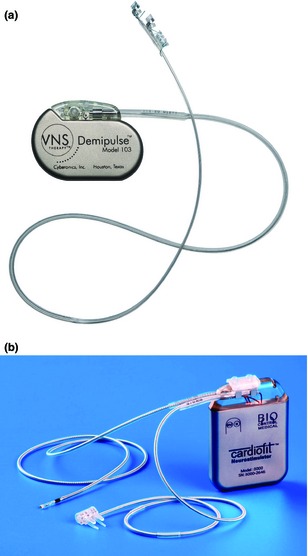
Implantable VNS systems: (a) VNS Therapy system and (b) CardioFit. (Reprinted with permission from (a) Cyberonics, Houston, TX, USA, and (b) BioControl Medical, Yehud, Israel).
Mood improvements observed in patients who received implantable VNS for refractory epilepsy 20, 21 led to investigations of treatment‐resistant depression. A large sham‐controlled, 10‐week trial in treatment‐resistant depression failed to find a statistical difference between the two treatments in terms of the 24‐item Hamilton Depression Rating Scale (HRSD24) response 22. However, a 1‐year open‐label extension (n = 205) found that the HRSD24 score improved significantly by 0.45 (standard error = 0.05) points per month (repeated measures t = 8.25, degrees of freedom 654, P < 0.001) 23. This led to US Food and Drug Administration (FDA) approval of implantable VNS for the adjunctive, long‐term treatment of chronic or recurrent depression in patients at least 18 years of age who are experiencing an episode of major depression and have not had an adequate response to four or more antidepressant treatments 24.
Implantable VNS provided efficacy benefits in small studies in refractory migraine and cluster headache (CH) 25, 26, heart failure 27, Alzheimer's disease 28, 29, treatment‐resistant anxiety disorders 30 and obesity 31. The exact mechanism by which VNS provides benefit across these widely different conditions is unknown. The diverse therapeutic potential of VNS, along with the AEs, cost and limited accessibility associated with implantable VNS, led to the development of new nVNS modulators that do not require surgery.
Safety and tolerability associated with the surgically implanted VNS Therapy system can be divided into two classes: device implantation related and VNS stimulation related 1, 3.
The most frequent surgical AEs include infection (3%–6% of patients), vocal cord paresis, lower facial weakness and, infrequently, bradycardia and asystole 32. Infection rarely prompts device removal 33. Vocal cord paresis and lower facial weakness have occurred in about 1% of patients each 32, 33. With surgical technique improvements, permanent voice alterations and lower facial weakness have become rare. Depending on the duration of use, replacement of the stimulus generator battery will be required, necessitating additional surgery.
Stimulation‐related AEs in refractory epilepsy and depression were similar and most frequently included voice alteration, cough, dyspnea, paresthesia, headache and pain (Table 1) 3. The frequency of these AEs declines with continued treatment 34. For example, voice alteration was present in 62% of patients with epilepsy receiving VNS at 3 months but in only 18.7% of patients at 5 years 3.
Table 1.
Adverse events (%) reported in clinical trials of the VNS Therapy system in patients with epilepsy or depression (reprinted with permission from Ben Menachem 3)
| Adverse event | 3 months | 12 months | 5‐year follow‐up | |
|---|---|---|---|---|
| Epilepsy | Depression | Epilepsy | Epilepsy | |
| Cough | 21 | 15 | 1.5 | |
| Voice alteration | 62 | 60 | 55 | 18.7 |
| Dyspnea | 16 | 23 | 13 | 2.3 |
| Pain | 17 | 27 | 15 | 4.7 |
| Paresthesia | 25 | 15 | 1.5 | |
| Headache | 20 | 30 | 16 | – |
| Pharyngitis | 9 | 10 | – | |
| Depression | 3 | 5 | – | |
| Infection | 4 | 3 | 6 | – |
| Deaths | 2 patients (1 SUDEP, 1 pneumonia) | 4 patients (1 SUDEP, 3 status epilepticus) | ||
SUDEP, sudden, unexpected, unexplained death in epilepsy.
Cardiac AEs associated with implantable VNS devices mainly occur in the operating room during initial device testing. These include bradycardia, ventricular asystole and complete heart block 35, 36, 37, 38. Only rarely have these emerged years after VNS initiation. One patient developed bradyarrhythmia characterized by sudden falls, pallor and unconsciousness lasting <10 s that occurred for the first time 2 years after VNS initiation. The attacks occurred during stimulation and stopped when the VNS device was turned off 39. Another report described intermittent self‐terminating complete heart block occurring every 15–25 min and lasting 20–40 s that occurred 6.5 years after the implantation of a VNS device for the treatment of epilepsy 40. A third case reported periodic asystole 9 years after implantation 41.
VNS is not associated with central nervous system AEs such as fatigue, psychomotor retardation, cognitive dysfunction or suicidal ideation in patients with epilepsy. One suicide and seven suicide attempts in six patients occurred in the pivotal implantable VNS trial 23 in treatment‐resistant depression. No teratogenicity has been observed 42, 43. Patients treated with implantable VNS have noted improvements in feelings of well‐being, alertness, memory and thinking skills, as well as mood.
CardioFit
CardioFit (BioControl Medical Ltd., Yehud, Israel) (Fig. 2b) is an implantable VNS device being investigated in heart failure acting by preferential activation of vagal efferent fibers 44. The rationale of this approach has been reviewed elsewhere 45, 46. The stimulation is designed to correct the autonomic imbalance (sustained sympathetic overdrive and parasympathetic withdrawal) that is maladaptive in heart failure 27, 45.
An initial feasibility study evaluated the safety of CardioFit in eight patients with New York Heart Association (NYHA) class II–III heart failure over 6 months 27. CardioFit stimulation provided statistically significant improvements in NYHA II–III heart failure, especially at months 1 and 3 (P < 0.01), reduced left ventricular end systolic volume (P = 0.03) and improved 6‐min walking test (P = 0.04) and Minnesota quality of life measure (P = 0.001). Mild, transient voice alteration was the only implantation‐associated event 27. Stimulation‐associated AEs included cough (n = 4), pain at stimulation site (n = 4), mandibular pain (n = 3) and voice alteration (n = 2). No AEs were severe; all resolved with continued treatment 27.
Subsequently, a phase 2 open‐label, 6‐month study (n = 32) in patients with NYHA II–IV heart failure had similar findings. Statistically significant effects were found between baseline and 6‐month follow‐up for NYHA class improvement (P < 0.001), 6‐min walking (P = 0.0014), quality of life (P = 0.0001), left ventricular ejection fraction (P = 0.0003) and left ventricular end systolic volume index (P = 0.02).
In total, 26 serious AEs (SAEs) occurred in 13 patients. Two SAEs (acute pulmonary edema; surgical revision necessitated by a loose electrode connector on the stimulus generator) were implantation related 47. A third SAE, an episode of syncope associated with new onset atrial fibrillation and hypotension, was felt to be possibly related to the system. Other SAEs possibly related to the procedure or the system included syncope facilitated by dehydration (two episodes) or new onset atrial fibrillation and hypotension (one episode), and atrial fibrillation (two episodes in the same patient; one episode recurred after cardioversion) 47.
Effectiveness of non‐invasive VNS devices
NEMOS
NEMOS (Cerbomed, Erlangen, Germany) is an external device that provides transcutaneous VNS (tVNS) by using a dedicated intra‐auricular electrode (like an earphone) which stimulates the auricular branch of the vagus nerve (Fig. 3a) 48. In 2010, the device received the European clearance (CE mark) for epilepsy and is available in Germany, Austria, Switzerland and Italy. The patient controls VNS stimulation intensity within a defined range and self‐treatment sessions lasting 1–4 h three to four times daily and as necessary (e.g. before a seizure) are recommended. Users adjust the current until they feel a slight discomfort or tingling sensation at the stimulation site 48.
Figure 3.
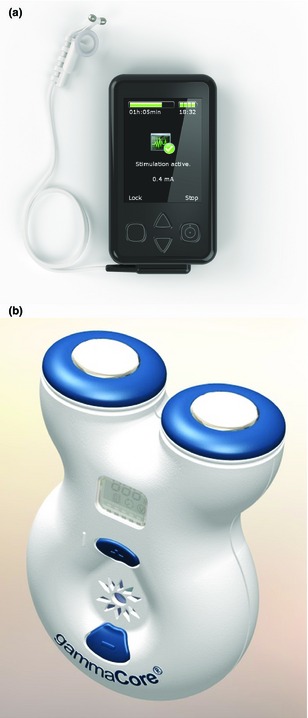
Non‐implantable VNS systems: (a) NEMOS (tVNS) and (b) gammaCore (nVNS). (Reprinted with permission from (a) Cerbomed, Erlangen, Germany, and (b) electroCore, Basking Ridge, NJ, USA).
A proof‐of‐concept study of NEMOS tVNS enrolled 10 patients with pharmacoresistant epilepsy who received treatment three times daily (1 h each) for 9 months 49. Three patients discontinued 49. Five of the seven patients who continued reported reductions in seizure frequency, but none reached the 50% reduction threshold for response. Two patients reported increased seizure frequency that remained constant over the entire study duration 49.
In another study in healthy volunteers (n = 48), tVNS increased mechanical and pressure pain threshold, reduced mechanical pain sensitivity and lowered pain ratings during sustained application of painful heat compared with sham treatment 50. There were no clinically relevant AEs.
Napadow et al. 51 compared the effect of NEMOS stimulation to non‐vagal auricular stimulation in patients (n = 18) with chronic pelvic pain. Although a numerical reduction in evoked pain intensity and temporal summation of mechanical pain was observed with NEMOS, the differences were not significant between the two methods. Anxiety was significantly reduced with NEMOS stimulation vs. non‐vagal auricular stimulation. No significant effect of stimulation, time or interaction on heart rate or heart rate variability (P > 0.7) or on respiratory rate (P > 0.8) was observed.
gammaCore
gammaCore (electroCore LLC, Basking Ridge, NJ, USA) is a handheld, self‐contained nVNS device under investigation for headache, epilepsy and gastrointestinal disorders. It consists of a portable stimulator with a battery, signal‐generating and ‐amplifying electronics and a digital control user interface that controls signal amplitude (Fig. 3b). Two stainless steel round discs function as skin contact surfaces that deliver a proprietary, low‐voltage electrical signal to the cervical vagus nerve. The device delivers a programmable number of stimulation cycles, each lasting 120 s 52.
Evidence for gammaCore nVNS efficacy comes from small studies in intractable CH 53, episodic migraine 54 and chronic migraine 55. In CH, gammaCore nVNS delivered both acutely for CH attacks and as a twice‐daily preventive treatment (median 12 weeks) was tested over a median of 12 weeks in 31 evaluable adults (12 with chronic CH, 10 as medically intractable CH, and nine with episodic CH). Overall, 18 of 21 patients reported improvement (51% mean improvement from baseline) and three reported no change. Seventeen were able to stop, reduce or significantly reduce their prior abortive treatment use 53. AEs included local discomfort, a mild skin irritation secondary to the conductive gel and worsening of pain in one subject 52.
An open‐label pilot study 4 of gammaCore nVNS in episodic migraine (n = 30) investigated applying two 90‐s stimulations administered 15 min apart during migraine. Overall, 27 patients treated 80 migraines. Of 19 patients with moderate or severe pain at the time of treatment of their first attack, nine (47%) reported pain relief and four (21%) reported being pain free 2 h after treatment. In 54 migraine attacks that were moderate to severe at the time of treatment, 2‐h pain relief was achieved in 23 (43%) attacks and 2‐h pain‐free status was achieved in 12 (22%). Treatment‐related AEs, all mild or moderate, included transient muscle stiffness/pain (n = 7) and dizziness (n = 2); all AEs except one (neck stiffness treated with a nonsteroidal anti‐inflammatory drug) resolved without treatment 4.
Moscato and Moscato 55 evaluated 73 patients with chronic migraine, 19 of whom had moderate to severe migraine pain with nausea, phonophobia and photophobia at the time of evaluation and received gammaCore nVNS in two 90‐s treatments administered 15 min apart. At 2 h, mean visual analog scale pain scores were significantly reduced from baseline (P < 0.05); nine of 19 patients were pain free, six had reduced pain and four remained unchanged. AEs included two reports of brief paresthesia, which resolved within a few minutes 55. gammaCore is now being evaluated in four multicenter, randomized, controlled trials in the EU and North America in primary headache disorders; to date, no significant serious device‐related AEs have been reported. Table 2 summarizes the clinical studies of new implantable and non‐implantable VNS devices covered in this review 4, 27, 47, 49, 50, 52, 53, 55, 56, 57, 58.
Table 2.
Summary of clinical studies utilizing implantable or non‐invasive VNS delivery cited in this review
| Reference | Indication studied | n | Stimulation schedule; location | Efficacy | Safety/tolerability |
|---|---|---|---|---|---|
| Schwartz et al. 27 | Severe congestive heart failure | 8 | 2–10 s on, 6–30 s off for 6 months; right cervical vagus nerve | Significant improvements in NYHA class (P < 0.01), QOL on Minnesota Living With Heart Failure questionnaire (P = 0.001), and left ventricular end systolic volume (P = 0.03) |
Implantation‐related AE: voice alteration (hoarseness) Stimulation‐related AEs: cough; pain at stimulation site, mandible and ear; voice alteration |
| De Ferrari et al. 47 | Chronic heart failure | 32 (8 from feasibility phase 27 and 24 from multicenter international phase 47) | Duty cyclea ≤25% (e.g. maximum 10 s on, 30 s off) for 6 months; right cervical vagus nerve |
At 3 and 6 months: 56% and 59% of patients improved by ≥1 NYHA class (P ≤ 0.001) At 6 months: significant improvements in 6‐min walk test (P = 0.0014), QOL on Minnesota Living with Heart Failure questionnaire (P = 0.0001), LVEF (P ≤ 0.0003) and LVESVI (P = 0.02) |
Implantation‐related SAEs: acute pulmonary edema (1 event), surgical revision (1 event) Other possibly related SAEs: dehydration‐related syncope (2 events); syncope resulting from new‐onset atrial fibrillation and hypotension; atrial fibrillation (2 events; 1 was a return to atrial fibrillation after cardioversion) AEs: pain at site of stimulation (n = 6), cough (n = 5), dysphonia (n = 4), mandibular pain (n = 3) and ECG stimulus artifact (n = 1) |
| Ben‐Menachem et al. 57 | Refractory focal epilepsy | 5 | 12–15 months after implantation: amplitude was 1.5–2.0 mA, frequency was 20 Hz, duty cyclea was 30 s on, 1.8–3 min off (14.3%–20.3%) with a pulse width of 0.3 ms and a quasi‐trapezoidal pulse shape; left cervical vagus nerve | Seizure frequency reduction of 50% in 2 patients and 25% in 2 patients; rate unchanged in 1 patient | Cough and/or hoarseness not noted until stimulation of 2 mA reached |
| Stefan et al. 49 | Pharmacoresistant epilepsy | 10 | 3 times daily (1 h each) for 9 months; left auricular branch of vagus nerve |
50% reduction threshold not reached; seizure frequency was reduced by 45% and 48% in 2 patients and increased in 2 patients |
3 patients discontinued; AEs included hoarseness, headache and constipation |
| Busch et al. 50 | Healthy volunteers | 48 | Stable stimulation duration of ~1 h; left auricular branch of vagus nerve | tVNS increased pain threshold and lowered pain sensitivity and pain ratings | No discontinuations or SAEs; AEs included stimulation site sensations of slight pain, pressure, prickling, itching or tickling in 39 patients with active stimulation |
| Hein et al. 58 | Major depression | 37 | 15 min once or twice daily, 5 day/week for 2 weeks; bilateral transauricular vagus nerve | Significant reduction (P < 0.0001) in Beck Depression Inventory (self‐report) but not in clinician‐rated Hamilton Depression Rating Scale between active and sham treatments | No vital sign changes; no unpleasant sensations or irritations |
| Nesbitt et al. 52, 53 | Intractable CH | 21 | Acute stimulation of 2–4 cycles (90 s each) to abort CH attacks and twice daily as preventive; cervical vagus nerve, ipsilateral to pain |
Overall improvement: estimated subjective improvement of 51% in 18 patients; no change in 3 patients Abortive treatment: 47% of acute attacks were terminated and 27% substantially improved in 15 min Preventive treatment: reduction in 24‐h attack frequency (4.68 ± 2.36 to 2.54 ± 2.12; P < 0.0005) |
AEs included worsening of pain in 1 patient; skin irritation, local skin reaction to conductive gel |
| Goadsby et al. 4 | Episodic migraine | 30 | Two 90‐s stimulations 15 min apart; right cervical vagus nerve | Pain relief noted at 2 h for 46 of 79 migraines (58%) treated by 26 patients; 2‐h pain free rate was 28% | AEs included transient muscle or local skin irritation and 2 reports of light‐headedness |
| Moscato and Moscato 55 | Chronic migraine | 19 | Two 90‐s stimulations 15 min apart; location not reported | Reduction (P < 0.05) in mean pain scores in overall group at 2 h; 9 patients were pain free, 6 had reduced pain and 4 were unchanged at 2 h | 2 brief episodes of paresthesia |
AE, adverse event; CH, cluster headache; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end systolic volume index; NYHA, New York Heart Association; QOL, quality of life; SAE, serious adverse event; tVNS, transcutaneous VNS; VNS, vagus nerve stimulation.
Duty cycle is the percentage of time that stimulation is on.
Discussion − safety and tolerability
VNS is well tolerated in the treatment of refractory epilepsy and depression 1. Most AEs resolve after 1–2 years of continued treatment 3. Implantable VNS is associated with surgically related AEs, such as infection and dysrhythmias; stimulation‐associated AEs include cough, paresthesia, pain and voice alteration, which generally decrease in prevalence over time. Voice alteration, a common and particularly disturbing AE that may continue in nearly 20% of patients at 5 years, may be a consequence of the continuous on−off stimulation cycle seen with implantable VNS and is stimulus dose dependent. nVNS devices could be expected to provide an improved safety profile because they do not require surgical implantation and provide shorter durations of stimulation compared with the constantly cycling stimulation with implantable VNS.
Efficacy could not be compared between these modalities at the time of this review because of the different stages of development of the various delivery systems. One consistent observation, however, is that efficacy and possible AEs, at least for epilepsy and depression, improve with time over a period of about 18 months 23, 32, 34, 59, 60, 61.
Implanted VNS devices are currently approved for the treatment of refractory epilepsy and depression; past and ongoing investigations in other indications have provided signals of the therapeutic potential in a wide variety of conditions. AEs, amongst other factors stemming from the surgical procedure, are negative aspects of implantable VNS and could be eliminated entirely through the use of nVNS delivery devices. The less frequent stimulation schedules used with nVNS may reduce the overall incidence of stimulation‐associated AEs. Without a requirement for an expensive and potentially risky surgical procedure, nVNS may facilitate the earlier use of therapeutic VNS without the prerequisite of achieving a ‘treatment‐refractory’ status in the condition of interest. Results from ongoing clinical studies are awaited to help inform appropriate use.
Disclosure of conflicts of interest
Dr Ben‐Menachem reports serving on an advisory board for electroCore and as a consultant for Bial, BioControl, Esai, UCB Pharma; she also serves as an editor of Acta Neurologica Scandinavica. Dr Revesz has no conflict of interest related to the content of this article. Dr Silberstein reports serving on an advisory board for electroCore. Dr Simon reports being an employee of electroCore and has numerous issued patents and pending patent applications related to the gammaCore device.
Acknowledgements
Medical writing support was provided by John H. Simmons, MD, of Peloton Advantage, LLC, and funded by electroCore LLC.
References
- 1. Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol 2010; 27: 130–138. [DOI] [PubMed] [Google Scholar]
- 2. Ben‐Menachem E, Manon‐Espaillat R, Ristanovic R, et al Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia 1994; 35: 616–626. [DOI] [PubMed] [Google Scholar]
- 3. Ben‐Menachem E. Vagus nerve stimulation, side effects, and long‐term safety. J Clin Neurophysiol 2001; 18: 415–418. [DOI] [PubMed] [Google Scholar]
- 4. Goadsby P, Lipton R, Cady R, Mauskop A, Grosberg B. Non‐invasive vagus nerve stimulation (nVNS) for acute treatment of migraine: an open‐label pilot study [abstract S40.004]. Presented at Annual Meeting of the American Academy of Neurology, 16−23 March 2013, San Diego, CA.
- 5. Jurgens TP, Leone M. Pearls and pitfalls: neurostimulation in headache. Cephalalgia 2013; 33: 512–525. [DOI] [PubMed] [Google Scholar]
- 6. Hatton KW, McLarney JT, Pittman T, Fahy BG. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg 2006; 103: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 7. Krahl SE. Vagus nerve stimulation for epilepsy: a review of the peripheral mechanisms. Surg Neurol Int 2012; 3: S47–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foley JO, Dubois F. Quantitative studies of the vagus nerve in the cat, I: The ratio of sensory and motor studies. J Comp Neurol 1937; 67: 49–67. [Google Scholar]
- 9. Massey BT. Physiology of oral cavity, pharynx and upper esophageal sphincter. GI Motility Online 2006. http://www.nature.com/gimo/contents/pt1/full/gimo2.html (accessed 09/26/2014).
- 10. Tewfik TL, Meyers AD. Vagus nerve anatomy. http://emedicine.medscape.com/article/1875813-overview (accessed 01/29/2014).
- 11. Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat 2011; 42: 288–296. [DOI] [PubMed] [Google Scholar]
- 12. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17. [DOI] [PubMed] [Google Scholar]
- 13. Lockard JS, Congdon WC, DuCharme LL. Feasibility and safety of vagal stimulation in monkey model. Epilepsia 1990; 31 (Suppl. 2): S20–S26. [DOI] [PubMed] [Google Scholar]
- 14. Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia 1990; 31 (Suppl. 2): S7–S19. [DOI] [PubMed] [Google Scholar]
- 15. Woodbury JW, Woodbury DM. Vagal stimulation reduces the severity of maximal electroshock seizures in intact rats: use of a cuff electrode for stimulating and recording. Pacing Clin Electrophysiol 1991; 14: 94–107. [DOI] [PubMed] [Google Scholar]
- 16. Zabara J. Inhibition of experimental seizures in canines by repetitive vagal‐stimulation. Epilepsia 1992; 33: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 17. Vagus Nerve Stimulation Study Group . A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995; 45: 224–230. [DOI] [PubMed] [Google Scholar]
- 18. Uthman BM, Wilder BJ, Penry JK, et al Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993; 43: 1338–1345. [DOI] [PubMed] [Google Scholar]
- 19. Cyberonics Inc. 2013. Annual Report. http://ir.cyberonics.com/annuals.cfm (accessed 03/05/2014).
- 20. Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000; 42: 203–210. [DOI] [PubMed] [Google Scholar]
- 21. Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000; 1: 93–99. [DOI] [PubMed] [Google Scholar]
- 22. Rush AJ, Marangell LB, Sackeim HA, et al Vagus nerve stimulation for treatment‐resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 2005; 58: 347–354. [DOI] [PubMed] [Google Scholar]
- 23. Rush AJ, Sackeim HA, Marangell LB, et al Effects of 12 months of vagus nerve stimulation in treatment‐resistant depression: a naturalistic study. Biol Psychiatry 2005; 58: 355–363. [DOI] [PubMed] [Google Scholar]
- 24. VNS Therapy System Physician's Manual. Houston, TX: Cyberonics Inc., 2013. http://dynamic.cyberonics.com/manuals/ (accessed 01/05/2015). [Google Scholar]
- 25. Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002; 22: 482–484. [DOI] [PubMed] [Google Scholar]
- 26. Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia 2005; 25: 82–86. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz PJ, De Ferrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 2008; 10: 884–891. [DOI] [PubMed] [Google Scholar]
- 28. Merrill CA, Jonsson MA, Minthon L, et al Vagus nerve stimulation in patients with Alzheimer's disease: additional follow‐up results of a pilot study through 1 year. J Clin Psychiatry 2006; 67: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 29. Sjogren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben‐Menachem E. Cognition‐enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: a pilot study. J Clin Psychiatry 2002; 63: 972–980. [DOI] [PubMed] [Google Scholar]
- 30. George MS, Ward HE Jr, Ninan PT, et al A pilot study of vagus nerve stimulation (VNS) for treatment‐resistant anxiety disorders. Brain Stimul 2008; 1: 112–121. [DOI] [PubMed] [Google Scholar]
- 31. Roslin M, Kurian M. VNS in the treatment of morbid obesity In: Schacter SC, Schmidt D, eds. VNS. Martin‐Dunitz: London, 2012: 113–121. [Google Scholar]
- 32. Ben‐Menachem E. Vagus‐nerve stimulation for the treatment of epilepsy. Lancet Neurol 2002; 1: 477–482. [DOI] [PubMed] [Google Scholar]
- 33. Handforth A, DeGiorgio CM, Schachter SC, et al Vagus nerve stimulation therapy for partial‐onset seizures: a randomized active‐control trial. Neurology 1998; 51: 48–55. [DOI] [PubMed] [Google Scholar]
- 34. Morris GL III, Mueller WM, the Vagus Nerve Stimulation Study Group E01‐E05 . Long‐term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology 1999; 53: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 35. Tatum WO, Moore DB, Stecker MM, et al Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology 1999; 52: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 36. Ali II, Pirzada NA, Kanjwal Y, et al Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav 2004; 5: 768–771. [DOI] [PubMed] [Google Scholar]
- 37. Ardesch JJ, Buschman HP, van der Burgh PH, Wagener‐Schimmel LJ, van der Aa HE, Hageman G. Cardiac responses of vagus nerve stimulation: intraoperative bradycardia and subsequent chronic stimulation. Clin Neurol Neurosurg 2007; 109: 849–852. [DOI] [PubMed] [Google Scholar]
- 38. Schuurman PR, Beukers RJ. Ventricular asystole during vagal nerve stimulation. Epilepsia 2009; 50: 967–968. [DOI] [PubMed] [Google Scholar]
- 39. Amark P, Stodberg T, Wallstedt L. Late onset bradyarrhythmia during vagus nerve stimulation. Epilepsia 2007; 48: 1023–1024. [DOI] [PubMed] [Google Scholar]
- 40. Borusiak P, Zilbauer M, Cagnoli S, Heldmann M, Jenke A. Late‐onset cardiac arrhythmia associated with vagus nerve stimulation. J Neurol 2009; 256: 1578–1580. [DOI] [PubMed] [Google Scholar]
- 41. Iriarte J, Urrestarazu E, Alegre M, et al Late‐onset periodic asystolia during vagus nerve stimulation. Epilepsia 2009; 50: 928–932. [DOI] [PubMed] [Google Scholar]
- 42. Ben‐Menachem E, Hellstrom K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 1999; 52: 1265–1267. [DOI] [PubMed] [Google Scholar]
- 43. Husain MM, Stegman D, Trevino K. Pregnancy and delivery while receiving vagus nerve stimulation for the treatment of major depression: a case report. Ann Gen Psychiatry 2005; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. CardioFit Pilot Study . Promising results from the CardioFit pilot study. http://www.biocontrol-medical.com/health_pros.php?ID=23 (accessed 02/10/2014).
- 45. Sabbah HN. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Clevel Clin J Med 2011; 78 (Suppl. 1): S24–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abraham WT, De Ferrari GM. Novel non‐pharmacological approaches to heart failure. J Cardiovasc Transl Res 2014; 7: 263–265. [DOI] [PubMed] [Google Scholar]
- 47. De Ferrari GM, Crijns HJ, Borggrefe M, et al Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32: 847–855. [DOI] [PubMed] [Google Scholar]
- 48. NEMOS® t‐VNS for treatment of drug‐resistant epilepsy. http://cerbomed.com/upload/Brochure_Epilepsy_Patients_EN.pdf (accessed 01/29/2014).
- 49. Stefan H, Kreiselmeyer G, Kerling F, et al Transcutaneous vagus nerve stimulation (t‐VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 2012; 53: e115–e118. [DOI] [PubMed] [Google Scholar]
- 50. Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception – an experimental study. Brain Stimul 2013; 6: 202–209. [DOI] [PubMed] [Google Scholar]
- 51. Napadow V, Edwards RR, Cahalan CM, et al Evoked pain analgesia in chronic pelvic pain patients using respiratory‐gated auricular vagal afferent nerve stimulation. Pain Med 2012; 13: 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nesbitt AD, Marin JCA, Tomkins E, Ruttledge MH, Goadsby PJ. Non‐invasive vagus nerve stimulation for the treatment of cluster headache: a cohort series with extended follow‐up [abstract; oral presentation]. Presented at Biennial World Congress of the International Neuromodulation Society, 8−13 June 2013, Berlin, Germany.
- 53. Nesbitt AD, Marin JCA, Tomkins E, Ruttledge MH, Goadsby PJ. Non‐invasive vagus nerve stimulation for the treatment of cluster headache: a cohort study [abstract P141]. Cephalalgia 2013; 33: 107. [Google Scholar]
- 54. Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K. Effect of noninvasive vagus nerve stimulation on acute migraine: an open‐label pilot study. Cephalalgia 2014; 34: 986–993. [DOI] [PubMed] [Google Scholar]
- 55. Moscato D, Moscato FR. Treatment of chronic migraine by means of vagal stimulator [abstract]. J Headache Pain 2013; 14 (Suppl): 56–57.23815607 [Google Scholar]
- 56. El Tahry R, Raedt R, Mollet L, et al A novel implantable vagus nerve stimulation system (ADNS‐300) for combined stimulation and recording of the vagus nerve: pilot trial at Ghent University Hospital. Epilepsy Res 2010; 92: 231–239. [DOI] [PubMed] [Google Scholar]
- 57. Ben‐Menachem E, Rydenhag B, Silander H. Preliminary experience with a new system for vagus nerve stimulation for the treatment of refractory focal onset seizures. Epilepsy Behav 2013; 29: 416–419. [DOI] [PubMed] [Google Scholar]
- 58. Hein E, Nowak M, Kiess O, et al Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm 2013; 120: 821–827. [DOI] [PubMed] [Google Scholar]
- 59. Nahas Z, Marangell LB, Husain MM, et al Two‐year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry 2005; 66: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 60. Siddiqui F, Herial NA, Ali II. Cumulative effect of vagus nerve stimulators on intractable seizures observed over a period of 3 years. Epilepsy Behav 2010; 18: 299–302. [DOI] [PubMed] [Google Scholar]
- 61. Ryzi M, Brazdil M, Novak Z, et al Long‐term vagus nerve stimulation in children with focal epilepsy. Acta Neurol Scand 2013; 127: 316–322. [DOI] [PubMed] [Google Scholar]


