Abstract
Recent investigations revealed mean diffusivity (MD) in gray matter and white matter areas is correlated with individual cognitive differences in healthy subjects and show unique properties and sensitivity that other neuroimaging tools donot have. In this study, we tested the hypothesis that the MD in the dopaminergic system is associated with individual differences in verbal creativity measured by divergent thinking (VCDT) and novelty seeking based on prior studies suggesting associations between these and dopaminergic functions. We examined this issue in a large sample of right‐handed healthy young adults. We used analyses of MD and a psychological measure of VCDT, as well as personality measures of the Temperament and Character Inventory (TCI). Our results revealed associations between higher VCDT and lower MD in the bilateral globus pallidus. Furthermore, not only higher novelty seeking, but also lower harm avoidance, higher self‐directedness, and higher self‐transcendence were robustly associated with lower MD in the right globus pallidus, whereas higher persistence was associated with lower MD in the left globus pallidus. These personality variables were also associated with VCDT. The globus pallidus receives the dopaminergic input from the substantia nigra and plays a key role in motivation which is critically linked to dopamine. These results suggested the MD in the globus pallidus, underlie the association between VCDT and multiple personalities in TCI including novelty seeking. Hum Brain Mapp 36:1808–1827, 2015. © 2015 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: mean diffusivity, creativity, divergent thinking, novelty seeking, harm avoidance, dopamine
INTRODUCTION
In diffusion tensor imaging (DTI), mean diffusivity (MD) characterizes the overall mean‐squared displacement of molecules (average ellipsoid size) and the overall presence of obstacles to diffusion [Le Bihan et al., 2001]. It increases with microscopic barrier disruption and extracellular fluid accumulation.
As summarized in our previous work [Takeuchi et al., 2011e], MD is often believed to be a measure of overall water content (or say, correlate with amount of water in the tissue) [Moseley et al., 2002]. Possible obstacles, such as the presence of fewer or smaller cellular structures (e.g., capillaries, synapses, and macromolecular proteins), may prevent free diffusion of water molecules and may also be expected to cause the value of MD to decrease [Ni et al., 2010]. In other words, MD decreases are likely to reflect an increase in tissue density due to the shape of neurons or glia, enhancement of tissue organization (strengthening of axonal or dendritic backbones and surrounding tissue) [Assaf and Pasternak, 2008]. Further, intervention paradigms that caused these tissue density increases, decreases MD [Sagi et al., 2012] and intervention paradigms that caused the synaptic decreases, increases MD [Abe et al., 2014], thus confirming these ideas. In addition, a wide range of clinical studies of neuronal degeneration have shown an increase in MD, which also confirmed this idea [Andreone et al., 2007; Kantarci et al., 2001; Nusbaum et al., 2001]. Given that these tissue changes are caused by neural plasticity and lead to neural function and cognitive changes [Hillman et al., 2008], and that individual differences in some of these tissue components are thought to underlie individual cognitive differences [Kanai and Rees, 2011], MD differences may underlie individual cognitive differences. Further, recent investigation revealed MD in gray matter and white matter areas is correlated with cognitive functions in young and healthy subjects which are unlikely to have neuronal degeneration so much [Fjell et al., 2012; Piras et al., 2010]. Further, not only cognitive functions, but also temperaments such as novelty seeking and harm avoidance have been shown to correlate with MD in gray matter and white matter areas [Bjørnebekk et al., 2012; Laricchiuta et al., 2014; Picerni et al., 2013].
Conversely, MD in the dopaminergic system's areas (MDDS) has been shown to be associated with several conditions that are known to be the altered dopaminergic system. Recently, MDDS has been shown to be more sensitive to the pathology of the dopaminergic system (Parkinson's disease) that cannot be detected by other magnetic resonance imaging (MRI) measures, such as brain structure volume, a measure sensitive to iron deposition, fractional anisotropy measures of DTI as well as measures of dopamine receptor binding of positron emission tomography (PET) [Péran et al., 2010; Seppi et al., 2004]. MDDS can also detect neural plasticity caused by treatment of this pathology with medication including dopamine agonists [Razek et al., 2011]. MDDS also showed a robust association with the motivational state [Takeuchi and Kawashima, 2013d], which has been robustly associated with the function of the dopaminergic system [Kaplan and Oudeyer, 2007]. Thus, somehow differences in MDDS seem to be linked with conditions associated with differences in the dopaminergic system.
Conversely, we and other laboratories have investigated neural correlates of individual differences of creativity measured by divergent thinking test (CDT; information retrieval and the call for a number of varied responses to a certain item [Guilford, 1967]). These include gray matter volume, fractional anisotropy (white matter structural integrity), resting state functional connectivity, resting state cerebral blood flow, brain activity during tasks [Jung et al., 2009, 2010a, b; Takeuchi et al., 2010b, c; 2011a, c, 2012a; Wei et al., 2014]. These include DTI indices in white matter areas [Jung et al., 2010a; Takeuchi et al., 2010c]. But so far, it has not been revealed the gray matter's MD correlates of CDT. Considering, MD has been shown to be correlated with temperaments that correlate with CDT such as novelty seeking and harm avoidance [Chavez‐Eakle et al., 2006], CDT may well correlate with MD. Further, other than studies described above, recent investigations, increasingly revealed gray matter's MD's unique importance, due to its reflection of physiological property [Sagi et al., 2012] as well as higher sensitivity toward neuropathology than other imaging tools [Péran et al., 2010].
Previous multiple review literatures as well as our studies suggested dopaminergic systems play a key role in creativity [Flaherty, 2005; Heilman et al., 2003; Takeuchi et al., 2010b], and creativity is associated with conditions that have been suggested to be associated with altered dopaminergic functions, such as novelty seeking. As summarized similarly in our previous study [Takeuchi et al., 2013b], evidence linking creativity and dopamine can be classified as (a) associations between creativity and schizotypy [Cooper, 1998; Eysenck and Furnham, 1993; Kline and Cooper, 1986; O'Reilly et al., 2001], which is associated with dopamine‐related genes [Ettinger et al., 2006] and overactivity of subcortical dopaminergic systems [Kirrane and Siever, 2000], (b) positive associations between CDT and novelty seeking [Chavez‐Eakle et al., 2006], while novelty seeking have been positively associated with dopaminergic functions [Bódi et al., 2009; Kaasinen et al., 2004; Schinka et al., 2002; Suhara et al., 2001; Tomer and Aharon‐Peretz, 2004], (c) associations between creativity and motivation that are not caused by external incentives [Prabhu et al., 2008], which has been associated with dopaminergic functions [Kaplan and Oudeyer, 2007], (d) associations between CDT and extraversion [King et al., 1996], which has been associated with dopaminergic functions [Ashby and Isen, 1999; Depue and Collins, 1999], (e) dopamine's antagonist's effects to suppress creativity [Flaherty, 2005] as well as dopamine's effects to suppress latent inhibition (a behavioral index of the ability to habituate to sensations)[Ellenbroek et al., 1996; Swerdlow et al., 2003], while reduced latent inhibition is associated with creativity among intelligent subjects [Carson et al., 2003], (f) the association between dopamine related gene and CDT [Mayseless et al., 2013], and (g) reduced CDT in the pathology of the dopaminergic system [Drago et al., 2009] as well as recovery of artistic creativity in response to dopaminergic agonist [Kulisevsky et al., 2009].
Thus, we hypothesized MD in the regions of the dopaminergic system would correlate with creativity and these have common overlaps of correlates of temperament that associate with creativity and dopaminergic functions (namely, novelty seeking). In this study, we aimed to test these hypotheses and investigate the associations between verbal creativity measured by divergent thinking (VCDT) as well as the temperament that associate with creativity and MD, particularly MDDS. Using data from a large sample, we answered this question directly using data of VCDT, and also data of dopamine‐related temperament [Tomer and Aharon‐Peretz, 2004] to determine whether any of these factors was associated with MD and how they were associated using path analyses.
METHODS
Subjects
Eight hundred ninety‐five healthy, right‐handed individuals (507 men and 388 women) participated in this study as part of our ongoing project to investigate the associations among brain imaging, cognitive functions, aging, genetics, and daily habits [Sassa et al., 2012; Takeuchi et al., 2011d, e, 2012b, 2013a; Taki et al., 2011a, c]. Some of the data from these 895 subjects were used in our previous study investigating the association between VCDT and neural mechanisms [Takeuchi et al., 2010b, c, 2011a, c, 2012b]. Some of the subjects who took part in this study also became subjects of our intervention studies (psychological data and imaging data recorded before the intervention were used in this study) [Takeuchi et al., 2011b, f, 2012]. Psychological tests and MRI scans not described in this study were performed together with those described in this study. The mean age of subjects was 20.8 years (standard deviation [SD], 1.8). All subjects were university students, postgraduates, or university graduates of less than one year's standing. All subjects had normal vision and none had a history of neurological or psychiatric illness. Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971]. Written informed consent was obtained from each subject. For nonadult subjects, written informed consent was obtained from their parents (guardians). This study was approved by the Ethics Committee of Tohoku University.
Assessment of Psychometric Measures of General Intelligence
Raven's Advanced Progressive Matrix (RAPM) [Raven, 1993] has been the psychometric measure shown to be most correlated with general intelligence, making it the best measure of general intelligence. Data for this test were collected from all 895 subjects. The score of this test (number of correct answers in 30 min) was used as a psychometric index of individual intelligence. The RAPM was administered in a group setting in this study. The RAPM tests can be administered individually by a psychologist or trained test administrator, or administered on a group basis [Raven, 1993]. For more details of how RAPM was performed in our study, see our previous works [Takeuchi et al., 2010b, c].
Creativity Assessment
As was the case with our previous studies [Takeuchi et al., 2010b, c, 2011a, c, 2012b], the S–A creativity test [Society_For_Creative_Minds, 1969] was used to assess VCDT. Guilford generated the draft plan and supervised the development of the test, after which the test was standardized for Japanese speakers [Society_For_Creative_Minds, 1969].
As described in our previous studies [Takeuchi et al., 2010b, c], the test is used to evaluate verbal creativity through DT [Society_For_Creative_Minds, 1969] and it involves three types of tasks. Each task is preceded by 2 min of practice involving two questions with a 5‐min time limit. The first task requires subjects to generate unique ways of using typical objects (e.g., “Other than reading, how can we use newspapers?” An example answer is “We can use them to wrap things”). The second task requires subjects to imagine desirable functions of ordinary objects (e.g., “What are the characteristics of a good TV? Write down as many characteristics as possible.” An example answer is “A TV that can receive broadcasts from all over the world”). The third task requires subjects to imagine the consequences of “unimaginable things” happening (e.g., “What would happen if all the mice in the world disappeared?” An example answer is “The world would become more hygienic”). Each task requires subjects to generate as many answers as possible. The S–A creativity test provides a total creativity score, which was used in this study, as well as scores for the following dimensions of the creative process: (a) Fluency—Fluency is measured by the number of relevant responses to questions and is related to the ability to produce and consider many alternatives. Fluency scores are determined by the total number of questions answered after excluding inappropriate responses or responses that are difficult to understand. (b) Flexibility—Flexibility is the ability to produce responses from a wide perspective. Flexibility scores are determined by the sum of the (total) number of category types that responses are assigned based on a criteria table or an almost equivalent judgment. (c) Originality—Originality is the ability to produce ideas that differ from those of others. Originality scoring is based on the sum of idea categories that are weighted based on a criteria table or an almost equivalent judgment. (d) Elaboration—Elaboration is the ability to produce detailed ideas [Society_for_Creative_Mind, 1969]. Elaboration scores are determined by the sum of responses that are weighted based on a criteria table or an almost equivalent judgment. These four dimensions correspond to the same concepts as those of the Torrance tests of creative thinking [Torrance, 1966].
As described in our previous studies [Takeuchi et al., 2010b, c], the total creativity score is the sum of the score of originality and that of elaboration in the version of the S–A creativity test [Society_For_Creative_Minds, 1969] that we used (for explanations of test versions, please see Supporting Information Methods). This is because the scores for Fluency and Flexibility are highly correlated with those for Elaboration [Society_For_Creative_Minds, 1969]. We used only the total score in this study (see Supporting Information Methods for the reasons). Scoring of the tests was performed by the Tokyo Shinri Corporation. Please refer to the appendix of our previous study [Takeuchi et al., 2010b, c] for the sample and the manner in which the tests were scored.
As described in our previous study [Takeuchi et al., 2010c], scores of the S–A creativity test have been shown to be significantly correlated with various other external measures such as various personality factors and problem solving abilities in daily life, suggesting its ability to predict performance in everyday situations [Shimonaka and Nakazato, 2007]. Furthermore, scores of the S–A creativity test, have been shown to be significantly correlated with frequency of visual hypnagogic experiences which in turn correlated with the vividness of mental imagery and neuroticism [Watanabe, 1998]. Furthermore, our previous study showed that scores on the S–A creativity test were positively correlated with extraversion, novelty seeking, motivational state, and daily physical activity level, which is consistent with the findings obtained based on the other creativity measures of DT [Takeuchi et al., 2013b]. Data for this test were collected from all 895 subjects.
Assessment of Temperaments
The personality traits of each subject were measured using the Japanese version of the Temperament and Character Inventory (TCI) [Cloninger et al., 1993; Kijima et al., 1996]. Each subject completed a 240‐item TCI questionnaire. Scores for four personality traits were calculated from the responses to these items: novelty seeking, harm avoidance, reward dependence, persistence, self‐directedness, self‐transcendence, and cooperativeness. We analyzed personalities other than novelty seeking to investigate the specificity of the results.
Data for this test were collected from 776 subjects. Cronbach coefficients alpha of the scale scores of Japanese version of TCI were substantially high and construct validity has been shown [Kijima et al., 2000].
Behavioral Data Analysis
The behavioral data were analyzed using SPSS 22.0 statistical software (SPSS, Chicago, IL). Sex differences in demographic variables were tested using two‐tailed t‐tests. In each analysis, P < 0.05 was considered statistically significant. Associations among demographic variables were analyzed using multiple regression analyses; P < 0.05, in each analysis was considered statistically significant in these analyses.
Image Acquisition and Analysis
All MRI data acquisition was conducted with a 3‐T Philips Achieva scanner. Diffusion‐weighted data were acquired using a spin‐echo echoplanar imaging (EPI) sequence [repetition time (TR) = 10,293 ms, echo time (TE) = 55 ms, field of view (FOV) = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices, SENSE reduction factor = 2, number of acquisitions = 1] with an 8‐ch head‐coil. The diffusion weighting was isotropically distributed along 32 directions (b value = 1,000 s/mm2). In addition, three images with no diffusion weighting (b value = 0 s/mm2; b = 0 images) and one b = 0 image were acquired from 768 and 127 subjects, respectively, using a spin‐echo EPI sequence (TR = 10,293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices). There are acquisitions for phase correction and for signal stabilization and these are not used as reconstructed images. Fractional anisotropy (FA) and MD maps were calculated from the collected images using a commercially available diffusion tensor analysis package on the MR consol as has been done in previous studies [Takeuchi et al., 2010a, c, 2011e, 2013a, c] and this procedure generates results congruent with previous studies [Taki et al., 2011c]. Calculations were performed according to a previously proposed method [Le Bihan et al., 2001].
Preprocessing of Structural Data
Preprocessing of the structural data was performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks, Natick, MA). First, using the previously validated [Takeuchi et al., 2013c] twisted methods and the new segmentation algorithm implemented in SPM8 and the information of both FA images and MD images, we segmented FA images and MD images of subjects (see Supporting Information Methods for details and this previous study for the validation). Then using the diffeomorphic anatomical registration through exponentiated lie algebra registration process implemented in SPM8, raw FA images, MD images, the GM segmentation map [GM concentration (density or segmentation) (GMC) map], the WM segmentation map [WM concentration (density, or segmentation) (WMC) map], and the cerebral spinal fluid (CSF) segmentation map [CSF concentration (density, or segmentation (CSFC) map] from the aforementioned second new segmentation process were normalized to give images with 1.5 × 1.5 × 1.5 mm3 voxels. Subsequently, from the normalized images of the (a) MD map, (b) GMC map, (c) WMC map, and (d) CFSC map, areas that were not strongly likely to be gray matter or white matter in our custom template (defined by “gray matter tissue probability + white matter tissue probability < 0.99” in the template) were removed (to exclude the strong effects of CSF on MD). These images were then smoothed by convolving them with an isotropic Gaussian kernel of 6‐mm full width at half maximum. For more details of these procedures, see Supporting Information Methods.
Whole‐Brain Statistical Analysis
Models of whole‐brain multiple regression analyses for investigating the effects of individual cognitive differences on MD
We performed multimodality voxelwise multiple regression analyses that adjusted for the effects of GMC, WMC, and CSFC to investigate associations between MD and the psychological variables. To perform these analyses, we used the biological parametric mapping toolbox of SPM5 [Casanova et al., 2007]. We performed eight whole‐brain multiple regression analyses. In all of these analyses, the dependent variable at each voxel was the MD value at that voxel and the independent variables included the GMC value, WMC value, and CSFC value at that voxel, as well as the number of b = 0 images, age, sex, and RAPM score. In addition, each multiple regression analysis included one of the following variables as an independent variable: S–A creativity test score, novelty‐seeking score, harm avoidance score, reward dependence score, persistence score, self‐directedness score, self‐transcendence score, and cooperativeness score. The analyses including the S–A creativity test scores were performed using data from 895 subjects. The analyses that included the personality scores were performed using data from 776 subjects. For the reasons why GMC, WMC, and CSFC were adjusted in a voxel‐by‐voxel manner, why it was not appropriate to include eight variables in one model, and why the number of b = 0 images was included in the model, see Supporting Information Methods.
Models of whole‐brain analyses of covariance for investigating the effects of interaction between sex and individual cognitive differences on MD
Next, we investigated whether the relationships between MD and the S–A creativity test score as well as that between MD and the seven personality scores differed between sexes (i.e., whether the interaction between sex and each of these scores affected MD). For these investigations, in whole‐brain analyses, we used voxelwise analyses of covariance (ANCOVAs). We performed eight whole‐brain ANCOVAs. In all of these ANCOVAs, sex difference was a group factor (using the full‐factorial option of SPM8). Furthermore, in these ANCOVAs, covariates included age, RAPM score, the number of b = 0 images (1 or 3), and one of the following variables, the S–A creativity test score, novelty‐seeking score, harm avoidance score, reward dependence score, persistence score, self‐directedness score, self‐transcendence score, and cooperativeness score. All these covariates, with the exception of the number of b = 0 images, were modeled so that each covariate's unique relationship with MD could be observed for each sex (using the interactions option of SPM8), which would allow the investigation of the interaction effects between sex and the covariates. The interaction effect between sex and scores of the cognitive measures of interest were assessed using t‐contrasts. In these interaction analyses, we used SPM8 instead of BPM (for reasons and considerations for the use of this method, see Supporting Information Methods). Analyses were limited to the gray + white matter mask created as described above.
Statistical thresholds for whole‐brain analyses
In all the whole‐brain analyses, we used cluster size‐based corrections for multiple comparisons (standard default cluster‐size tests in SPM). The voxel‐level cluster‐determining threshold is P < 0.001, uncorrected in all these analyses.
In the whole brain multiple regression analyses of the study's main focus and hypothesis (namely a negative correlation between MD and VCDT or novelty seeking), only clusters with P < 0.05 after correcting for multiple comparisons (controlling for familywise error) at the cluster size were considered statistically significant. For the remaining 14 contrasts of the whole‐brain multiple regression analyses, only clusters with P < 0.0035 (∼0.05/14) after correcting for multiple comparisons (controlling for familywise errors) at the cluster size were considered statistically significant to avoid Type I errors, as many statistical tests are available.
All whole‐brain ANCOVAs were exploratory and were not relevant to the main hypothesis of this study. Thus, for 16 contrasts of ANCOVAs, only clusters with P < 0.0031 (approximately 0.05/16) after correction for multiple comparisons (controlling for familywise errors) at the cluster size were considered statistically significant to avoid Type I errors for the same reasons as described above.
Analyses of Linear and Nonlinear Associations Between VCDT and MD in Regions of Interest
Subsequently, in the analyses of VCDT, regions of interest (ROI) analyses were applied to the main areas of the subcortical dopaminergic areas. These areas included the bilateral caudate, bilateral putamen, bilateral globus pallidus, and bilateral substantia nigra. We used SPSS 22.0 for these analyses. For the reasons for performing ROI analyses, see Supporting Information Methods.
Constructions for ROI
All ROIs were constructed using the WFU PickAtlas Tool (http://www.fmri.wfubmc.edu/cms/software#PickAtlas) [Maldjian et al., 2003, 2004]. The mask images of the ROIs were generated using the Brodmann area option of the PickAtlas Tool.
Mean value extraction from ROI
Subsequently, the mean MD, GMC, WMC, and CSFC values of these images were extracted from the aforementioned normalized images. Here, we limited the areas to extract these values from within the areas that showed “gray matter tissue probability + white matter tissue probability > 0.999” in the custom template mentioned above. A stricter threshold was chosen because we cannot remove the effects of GMC, WMC, and CSFC in the nonlinear analyses described below, as we cannot add those values as covariates.
Rationale and models for nonlinear analyses
First, we performed nonlinear ROI analyses considering a possible inverted U‐shaped association between dopamine level and creativity [Chermahini and Hommel, 2010, 2012]. As reported in our previous article [Taki et al., 2011b], to analyze whether the linear or quadratic function fits best the trajectory of MD with creativity, the correlation between MD and creativity was estimated using linear and quadratic functions in each ROI. These analyses were performed without covariates owing to technical limitations.
Statistical evaluation for the results of nonlinear analyses
We determined the best‐fit model by selecting the function that showed the smallest Akaike information criteria (AIC) [Akaike, 1974]. Information regarding the best‐fit model can be obtained based on AIC, whereas information regarding statistical significance cannot be obtained using this approach. However, as a reference, we described the P value for the significance level of the best‐fit model in Table 3. We did not assess the significance level or performed corrections for multiple comparisons here, as it is difficult to evaluate those parameters (multiple ROIs, testing for two models for each ROI, correlation between the two models, etc.). Moreover, these nonlinear analyses are unable to adjust the effects of basic variables such as age and sex.
Table 3.
Association between S–A creativity test score and mean MD in ROIs of the areas that belong to the dopaminergic system
| Areas | Best‐fit modela | Adjusted R 2 | P value of the best‐fit model (df) | Statistical values of the multiple regression (uncorrected P value, corrected P value, df) |
|---|---|---|---|---|
| Left putamen | Linear, negative | 0.002 | 0.110 (893) | 0.095, 0.099, 886 |
| Right putamen | Linear, negative | 0.001 | 0.150 (893) | 0.028, 0.058, 886 |
| Left globus pallidus | Linear, negative | 0.010 | 0.001 (893) | 0.003, 0.016, 886 |
| Right globus palidus | Linear, negative | 0.004 | 0.028 (893) | 0.005, 0.016, 886 |
| Left caudate | Linear, negative | <0.001 | 0.390 (893) | 0.110, 0.099, 886 |
| Right caudate | Quadratic, negative | 0.001 | 0.289 (892) | 0.096, 0.099, 886 |
| Left substantia nigra | Linear, negative | 0.001 | 0.209 (893) | 0.110, 0.099, 886 |
| Right substantia nigra | Quadratic, negative | <0.001 | 0.335 (892) | 0.284, 0.224, 886 |
Best‐fit model of the correlation between S–A creativity test score and mean MD in ROIs determined using the Akaike Information Criterion.
Linear multiple regression analyses of the ROI
After confirming the absence of a significant quadratic association between MD and VCDT, we performed ROI analyses of multiple linear regressions. In each of these analyses, the dependent variables were the mean MD value within each ROI, and the independent variables included the mean GMC value, mean WMC value, and mean CSFC value of the corresponding ROI as well as the number of b = 0 images, age, sex, RAPM score, and S–A creativity test score. Using the multiple regression analyses, we can adjust the effects of these variables.
Statistical thresholds for linear multiple regression analyses
Here, we applied one‐tailed analyses, because our hypotheses or study background did not include any data that suggested the possibility of positive associations between MD and VCDT. In these analyses, results with a threshold of P < 0.05, corrected for false discovery rate using the two‐stage sharpened method [Benjamini et al., 2006], were considered statistically significant. The correction for multiple comparisons using this method was applied to the results of the eight ROI analyses of multiple regressions mentioned above.
Path Analyses of the Associations Between MD in the Bilateral Globus Pallidus, Personalities, and VCDT
Rationales for the path analyses and variables
The results of the analyses described above suggest the overlap between MD correlates of personalities and those of VCDT in the bilateral globus pallidus (see Results section). Accordingly, we next proceeded to the path analyses using structural equation modeling (SEM), to identify the association among these variables. Here we performed two analyses. One analysis involved MD in the left globus pallidus as well as S–A creativity test score and persistence score, both of which proved to be correlated with MD in the left globus pallidus (see Results section). The other analysis involved MD in the right globus pallidus as well as S–A creativity test score and novelty‐seeking score, harm‐avoidance score, self‐directedness score, and self‐transcendence score, all of which correlated with MD in the right globus pallidus (see Results section).
Basic methods for path analyses
SEM was performed as similarly described in a previous study [Charlton et al., 2008]. SEM was performed using the Amos software (version 22.0, IBM, SPSS). Intercepts were allowed in the structural equations, and models were fitted using maximum likelihood methods.
Preconditions for the construction of initial models
Based on a literature review, we made a few assumptions to construct the initial models. First, as described in the previous study [Charlton et al., 2008], we assumed that MD affects the cognitive variables, and not the other way around. Second, we assumed that personality variables affect VCDT, and not the other way around [Takeuchi et al., 2013b].
Evaluations and improvement of models in the path analyses
Subsequently, as described in the previous study [Charlton et al., 2008], we considered whether the paths of relatively complex literature‐derived models shown in Figures 1 and 2 could be reduced by removing pathways that lacked statistically significant associations and the models could be improved. For the path analysis involving MD in the right globus pallidus, there were 64 initial models, as we could not presume the direction of paths between personalities (Fig. 2). To obtain a better model, we used stepwise removal or alternation procedure that fitted the model, as described in the previous studies [Charlton et al., 2008; Fjell et al., 2012]. The models were evaluated by comparing the fit of nested models that included and excluded a path in question and using AIC and statistics of fitness. Once a final model was obtained, regression coefficients were estimated for all the remaining paths. To check that the final model fitted the data adequately, two verifications were performed: (a) a test to check for the lack of fit was performed using χ 2 statistics and (b) the following fit indices were calculated: AIC, the comparative fit index (CFI), and the root mean square error of approximation (RMSEA). Based on the initial models, the paths with the highest P value were deleted recursively one‐by‐one, and the analyses were rerun after each path was removed until the model fit stopped improving.
Figure 1.
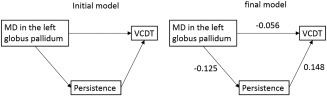
Path analysis involving MD in the left globus pallidus. Path analysis was used to test the nature of the relationships among MD in the globus pallidus, persistence, and VCDT. The model shown on the left is the initial model. It was planned that the paths with the highest P value were deleted recursively one‐by‐one, and that the analyses were rerun after each path was removed. The model that included all the paths remained the ones that showed the best fit. However, the direct path from MD in the left globus pallidus to VCDT did not reach significance (P = 0.117). Standardized regression weights for the significant paths are shown next to each path arrow.
Figure 2.
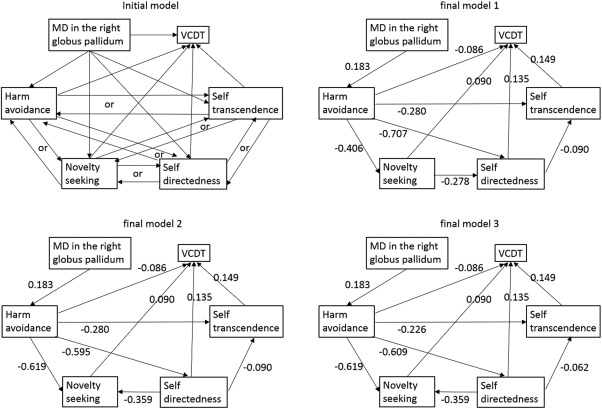
Path analysis involving the MD in the right globus pallidus. Path analysis was used to test the nature of the relationships among MD in the globus pallidus, harm avoidance, novelty seeking, self‐directedness, self‐transcendence, and VCDT. The model shown on the upper left is the initial model. Depending on the directionality of the paths among personalities, there were 64 initial models. From each initial model, the paths with the highest P value were deleted recursively one‐by‐one, and the analyses were rerun after each path was removed until the model fit stopped improving. Three of the final models showed the exact same statistics for the fit of the model. However, in all final models, the path from the harm avoidance to VCDT did not reach significance (P = 0.087), despite the fit of the model. Standardized regression weights for the significant paths are shown next to each path arrow.
RESULTS
Behavioral Data
Table 1 shows the average and SD of age and RAPM, S–A creativity, novelty seeking, harm avoidance, reward dependence, persistence, self‐directedness, self‐transcendence, and cooperativeness scores of the males and females included in our sample. Two‐tailed t‐tests showed that females had a significantly higher score on S–A creativity, reward dependence, self‐directedness, cooperativeness, and self‐transcendence, whereas males had a significantly higher score on RAPM. There were no other significantly different results. The results of these two‐tailed t‐tests are presented in Table 1.
Table 1.
Demographic variables of the study participants and results of the comparison between males and females using two‐tailed t‐tests
| Measure | Males | Females | P value | T value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (N = 895, M = 507, F = 388) | 20.89 | 1.94 | 20.72 | 1.69 | 0.151 | 1.438 |
| RAPMa (N = 895, M = 507, F = 388) | 28.77 | 3.72 | 28.16 | 3.73 | 0.016 | 2.423 |
| S–A creativity test (N = 895, M = 507, F = 388) | 36.28 | 10.66 | 38.58 | 9.77 | 0.001 | −3.352 |
| Novelty seeking (N = 776, M = 432, F = 344) | 21.01 | 5.56 | 21.04 | 5.76 | 0.948 | −0.065 |
| Harm avoidance (N = 776, M = 432, F = 344) | 18.91 | 7.51 | 19.31 | 6.57 | 0.434 | −0.783 |
| Reward dependence (N = 776, M = 432, F = 344) | 14.40 | 3.80 | 16.20 | 3.68 | 5.004 × 10−11 | −6.669 |
| Persistence (N = 776, M = 432, F = 344) | 4.53 | 2.04 | 4.56 | 1.96 | 0.818 | −0.231 |
| Self‐directedness (N = 776, M = 432, F = 344) | 23.42 | 7.26 | 24.53 | 6.76 | 0.028 | −2.196 |
| Cooperativeness (N = 776, M = 432, F = 344) | 26.25 | 5.94 | 28.37 | 6.01 | 1.168 × 10−6 | −4.902 |
| Self‐transcendence (N = 776, M = 432, F = 344) | 10.42 | 5.15 | 11.67 | 5.32 | 0.001 | −3.297 |
Raven's Advanced Progressive Matrices
After adjusting for the effects of age and sex, no significant correlations between RAPM and S–A creativity scores were observed among the 895 subjects (P = 0.607, t = 0.514, df = 891). Multiple regression analyses that used covariates of age, sex, and RAPM score and which tested the association between two variables among S–A creativity test, novelty seeking, harm avoidance, reward dependence, persistence, self‐directedness, cooperativeness, and self‐transcendence scores showed the presence of several significant correlations between these variables (see Table 2 for statistical results). In particular, the S–A creativity test score was significantly and positively correlated with the novelty‐seeking score, persistence score, cooperativeness score, and self‐transcendence score. Furthermore, the S–A creativity test score was significantly and negatively correlated with the harm avoidance score.
Table 2.
Matrix of statistical results (beta value, t value, P value, beginning at the top) of the multiple regression analyses performed between psychological variables and the covariates of age, sex, and score of RAPM (N = 776, df = 771)
| S–A creativity test | Novelty seeking | Harm avoidance | Reward dependence | Persistence | Self‐directedness | Cooperativeness | Self‐transcendence | |
|---|---|---|---|---|---|---|---|---|
| S–A creativity test | — | — | — | — | — | — | — | — |
| Novelty seeking | (0.142, 3.988, 7.30 × 10−5) | — | — | — | — | — | — | — |
| Harm avoidance | (−0.234, −6.564, 9.61 × 10−11) | (−0.407, −12.051, 9.18 × 10−31) | — | — | — | — | — | — |
| Reward dependence | (0.067, 1.824, 0.069) | (0.104, 2.819, 0.005) | (−0.089, −2.463, 0.014) | — | — | — | — | — |
| Persistence | (0.153, 4.289, 2.02 × 10−5) | (−0.182, −5.127, 3.72 × 10−7) | (−0.158, −4.540, 6.52 × 10−6) | (0.121, 3.486, 6.52 × 10−4) | — | — | — | |
| Self‐directedness | (0.184, 5.144, 3.41 × 10−7) | (−0.007, −0.186, 0.852) | (−0.579, −19.976, 7.21 × 10−72) | (0.098, 2.796, 0.005) | (0.270, 7.694, 4.36 × 10−14) | — | — | — |
| Cooperativeness | (0.084, 2.314, 0.021) | (−0.071, −1.934, 0.054) | (−0.306, −8.966, 2.30 × 10−18) | (0.415, 12.838, 2.51 × 10−34) | (0.186, 5.179, 1.79 × 10−28) | (0.385, 11.527, 4.36 × 10−14) | — | — |
| Self‐transcendence | (0.189, 5.299, 1.52 × 10−7) | (0.142, 3.932, 9.17 × 10−5) | (−0.243, −7.051 3.96 × 10−12) | (0.204, 5.915, 4.98 × 10−9) | (0.194, 5.462, 6.37 × 10−8) | (0.070, 1.957, 0.051) | (0.299, 8.790, 9.62 × 10−18) | — |
Whole Brain Analyses of Correlations Between VCDT and MD
After controlling for sex, age, number of b = 0 images, GMC, WMC, CSFC at each voxel, and RAPM score, a whole brain multiple regression analysis showed that the S–A creativity score was not significantly related to any of the regions (df = 886).
Investigation of the Linear and Nonlinear Associations Between VCDT and MD in the ROI
We investigated whether linear or quadratic functions fit better the association between S–A creativity test score and MD in ROIs. The associations between S–A creativity test score and MD in the areas of the dopaminergic system were best explained by the linear model that included the bilateral globus pallidus, in which the association became significant. When the quadratic model fit best, the P value was well above 0.1 (Table 3).
The present findings may be incongruent with the suggested inverted U‐shaped association between creativity and dopamine level [Chermahini and Hommel, 2010, 2012]. However, the alternative explanation is that an excessive level of dopamine neurotoxicity [Cheng et al., 1996], which may damage the neural tissues and increase MD, results in an association between MD and dopamine level that is not linear. Future studies involving very high and very low levels of dopamine, as measured using PET, may be needed to ascertain these suggestions and possibilities more firmly.
After confirming the lack of existence of a significant quadratic association between MD and VCDT, we performed ROI analyses of multiple linear regressions. After correcting for confounding variables and for multiple comparisons, the S–A creativity test score was significantly and negatively correlated with MD in the bilateral pallidus (Fig. 3 and Table 3).
Figure 3.
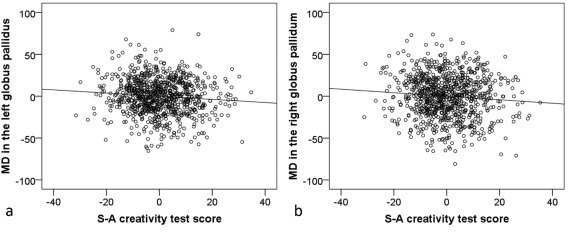
Associations between mean MD in ROIs and the S–A creativity test score. Residual plots with trend lines depicting the correlations between residuals in the multiple regression analyses using mean MD in the ROIs as the dependent variable and eight additional variables (RAPM score, total S–A creativity test score, age, sex, number of b = 0 images, mean GMC, mean WMC, and mean CSFC) as independent variables. (a) Association between the mean MD in the left globus pallidus and the S–A creativity score. (b) Association between mean MD in the right globus pallidus and the S–A creativity score.
Whole Brain Analyses of Correlations Between Personalities and MD
Next, we investigated the association between personality traits and MD. After controlling for sex, age, number of b = 0 images, GMC, WMC, CSFC at each voxel, and RAPM score, a whole brain multiple regression analysis showed that the novelty‐seeking score was significantly and negatively correlated with MD in the cluster that spread around the right anterior cingulate cortex, right middle cingulate cortex, and right corpus callosum; the cluster in the right cerebellum; and the cluster that spread around the right caudate, right putamen, right globus pallidus, and right insula (Fig. 4 and Table 4).
Figure 4.
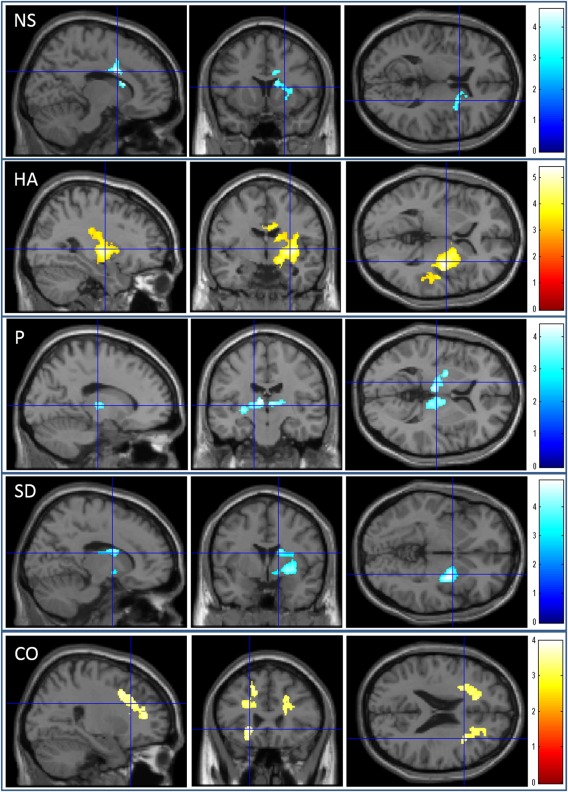
Regions with significant correlations between MD and the personality trait scores. The results shown were obtained using a threshold of the corrected cluster size tests and with an underlying voxel level of P < 0.001 uncorrected. Regions of significant correlation are overlaid on a “single subject” T1 SPM5 image. NS = novelty seeking. HA = harm avoidance. P = persistence. SD = self‐transcendence. CO = cooperativeness. The red color represents positive correlations. The blue color represents negative correlations. The color bars indicate the t scores. Regions that exhibited a significant negative correlation with novelty seeking were observed in the right basal ganglia, right anterior cingulate cortex, and adjacent areas. Regions that exhibited a significant positive correlation with harm avoidance were observed in the right basal ganglia, right middle cingulate cortex, and right posterior insula. Regions that exhibited a significant negative correlation with persistence were observed in the bilateral thalamus, left globus pallidus, and left putamen. Regions that exhibited a significant negative correlation with self‐transcendence were observed in the right basal ganglia, right insula, and adjacent regions. Regions that exhibited a significant positive correlation with cooperativeness were observed in the areas located around the anterior cingulate cortex, dorsolateral prefrontal cortex, insula, and orbitofrontal cortex in the left hemisphere, and the areas located around the anterior cingulate cortex and dorsolateral prefrontal cortex in the right hemisphere.
Table 4.
Brain regions that exhibited significant correlations between MD and the temperament subscales of TCI (df = 767)
| Area | x | y | z | T score of the peak | Corrected P value (cluster) |
|---|---|---|---|---|---|
| Negative correlation of novelty seeking | |||||
| Anterior cingulate cortex (R)/Middle cingulate cortex (R)/Body of the corpus callosum (R) | 13.5 | 7.5 | 28.5 | 4.58 | <0.001 |
| Cerebellum (R) | 33 | −48 | −30 | 4.37 | 0.002 |
| Caudate body and head (R)/Putamen(R)/Globus pallidus (R)/Insula(R) | 13.5 | 15 | 15 | 4.37 | <0.001 |
| Positive correlation of harm avoidance | |||||
| Caudate body and head (R)/Putamen(R)/Globus pallidus (R)/Insula(R)/Thalamus(R) | 34.5 | −9 | 1.5 | 5.38 | <0.001 |
| Middle cingulate cortex (R)/Body of the corpus callosum (R) | 12 | −10.5 | 30 | 4.30 | <0.001 |
| Insula (R)/Superior temporal gyrus (R) | 48 | −18 | 6 | 4.09 | <0.001 |
| Negative correlation of Persistence | |||||
| Thalamus (L)/Globus palludus (L)/Putamen (L) | −7.5 | −16.5 | 9 | 4.36 | <0.001 |
| Thalamus (R) | 12 | −24 | 6 | 3.99 | <0.001 |
No other significant results were observed. Clusters mostly included intermediate white matter areas.
The whole brain analyses that were performed using the same nuisance covariates revealed that the harm avoidance score was significantly and positively correlated with MD in the cluster that spread around the right caudate, right putamen, right globus pallidus, right insula, and right thalamus; the anatomical cluster that spread around the right middle cingulate cortex and the body of the right corpus callosum; and the anatomical cluster that spread around the right posterior insula and the right superior temporal gyrus (Fig. 4 and Table 4).
The whole brain analyses that were performed using the same nuisance covariates revealed that the reward dependence score was not significantly correlated with MD.
The whole brain analyses that were performed using the same nuisance covariates revealed that the persistence score was significantly and negatively correlated with MD in the anatomical cluster that spread around the left thalamus, left globus pallidus, and left putamen and the anatomical cluster in the right thalamus (Fig. 4 and Table 4).
The whole brain analyses that were performed using the same nuisance covariates revealed that the self‐directedness score was significantly and negatively correlated with MD in the anatomical cluster that spread around the right putamen, right globus pallidus, and right insula and the anatomical cluster that spread around the right caudate and right insula (Fig. 4 and Table 5).
Table 5.
Brain regions that exhibited significant correlations between MD and the character subscales of TCI (df = 767)
| Area | x | y | z | T score of the peak | Corrected P value (cluster) |
|---|---|---|---|---|---|
| Negative correlation of self‐directedness | |||||
| Putamen(R)/Globus pallidus (R)/Insula(R) | 31.5 | −3 | −3 | 4.90 | <0.001 |
| Caudate body (R)/Insula (R) | 15 | 4.5 | 18 | 4.32 | <0.001 |
| Positive correlation of cooperativeness | |||||
| Insula (L)/Orbitofrontal cortex (L) | −21 | 25.5 | −4.5 | 4.16 | 0.001 |
| Anterior cingulate cortex(L)/Insula(L)/Dorsolateral prefrontal cortex (L) | −28.5 | 40.5 | 9 | 3.98 | <0.001 |
| Anterior cingulate cortex(R)/Dorsolateral prefrontal cortex (R) | 33 | 15 | 22.5 | 3.81 | <0.001 |
No other significant results were observed.
The whole brain analyses that were performed using the same nuisance covariates revealed that the cooperativeness score was significantly and positively correlated with MD in the anatomical cluster that spread around the left insula and left orbitofrontal cortex; the anatomical cluster that spread around the left anterior cingulate cortex, left insula, left dorsolateral prefrontal cortex, and intermediate white matter areas; and the anatomical cluster that spread around the right anterior cingulate cortex and right dorsolateral prefrontal cortex (Fig. 4 and Table 5).
The whole brain analyses that were performed using the same nuisance covariates revealed that the self‐transcendence score was not significantly correlated with MD.
Interaction Effects Between Sex and VCDT as well as Personality Traits on MD
The analysis of covariance that was performed using data obtained from both sexes revealed the absence of interaction effects between the scores of any of the psychological measures and sex on MD (df = 886 in the case of the analysis of VCDT and df = 767 in the case of the analyses of personalities).
Path analyses of the Associations Among MD in the Bilateral Globus Pallidus, Personalities, and VCDT
Next, we proceeded to the path analyses to identify the association among these variables using data from 776 subjects.
The whole brain analyses revealed that MD in the left globus pallidus was correlated with the persistence score, and that MD in the right globus pallidus was correlated with the novelty‐seeking score, harm avoidance score, and self‐directedness score. Based on the purpose of the path analyses, we first investigated whether additional personality variables were correlated with MD in the globus pallidus via ROI analyses of multiple regressions. After correcting for confounding variables (age, sex, RAPM score, number of b = 0 images, mean GMC, mean WMC, and mean CSFC in each ROI), we found that the self‐transcendence score was significantly and negatively correlated with MD in the right globus pallidus (P = 0.007, t = −2.703). There were no additional significant correlations. Thus, in the path analysis involving the left globus pallidus, we considered the persistence score and the S–A creativity test score in the model. Moreover, in the path analysis involving the right globus pallidus, we considered the novelty‐seeking score, harm avoidance score, self‐directedness score, self‐transcendence score, and S–A creativity test score in the model.
To generate the model that involved MD in the left globus pallidus, the initial model included three paths. We confirmed that the removal of any paths worsened the fit of the model; thus, this initial model became the final model from the perspective of AIC (χ 2 statistic <0.001, CFI = 1.0, RMSEA = 0.043). In this model, lower MD directly led to higher persistence as well as higher VCDT; furthermore, lower MD indirectly led to higher VCDT via the path in which higher persistence led to higher VCDT. However, the P value of the path from MD in the left globus pallidus to VCDT was 0.117; therefore, whether the direct effect of this path exists remains unclear, despite the fit of the model.
To generate the model involving MD in the right globus pallidus, the 64 initial models created are presented in Figure 2. From each initial model (AIC = 54, CFI = 1.0, RMSEA = 0.205), the paths with the highest P value were deleted recursively one‐by‐one, and the analyses were rerun after each path was removed until the model fit stopped improving. Three of the final models showed the exact same statistics for the fit of the models (Fig. 2, χ 2 statistic = 1.604, df = 5, P = 0.901, AIC = 45.604, CFI = 1.0, RMSEA < 0.001). However, in all final models, the path from harm avoidance to VCDT did not reach significance (P = 0.087), despite the fit of the model. In all three final models, lower MD directly led to lower harm avoidance, which in turn led to higher novelty seeking, higher self‐directedness, and higher self‐transcendence. Among higher novelty seeking, higher self‐directedness, and higher self‐transcendence, all variables were associated with each other, with the exception of novelty seeking and self‐transcendence. Finally, lower harm avoidance, higher novelty seeking, higher self‐directedness, and higher self‐transcendence led to higher VCDT.
DISCUSSION
We investigated the associations between MD and VCDT as well as creativity‐related cognitions. First, consistent with the previous study [Chavez‐Eakle et al., 2006] that showed that VCDT was associated with all TCI personality variables (the association was negative only in the case of harm avoidance), in this study, VCDT was positively associated with novelty seeking, persistence, self‐directedness, self‐transcendence, and cooperativeness (although there was only a tendency for the association with reward dependence), and negatively associated with harm avoidance. Partly consistent with our hypothesis, we found that VCDT was associated with reduced MD in the bilateral globus pallidus, and that novelty seeking was negatively correlated with MD in the right putamen, right globus pallidus, and right caudate as well as in the area located around the right middle cingulate cortex. However, interestingly, harm avoidance was positively correlated with MD in areas that were very similar to areas with negative MD correlates of novelty seeking, together with additional areas. Furthermore, self‐directedness was also negatively correlated with MD in the right putamen, right globus pallidus, and right caudate. Finally, the ROI analysis revealed a significant negative correlation with MD in the right globus pallidus. Thus, these four personalities had common MD correlates with VCDT in the right globus pallidus. Persistence was negatively correlated with MD in the left putamen, left globus pallidus, and bilateral thalamus. Thus, persistence had common MD correlates with VCDT in the left globus pallidus. Also, cooperativeness was positively correlated with MD in the anterior parts of the brain. However, the path analysis revealed associations among MD in the bilateral globus pallidus, personalities, and VCDT. The results showed that lower MD in the left globus pallidus led to higher persistence, which in turn led to higher VCDT. Furthermore, the results showed that lower MD in the right globus pallidus led primarily to lower harm avoidance, which in turn led to higher self‐directedness, self‐transcendence, and novelty seeking, all of which then led to higher VCDT.
VCDT and novelty seeking, which have been associated with dopaminergic functioning, were associated with MD in the globus pallidus, which receives the input from the substantia nigra. Moreover, a reduced MD signal in the globus pallidus appeared to be associated with facilitated functioning. As summarized in our previous study [Takeuchi et al., 2010b] and in the Introduction of this article, several studies have shown that creativity is associated with personality or temperament traits, function, pharmacology, and cognitive and neural mechanisms associated with dopamine. In addition, as described in the Introduction, novelty seeking has been positively associated with dopaminergic functions [Bódi et al., 2009; Kaasinen et al., 2004; Schinka et al., 2002; Suhara et al., 2001; Tomer and Aharon‐Peretz, 2004]. In this study, in the globus pallidus, MD was negatively correlated with VCDT. The globus pallidus receives the dopaminergic input from the substantia nigra [Greenstein and Greenstein, 2000; Lindvall and Björklund, 1979] and is an important part of the dopaminergic system. As described below, the globus pallidus plays a key role in motivation, which has been robustly associated with the function of the dopaminergic system [Kaplan and Oudeyer, 2007]. Moreover, as described in the Introduction, MD decreases are likely to reflect an increase in tissue density due to the shape of neurons or glia and enhancement of tissue organization (strengthening of axonal or dendritic backbones and surrounding tissue), which are likely to lead to facilitated function. These results showed that VCDT and novelty seeking were associated with lower MD (which reflected the augmented function) in a part of the dopaminergic system, as hypothesized.
In this study, lower harm avoidance seemed to be primarily associated with lower MD in the right globus pallidus, and lower harm avoidance seemed to lead to higher self‐directedness, self‐transcendence, and novelty seeking, all of which then led to higher VCDT. The overlap between MD correlates of VCDT and MD correlates of these personalities also tended to be observed in the right putamen. However, this observation was different from our hypothesis, which attributed a central role to novelty seeking. However, the association between harm avoidance and these variables as well as dopamine was congruent with empirical evidence. Harm avoidance was most strongly and negatively correlated with VCDT in this study, which was consistent with a previous report that showed a robust negative association between VCDT and harm avoidance [Chavez‐Eakle et al., 2006]. Further, the association between harm avoidance and MDDS observed here was reported in the recent previous study using a smaller sample size [Laricchiuta et al., 2014]; this finding is also considered to be robust. Furthermore, our study found that higher MD values in the area including the right putamen were associated with higher harm avoidance, and previous PET studies have shown that harm avoidance is associated with the availability of dopaminergic receptors in the right putamen and caudate [Kaasinen et al., 2001; Kim et al., 2011]. Although harm avoidance has been originally hypothesized to be associated with the functions of the serotonergic system [Cloninger, 1985], an overwhelming body of empirical evidence suggests not only that higher harm avoidance is associated with loss of serotonergic functions but also that higher harm avoidance and lower novelty seeking are both associated with loss of dopaminergic function. For example, previous PET studies reported an association between dopamine receptor availability and harm avoidance [Frank et al., 2005; Kaasinen et al., 2001; Kim et al., 2011; Yasuno et al., 2001] as well as closely related personality measures [Laakso et al., 2003]. Furthermore, Parkinson's disease, which includes degeneration in the dopaminergic system, is associated with not only reduced novelty seeking but also higher harm avoidance [Kaasinen et al., 2001; Tomer and Aharon‐Peretz, 2004]. This may be partly associated with an indistinguishable interaction between dopaminergic functions and serotonergic functions and suggests that it is almost impossible to view two systems in isolation, as supported by numerous empirical evidence [Kahn and Davidson, 1993]. Both are anatomically closely connected [Törk, 1990] and highly functionally interactive [Kelland et al., 1990]. In particular, most serotonin receptors act to facilitate neuronal dopamine function, and the activated serotonergic system is literally directly linked to the inhibited dopaminergic system [Di Giovanni et al., 2008]. The robust negative correlation observed for novelty seeking and harm avoidance in the present and previous studies may be partly in line with this notion.
From the cognitive perspective, the motivational cognitive component may be the one key factor that links the associations among MD in the right globus pallidus, harm avoidance, novelty seeking, self‐directedness, self‐transcendence, and VCDT. The personality variables of TCI were robustly associated with each other in this study. In particular, the negative association observed between novelty seeking and harm avoidance as well as the negative association observed between harm avoidance and self‐directedness were very robust, as described in previous studies [Hansenne et al., 2005; Pélissolo and Lépine, 2000]. Thus, it is difficult to identify the key cognitive factor that underlies the associations among these variables. Using data of profile of mood states (POMS) [McNair et al., 1992; Takeuchi et al., 2014a] from 775 subjects (one datum was missing among the 776 subjects whose data was used in the analyses of personalities in this study), we reported previously that lower MD in the right putamen, right globus pallidus, right caudate, and bilateral thalamus as well as higher VCDT are robustly and negatively associated with higher vigor (motivational state) but not with depression, anger/hostility, anxiety, confusion, and fatigue [Takeuchi and Kawashima, 2013d]. Furthermore, our supplemental analysis using the data of the present 775 subjects showed robust associations between vigor and harm avoidance (strongest negative association, r = −0.27, P < 0.001, df = 774), novelty seeking (positive association, r = 0.13, P < 0.001, df = 774), self‐directedness (positive association, r = 0.22, P < 0.001, df = 774), and self‐transcendence (positive association, r = 0.20, P < 0.001, df = 774; it should be noted that, because POMS measures the state of mood in the previous week, the associations of trait variables are generally modest). Further, the constructs of harm avoidance involve such as components asthenia; these are likely to be associated with general motivational components, which in turn are associated with dopaminergic functions [Kaplan and Oudeyer, 2007]. Moreover, novelty seeking and self‐directedness include factors such as exploratory excitability and purposefulness, respectively, both of which are likely to lead to motivation. It is not easy to understand the association between self‐transcendence and motivation instantaneously; however, in fact, a previous study has shown a robust association between self‐transcendence and intrinsic motivation [Tanaka et al., 2009]. These claims may be supported by our available data of self‐fulfilling achievement motivation (achievement motivation directed at pursuing goals evaluated by one's own standards of achievement regardless of the values of others and society) [Horino and Mori, 1991; Takeuchi et al., 2014b] from 776 subjects. Self‐fulfilling achievement motivation was robustly associated with harm avoidance (r = −0.30, P < 0.001, df = 775), exploratory excitability of novelty seeking (r = 0.30, P < 0.001, df = 775), purposefulness of self‐directedness (r = 0.36, P < 0.001, df = 775), and self‐transcendence (r = 0.42, P < 0.001, df = 775). Considering all these observations, the motivational cognitive component may be the key factor that links the association among MD in the right globus pallidus, harm avoidance, novelty seeking, self‐directedness, self‐transcendence, and VCDT. Consistently, evidence from various fields points out that the globus pallidus and its adjacent area play a key role in motivation [Smith and Nichols, 2009; Takeuchi et al., 2014b]. This may be another reason.
The pathway from the left globus pallidus to persistence and on to VCDT may be in line with the notion that the motivational factor is the key that links the associations observed among lower MD in the globus pallidus, personalities, and VCDT. Persistence is perseverance despite frustration and fatigue as well as a tendency toward a persistent pursuit of desired goals [Cloninger et al., 1993; Mardaga and Hansenne, 2007]. Persistent individuals are eager, ambitious, and determined overachievers [Cloninger, 1994]. Thus, persistence is, by definition, related to a certain form of motivation. However, our aforementioned study did not find a significant association between MD in the left globus pallidus and the vigor subscale of POMS. Moreover, as described in the Results section, although four other personality factors related to VCDT were associated with MD in the right globus pallidus, persistence was not, and persistence was the only personality factor that was associated with MD in the left globus pallidus. The reason underlying this discrepancy is not clear, and we are not aware of previous neuroscience studies that can directly explain the discrepancy regarding the laterality of the globus pallidus and the nature of motivation. It is known that structural asymmetry exists in the globus pallidus [Kooistra and Heilman, 1988]. Furthermore, asymmetry of the dopamine level is also present in the globus pallidus [Glick et al., 1982]. A functional imaging study has suggested that the left globus pallidus is involved in the attentional control of information flow in the mind [McNab and Klingberg, 2007]. Furthermore, a lesion in the left globus pallidus, but not that in the right globus pallidus, is associated with transient decline of frontal lobe cognitive function. Considering all these relevant findings, the left globus pallidus may be involved in the motivation to shut out distractors and regulate persistent behaviors. However, related evidence is lacking, and future studies are needed to elucidate the nature of the laterality of the globus pallidus more thoroughly.
MD in various other regions was related to personality variables but not to VCDT; these areas may be related to each personality factor via their functions. Higher cooperativeness was associated with higher MD primarily in the white matter areas located around the anterior part of the anterior cingulate to the insula as well as adjacent areas. As described in the Introduction section, it has been considered that higher MD reflects a lower density of neural tissues, which prohibits free water diffusion. Conversely, the area from the anterior cingulate to the anterior insula is the area that forms the salience network [Seeley et al., 2007]. This network may integrate interoceptive information with emotional salience [Seeley et al., 2007; Taylor et al., 2009] and is thought to be involved in anxiety [Seeley et al., 2007] and social anxiety [Liao et al., 2010]. Thus, we speculate that a lower amount of tissues in the pathway may lead to this type of cognitive factor (social anxiety), which in turn may prohibit prosociality and cooperativeness. Persistence was negatively correlated with MD in the thalamus, which forms a circuit with basal ganglia and cortical areas. Among the circuit, the thalamus is considered to serve as an important center of integration of the networks that underlie the ability to modulate behaviors [Haber and Calzavara, 2009]. Further, higher novelty seeking and lower harm avoidance were associated with lower MD in the anterior part of the middle cingulate as well as in the caudate and adjacent areas. One of the common roles of these areas is various types of reward processing [Liu et al., 2011]. Also, higher harm avoidance was associated with higher MD in the area located around the posterior insula. This area is involved in a certain form of feeling that motivates individuals to take actions such as love, hatred, and motivation based on competition [Haruno and Kawato, 2006; Takeuchi et al., 2014b; Zeki and Romaya, 2008]. Although these functions are not necessarily associated with VCDT, these regions may be associated with personality variables.
There was at least one limitation to this study: to measure creativity, we used only the S–A creativity test, which is a measure of VCDT, and did not use tests for figural creativity measured by divergent thinking. Furthermore, as summarized by Jung et al. [2010b], several cognitive processes are important for creativity or creative measures, such as flow [Csikszentmihalyi, 1997], insight [Jung‐Beeman et al., 2004], perseverance in the face of social acceptance or resistance, such as that of personality variables, creative achievements, and remote association of ideas. Focusing on divergent thinking as a measure of creativity is common in imaging studies performed in several laboratories [Gansler et al., 2011; Zhu et al., 2013] but not in those performed in laboratories that used other measures [Fink et al., 2014; Jung et al., 2010b]. In terms of the associations with TCI measures, the performance of VCDT and figural creativity measured by divergent thinking showed quite similar patterns of psychological correlations [Chavez‐Eakle et al., 2006]. Furthermore, as described previously [Takeuchi et al., 2012a], we focused on the DT test as a measure of creativity in this study, because, currently, DT tests dominate as a measure of creativity in this field [Dietrich, 2007]. DT has been proposed to be a key aspect of creativity [Guilford, 1967], and a meta‐analysis [Kim, 2008] has demonstrated that DT scores exhibit a significantly stronger relationship with creative achievement than scores on intelligence tests, thus supporting the validity of DT as predictive of creative ability. To the best of our knowledge, no other creative measures used in laboratory settings have shown this level of validity. However, different creative measures have overlapping and distinct neural correlates [Fink et al., 2014], which may also hold true for MD measures. Additional studies are needed to investigate the MD correlates of other creative measures.
In summary, our study was based on the hypothesis of a correlation between MDDS, novelty seeking (which is the typical personality factor associated with the dopaminergic system), and VCDT. Our results revealed associations between higher VCDT and lower MD in the bilateral globus pallidus, which receives the dopaminergic input from the substantia nigra and plays a key role in motivation. Furthermore, not only higher novelty seeking, but also lower harm avoidance, higher self‐directedness, and higher self‐transcendence were robustly associated with lower MD in the right globus pallidus, whereas higher persistence was associated with lower MD in the left globus pallidus, and all these variables were associated with the higher motivational cognitive component. The path analysis suggested that lower MD in the left globus pallidus led to higher persistence, which led to higher VCDT, and that lower MD in the right globus pallidus was primarily associated with lower harm avoidance, which in turn led to higher self‐directedness, higher self‐transcendence, and higher novelty seeking, all of which led to higher VCDT. These analyses elucidated the neural mechanisms that underlie the association between VCDT and multiple personalities in TCI and MD in the globus pallidus, which plays a key role in motivation and underlies these associations.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors respectfully thank Yuki Yamada for operating the MRI scanner, Yuriko Suzuki from Philips for advice on the DWI and Haruka Nouchi for being an examiner of psychological tests. The authors also thank study participants, the other examiners of psychological tests, and all of our colleagues in Institute of Development, Aging and Cancer and in Tohoku University for their support. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
REFERENCES
- Abe M, Fukuyama H, Mima T (2014): Water diffusion reveals networks that modulate multiregional morphological plasticity after repetitive brain stimulation. Proc Natl Acad Sci USA 111:4608–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H (1974): A new look at the statistical model identification. IEEE Trans Automat Control 19:716–723. [Google Scholar]
- Andreone N, Tansella M, Cerini R, Versace A, Rambaldelli G, Perlini C, Dusi N, Pelizza L, Balestrieri M, Barbui C (2007): Cortical white‐matter microstructure in schizophrenia Diffusion imaging study. Br J Psychiatry 191:113–119. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM (1999): A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev 106:529–550. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O (2008): Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: A review. J Mol Neurosci 34:51–61. [DOI] [PubMed] [Google Scholar]
- Bódi N, Kéri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibó G, Takáts A, Bereczki D, Gluck MA (2009): Reward‐learning and the novelty‐seeking personality: A between‐and within‐subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain 132:2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D (2006): Adaptive linear step‐up procedures that control the false discovery rate. Biometrika 93:491–507. [Google Scholar]
- Bjørnebekk A, Westlye LT, Fjell AM, Grydeland H, Walhovd KB (2012): Social reward dependence and brain white matter microstructure. Cereb Cortex 22:2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson SH, Peterson JB, Higgins DM (2003): Decreased latent inhibition is associated with increased creative achievement in high‐functioning individuals. J Pers Soc Psychol 85:499–506. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA (2007): Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 34:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton R, Landau S, Schiavone F, Barrick T, Clark C, Markus H, Morris R (2008): A structural equation modeling investigation of age‐related variance in executive function and DTI measured white matter damage. Neurobiol Aging 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- Chavez‐Eakle RA, del Carmen Lara M, Cruz‐Fuentes C (2006): Personality: A possible bridge between creativity and psychopathology? Creat Res J 18:27–38. [Google Scholar]
- Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y (1996): Differential neurotoxicity induced by l‐DOPA and dopamine in cultured striatal neurons. Brain Res 743:278–283. [DOI] [PubMed] [Google Scholar]
- Chermahini SA, Hommel B (2010): The (b) link between creativity and dopamine: Spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition 115:458–465. [DOI] [PubMed] [Google Scholar]
- Chermahini SA, Hommel B (2012): More creative through positive mood? Not everyone! Front Hum Neurosci 6:article 319. [DOI] [PMC free article] [PubMed]
- Cloninger CR (1985): A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev 4:167–226. [PubMed] [Google Scholar]
- Cloninger CR (1994): Temperament and personality. Curr Opin Neurobiol 4:266–273. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR (1993): A psychobiological model of temperament and character. Arch Gen Psychiatry 50:975–990. [DOI] [PubMed] [Google Scholar]
- Cooper SH (1998): Changing notions of defense within psychoanalytic theory. J Pers 66:947–964. [Google Scholar]
- Csikszentmihalyi M. 1997. Creativity: Flow and the Psychology of Discovery and Invention. New York: Harper Collins. [Google Scholar]
- Depue RA, Collins PF (1999): Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 22:491–517. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Esposito E. 2008. Serotonin‐Dopamine Interaction: Experimental Evidence and Therapeutic Relevance. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Dietrich A (2007): Who's afraid of a cognitive neuroscience of creativity? Methods 42:22–27. [DOI] [PubMed] [Google Scholar]
- Drago V, Foster P, Skidmore F, Heilman K (2009): Creativity in Parkinson's disease as a function of right versus left hemibody onset. J Neurol Sci 276:179–183. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR (1996): Prepulse inhibition and latent inhibition: The role of dopamine in the medial prefrontal cortex. Neuroscience 75:535–542. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Joober R, De Guzman R, O'Driscoll GA (2006): Schizotypy, attention deficit hyperactivity disorder, and dopamine genes. Psychiatry Clin Neurosci 60:764–767. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Furnham A (1993): Personality and the Barron‐Welsh Art Scale. Percept Mot Skills 76:837–838. [DOI] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Hutterer L, Steiner E, Benedek M, Weber B, Reishofer G, Papousek I, Weiss EM (2014): Gray matter density in relation to different facets of verbal creativity. Brain Struct Funct 219:1263–1269. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB (2012): A multi‐modal investigation of behavioral adjustment: Post‐error slowing is associated with white matter characteristics. Neuroimage 61:195–205. [DOI] [PubMed] [Google Scholar]
- Flaherty AW (2005): Frontotemporal and dopaminergic control of idea generation and creative drive. J Comp Neurol 493:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S (2005): Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C] raclopride. Biol Psychiatry 58:908–912. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Moore DW, Susmaras TM, Jerram MW, Sousa J, Heilman KM (2011): Cortical morphology of visual creativity. Neuropsychologia 49:2527–2532. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB (1982): Lateral asymmetry of neurotransmitters in human brain. Brain Res 234:53–63. [DOI] [PubMed] [Google Scholar]
- Greenstein B, Greenstein A. 2000. Color Atlas of Neuroscience: Neuroanatomy and Neurophysiology. New York: George Thieme Verlag. [Google Scholar]
- Guilford JP. 1967. The Nature of Human Intelligence. New York: McGraw‐Hill Companies. [Google Scholar]
- Haber SN, Calzavara R (2009): The cortico‐basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansenne M, Delhez M, Cloninger CR (2005): Psychometric properties of the temperament and character inventory–revised (TCI–R) in a Belgian sample. J Pers Assess 85(1):40–49. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kawato M (2006): Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. J Neurophysiol 95:948–959. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Nadeau SE, Beversdorf DO (2003): Creative innovation: Possible brain mechanisms. Neurocase 9:369–379. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF (2008): Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9:58–65. [DOI] [PubMed] [Google Scholar]
- Horino M, Mori K (1991): The effects of achievement motivation on relationships between depression and social support. Jpn J Educ Psychol 39:308–315. [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Flores RA, Smith SM, Caprihan A, Yeo RA (2009): Biochemical support for the “threshold” theory of creativity: a magnetic resonance spectroscopy study. J Neurosci 29:5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ (2010a): White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS ONE 5:e9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Bockholt HJ, Flores RA, Smith SM, Chavez RS, Haier RJ (2010b): Neuroanatomy of creativity. Hum Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel‐Liu S, Greenblatt R, Reber PJ, Kounios J (2004): Neural activity when people solve verbal problems with insight. PLoS Biol 2:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, Rinne JO (2001): Personality traits and brain dopaminergic function in Parkinson's disease. Proc Natl Acad Sci USA 98:13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Någren K, Rinne JO (2004): Insular dopamine D2 receptors and novelty seeking personality in Parkinson's disease. Mov Disord 19:1348–1351. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Davidson M (1993): Serotonin, dopamine and their interactions in schizophrenia. Psychopharmacology 112:S1–S4. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G (2011): The structural basis of inter‐individual differences in human behaviour and cognition. Nat Rev Neurosci 12:231–242. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG (2001): Mild cognitive impairment and Alzheimer disease: Regional diffusivity of water. Radiology 219:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Oudeyer P‐Y (2007): In search of the neural circuits of intrinsic motivation. Front Neurosci 1:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Freeman A, Chiodo L (1990): Serotonergic afferent regulation of the basic physiology and pharmacological responsiveness of nigrostriatal dopamine neurons. J Pharmacol Exp Ther 253:803–811. [PubMed] [Google Scholar]
- Kijima N, Saito R, Takeuchi M, Yoshino A, Ono Y, Kato M, Kitamura T (1996): Cloninger's seven‐factor model of temperament and character and Japanese version of temperament and character inventory (TCI). Arch Psychiatr Diagn Clin Evaluat 7:379–399. [Google Scholar]
- Kijima N, Tanaka E, Suzuki N, Higuchi H, Kitamura T (2000): Reliability and validity of the Japanese version of the Temperament and Character Inventory. Psychol Rep 86:1050–1058. [DOI] [PubMed] [Google Scholar]
- Kim KH (2008): Meta‐analyses of the relationship of creative achievement to both IQ and divergent thinking test scores. J Creat Behav 42:106–130. [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH (2011): Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: A high resolution PET study. Biol Psychol 87:164–167. [DOI] [PubMed] [Google Scholar]
- King LA, Walker LM, Broyles SJ (1996): Creativity and the five‐factor model. J Res Pers 30:189–203. [Google Scholar]
- Kirrane RM, Siever LJ (2000): New perspectives on schizotypal personality disorder. Curr Psychiatry Rep 2:62–66. [DOI] [PubMed] [Google Scholar]
- Kline P, Cooper C (1986): Psychoticism and creativity. J Genet Psychol 147:183–188. [DOI] [PubMed] [Google Scholar]
- Kooistra CA, Heilman KM (1988): Motor dominance and lateral asymmetry of the globus pallidus. Neurology 38:388–390. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pagonabarraga J, Martinez‐Corral M (2009): Changes in artistic style and behaviour in Parkinson's disease: Dopamine and creativity. J Neurol 256:816–819. [DOI] [PubMed] [Google Scholar]
- Laakso A, Wallius E, Kajander J, Bergman J, Eskola O, Solin O, Ilonen T, Salokangas RK, SyvÃĪlahti E, Hietala J (2003): Personality traits and striatal dopamine synthesis capacity in healthy subjects. Am J Psychiatry 160:904–910. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Petrosini L, Piras F, Cutuli D, Macci E, Picerni E, Chiapponi C, Caltagirone C, Spalletta G (2014): Linking novelty seeking and harm avoidance personality traits to basal ganglia: Volumetry and mean diffusivity. Brain Struct Funct 219:793–803. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001): Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging 13:534–546. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S (2010): Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52:1549–1558. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björklund A (1979): Dopaminergic innervation of the globus pallidus by collaterals from the nigrostriatal pathway. Brain Res 172:169–173. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011): Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Mardaga S, Hansenne M (2007): Relationships between Cloninger's biosocial model of personality and the behavioral inhibition/approach systems (BIS/BAS). Pers Individ Dif 42:715–722. [Google Scholar]
- Mayseless N, Uzefovsky F, Shalev I, Ebstein RP, Shamay‐Tsoory SG (2013): The association between creativity and 7R polymorphism in the dopamine receptor D4 gene (DRD4). Front Hum Neurosci 7:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T (2007): Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11:103–107. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. 1992 Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Moseley M, Bammer R, Illes J (2002): Diffusion‐tensor imaging of cognitive performance. Brain Cogn 50:396–413. [DOI] [PubMed] [Google Scholar]
- Ni J, Chen S, Liu J, Huang G, Shen T, Chen X (2010): Regional diffusion changes of cerebral grey matter during normal aging–A fluid‐inversion prepared diffusion imaging study. Eur J Radiol 75:134–138. [DOI] [PubMed] [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW (2001): Regional and global changes in cerebral diffusion with normal aging. Am J Neuroradiol 22:136–142. [PMC free article] [PubMed] [Google Scholar]
- O'Reilly T, Dunbar R, Bentall R (2001): Schizotypy and creativity: an evolutionary connection? Pers Individ Dif 31:1067–1078. [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pélissolo A, Lépine J‐P (2000): Normative data and factor structure of the temperament and character inventory (TCI) in the French version. Psychiatry Res 94:67–76. [DOI] [PubMed] [Google Scholar]
- Péran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, Celsis P, Rascol O, Démonet J‐F, Stefani A (2010): Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 133:3423–3433. [DOI] [PubMed] [Google Scholar]
- Picerni E, Petrosini L, Piras F, Laricchiuta D, Cutuli D, Chiapponi C, Fagioli S, Girardi P, Caltagirone C, Spalletta G (2013): New evidence for the cerebellar involvement in personality traits. Front Behav Neurosci 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Caltagirone C, Spalletta G (2010): Working memory performance and thalamus microstructure in healthy subjects. Neuroscience 171:496–505. [DOI] [PubMed] [Google Scholar]
- Prabhu V, Sutton C, Sauser W (2008): Creativity and certain personality traits: Understanding the mediating effect of intrinsic motivation. Creat Res J 20:53–66. [Google Scholar]
- Raven J. 1993. Manual for Raven's Progressive Matrices And Vocabulary Scales. Oxford: Oxford Psychologists Press. [Google Scholar]
- Razek AA, Elmongy A, Hazem M, Zakareyia S, Gabr W (2011): Idiopathic Parkinson disease effect of levodopa on apparent diffusion coefficient value of the brain. Acad Radiol 18:70–73. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur‐Moryosef S, Blumenfeld‐Katzir T, Assaf Y (2012): Learning in the fast lane: New insights into neuroplasticity. Neuron 73:1195–1203. [DOI] [PubMed] [Google Scholar]
- Sassa Y, Taki Y, Takeuchi H, Hashizume H, Asano M, Asano K, Wakabayashi A, Kawashima R (2012): The correlation between brain gray matter volume and empathizing and systemizing quotients in healthy children. Neuroimage 60:2035–2041. [DOI] [PubMed] [Google Scholar]
- Schinka J, Letsch E, Crawford F (2002): DRD4 and novelty seeking: Results of meta‐analyses. Am J Med Genet 114:643–648. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppi K, Schocke MF, Donnemiller E, Esterhammer R, Kremser C, Scherfler C, Diem A, Jaschke W, Wenning GK, Poewe W (2004): Comparison of diffusion‐weighted imaging and [123I] IBZM‐SPECT for the differentiation of patients with the Parkinson variant of multiple system atrophy from those with Parkinson's disease. Mov Disord 19:1438–1445. [DOI] [PubMed] [Google Scholar]
- Shimonaka Y, Nakazato K (2007): Creativity and factors affecting creative ability in adulthood and old age. Jpn J Educ Psychol 55:231–243. [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Society_For_Creative_Minds . 1969. Manual of S‐A Creativity Test. Tokyo: Tokyo Shinri Corporation. [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K (2001): Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage 13:891–895. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Sharp R, Auerbach PP (2003): Dopamine agonists disrupt visual latent inhibition in normal males using a within‐subject paradigm. Psychopharmacology 169:314–320. [DOI] [PubMed] [Google Scholar]
- Törk I (1990): Anatomy of the Serotonergic Systema. Ann N Y Acad Sci 600:9–34. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R (2010a): Training of working memory impacts structural connectivity. J Neurosci 30:3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010b): Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel‐based morphometry Neuroimage 51:578–585. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010c): White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51:11–18. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011a): Cerebral blood flow during rest associates with general intelligence and creativity. PLoS ONE 6:e25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011b): Effects of training of processing speed on neural systems. J Neurosci 31:12139–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011c): Failing to deactivate: the association between brain activity during a working memory task and creativity. Neuroimage 55:681–687. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011d): Regional gray matter density associated with emotional intelligence: Evidence from voxel‐based morphometry. Hum Brain Mapp 32:1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011e): Verbal working memory performance correlates with regional white matter structures in the fronto‐parietal regions. Neuropsychologia 49:3466–3473. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011f): Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS ONE 6:e23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2012a): The association between resting functional connectivity and creativity. Cereb Cortex 22:2921–2929. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2012b): Regional gray and white matter volume associated with Stroop interference: Evidence from voxel‐based morphometry. Neuroimage 59:2899–2907. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2013a): White matter structures associated with emotional intelligence: Evidence from diffusion tensor imaging. Hum Brain Mapp 34:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sekiguchi A, Nouchi R, Kotozaki Y, Nakagawa S, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T (2013b): Association of hair iron levels with creativity and psychological variables related to creativity. Front Hum Neurosci 7:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Thyreau B, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2013c): White matter structures associated with empathizing and systemizing in young adults. Neuroimage 77:222–236. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kawashima R (2013d): Neural bases of individual differences of creativity measured by the divergent thinking test (symposium), Washington DC. [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sekiguchi A, Kotozaki Y, Nakagawa S, Miyauchi CM, Sassa Y, Kawashima R (2014a): Working memory training improves emotional states of healthy individuals. Front Syst Neurosci 8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, Miyauchi C, Yokoyama R, Iizuka K, Hashizume H, Nakagawa S (2014b): Regional gray matter density is associated with achievement motivation: Evidence from voxel‐based morphometry. Brain Struct Funct 219:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2012): Effects of processing speed training on cognitive functions and neural systems. Rev Neurosci 23:289–301. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Asano M, Asano K, Kotozaki Y, Nouchi R, Wu K, Fukuda H (2011a): Correlation among body height, intelligence, and brain gray matter volume in healthy children. Neuroimage 59:1023–1027. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Wu K, Asano M, Asano K, Fukuda H, Kawashima R (2011b): Correlation between gray matter density‐adjusted brain perfusion and age using brain MR images of 202 healthy children. Hum Brain Mapp 32:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K (2011c): Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage 60:471–475. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Hashizume H, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K (2013): Linear and curvilinear correlations of brain white matter volume, fractional anisotropy, and mean diffusivity with age using voxel‐based and region of interest analyses in 246 healthy children. Hum Brain Mapp 34:1842–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Mizuno K, Fukuda S, Tajima S, Watanabe Y (2009): Personality traits associated with intrinsic academic motivation in medical students. Med Educ 43:384–387. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD (2009): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Aharon‐Peretz J (2004): Novelty seeking and harm avoidance in Parkinson's disease: Effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry 75:972–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance EP. 1966. Torrance Tests of Creative Thinking: Bensenville, IL: Scholastic Testing Service. [Google Scholar]
- Watanabe T (1998): A study on the individual differences of the experience of hypnagogic imagery. Shinrigaku Kenkyu 68:478–483. [DOI] [PubMed] [Google Scholar]
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J (2014): Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex 51:92–102. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K (2001): Relation among dopamine D 2 receptor binding, obesity and personality in normal human subjects. Neurosci Lett 300:59–61. [DOI] [PubMed] [Google Scholar]
- Zeki S, Romaya JP (2008): Neural correlates of hate. PLoS One 3:e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang Q, Qiu J (2013): Relating inter‐individual differences in verbal creative thinking to cerebral structures: An optimal voxel‐based morphometry study. PLoS One 8:e79272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


