Abstract
We aimed to compare the efficacy and safety of lobeglitazone and pioglitazone as add‐ons to metformin in patients with type 2 diabetes. Patients who were inadequately controlled by metformin were randomized and treated once daily with either lobeglitazone (0.5 mg, n = 128) or pioglitazone (15 mg, n = 125) for 24 weeks, with a 28‐week extension trial of lobeglitazone treatment in patients who consented. The primary endpoint was the change in glycated haemoglobin (HbA1c) concentration from baseline to week 24. At week 24, the mean change from baseline in HbA1c was −0.74% for the lobeglitazone group and −0.74% for the pioglitazone group, with a mean difference of 0.01% [95% confidence interval (CI) of difference, −0.16 to 0.18]. The effects of lobeglitazone on lipid variables and the adverse events associated with lobeglitazone were similar to those observed with pioglitazone. Lobeglitazone was not inferior to pioglitazone as an add‐on to metformin in terms of their efficacy and safety.
Keywords: antidiabetic drug, clinical trial, phase III study, randomised trial, thiazolidinediones
Introduction
Despite the continuing debate about the safety of thiazolidinedione (TZD) 1, 2, 3 and attempts to develop new classes of insulin sensitizers 4, 5, there is still a clinical need for a novel TZD. Lobeglitazone (CKD‐501; Chong Kun Dang Pharmaceutical Corp., Seoul, Korea) is a novel peroxisome proliferator‐activated receptor (PPAR)‐γ agonist with a TZD moiety and substituted pyrimidines 6. These substituted pyrimidines were selected on their empirical effects on triglyceride accumulation in adipocytes in vitro and their glucose‐lowering and lipid‐modulating activities in diabetic mice in vivo 7, 8. Unlike pioglitazone, urinary excretion of lobeglitazone is negligible in humans 9; moreover, no bladder cancer has been observed in 2‐year carcinogenicity studies in mice 10 and rats 11. In pharmacokinetic studies in healthy adults, lobeglitazone was well‐tolerated and did not significantly affect the pharmacokinetics of metformin or vice versa 12. The aim of the present study was therefore to compare the efficacy and safety of lobeglitazone and pioglitazone as add‐ons to metformin for patients with type 2 diabetes.
Patients and Methods
This was a 24‐week, randomized, double‐blind, parallel‐group, active‐controlled, phase III clinical trial conducted from June 2010 to April 2012 in 18 centres in the Republic of Korea. Patients were recruited by the investigators from the outpatient clinic of each centre. Advertisements in newspapers and subways were used to facilitate patient recruitment. An additional 28‐week extension phase of lobeglitazone treatment was conducted for patients who consented to further assessment of safety profiles. The clinical trial protocol was approved by the institutional review board of each centre. This trial complied with the Declaration of Helsinki and is registered with clinicaltrials.gov (NCT01106131). All patients in the present study provided written informed consent.
Patients aged 18–80 years (body mass index 21–40 kg/m2) with inadequately controlled type 2 diabetes [glycated haemoglobin (HbA1c) concentration 7–10% (53–86 mmol/mol)], and a fasting C‐peptide concentration >1.0 ng/ml, despite a stable regimen of metformin (≥1000 mg/day; unchanged for at least 2 months before randomization) were eligible for this study. The exclusion criteria for this study are available in File S1.
After a 2‐week, single‐blind, placebo run‐in period, eligible patients were randomized (1 : 1) to receive once‐daily lobeglitazone (0.5 mg) plus pioglitazone placebo or once‐daily pioglitazone (15 mg) plus lobeglitazone placebo as an add‐on therapy to metformin (≥1000 mg/day) for 24 weeks. The primary endpoint was the change in HbA1c concentration between baseline and week 24. This trial was designed as a non‐inferiority trial to establish whether lobeglitazone was not inferior to pioglitazone in terms of the primary endpoint. The 95% lower confidence limit for the between‐treatment difference was estimated and compared with the predefined −0.4% non‐inferiority margin. Details of the study protocol and statistical methods are available in File S1.
Results
Baseline Characteristics
A total of 253 patients were randomized and comprised the full analysis set. Of these patients, 128 were treated with lobeglitazone and 125 were treated with pioglitazone. Overall, 222 patients (87.7%) completed the treatment period (Figure S1, File S1). The baseline characteristics did not differ between the two groups (Table S1, File S1). No initial differences were observed between the groups regarding the distribution of co‐administered drugs, liver function test results, bone densitometry scans, or N‐terminal pro‐brain natriuretic peptide levels (data not shown). Three patients in the pioglitazone group were given increased doses of pioglitazone as a result of hyperglycaemia [HbA1c >8.0% (64 mmol/mol), fasting plasma glucose >11.1 mmol/L] at week 12.
Primary and Secondary Efficacy Endpoints
The reduction in HbA1c concentration between baseline and week 24 was 0.74% in the lobeglitazone group and 0.74% in the pioglitazone group (Figure 1). The mean difference between these groups was 0.01% [95% confidence interval (CI) of difference, −0.16 to 0.18]. The change in HbA1c level from baseline was significant in both groups (p < 0.001). The proportion of patients who achieved an HbA1c concentration of <7.0% was 47.7% for the lobeglitazone group and 48.0% for the pioglitazone group (p = 0.956).
Figure 1.
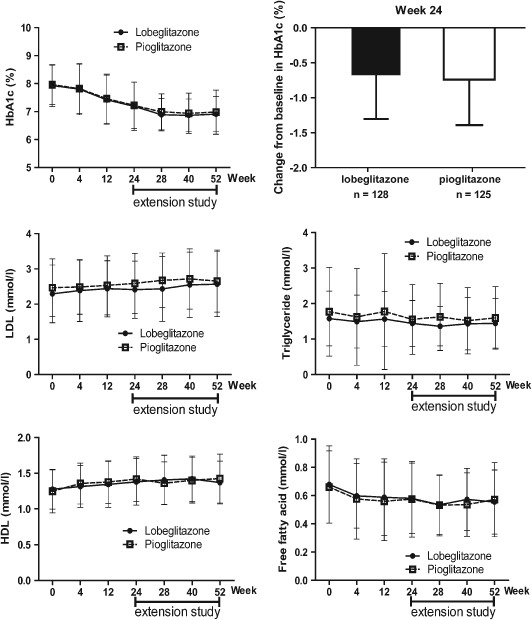
Mean changes in glycated haemoglobin (HbA1c) and lipid variables from baseline (lobeglitazone group, n = 128; pioglitazone group, n = 125). For the extension study, the results obtained for the 83 patients who continued lobeglitazone (lobeglitazone group) and the 94 patients who switched to lobeglitazone from pioglitazone (pioglitazone group) are shown.
Significant decreases in fasting plasma glucose, homeostatic model assessment of insulin resistance, fasting insulin and fasting C‐peptide levels, in addition to increases in homeostatic model assessment of β‐cell function, were observed in both groups; no differences were observed between the two groups (Table 1, Figure 1; Figure S2, File S1). Significant increases in HDL cholesterol and adiponectin levels, in addition to decreases in free fatty acid and triglyceride levels, were observed in both groups. The changes in LDL cholesterol levels were similar in each group (Table 1 and Figure 1).
Table 1.
Effects of lobeglitazone versus pioglitazone on glucometabolic and lipid variables between baseline and 24 weeks
| Variable | Lobeglitazone (n = 128) | Pioglitazone (n = 125) | p* | ||
|---|---|---|---|---|---|
| Baseline | Week 24 | Baseline | Week 24 | ||
| HbA1c, % (mmol/mol) | 7.93 ± 0.75 | 7.19 ± 0.86 § | 7.96 ± 0.70 | 7.22 ± 0.83 § | 0.858 |
| (63 ± 8) | (55 ± 9) | (63 ± 8) | (55 ± 9) | ||
| Fasting glucose, mmol/l | 8.57 ± 2.61 | 7.31 ± 2.22 § | 8.67 ± 2.17 | 7.20 ± 1.60 § | 0.433 |
| Fasting insulin, pmol/l | 67.85 ± 41.18 | 57.85 ± 26.18 ‡ | 67.37 ± 36.46 | 54.45 ± 24.17 § | 0.232 |
| Fasting C‐peptide, nmol/l | 0.76 ± 0.36 | 0.67 ± 0.25 § | 0.77 ± 0.37 | 0.65 ± 0.28 § | 0.502 |
| HOMA‐β | 44.24 ± 26.49 | 52.02 ± 34.88 § | 42.83 ± 28.02 | 51.08 ± 45.01 † | 0.916 |
| HOMA‐IR | 3.89 ± 3.69 | 2.69 ± 1.29 § | 3.79 ± 2.62 | 2.54 ± 1.36 § | 0.358 |
| Total cholesterol, mmol/l | 4.19 ± 0.90 | 4.37 ± 0.90 ‡ | 4.39 ± 0.99 | 4.58 ± 0.91 ‡ | 0.410 |
| Triglyceride, mmol/l | 1.58 ± 0.77 | 1.44 ± 0.65 † | 1.77 ± 1.25 | 1.55 ± 0.98 † | 0.783 |
| LDL cholesterol, mmol/l | 2.29 ± 0.82 | 2.41 ± 0.81 † | 2.46 ± 0.82 | 2.58 ± 0.85 † | 0.530 |
| Small dense LDL cholesterol (%) | 4.02 ± 4.68 | 2.79 ± 4.53 § | 5.07 ± 5.53 | 4.01 ± 5.14 † | 0.204 |
| HDL cholesterol, mmol/l | 1.28 ± 0.31 | 1.40 ± 0.35 § | 1.27 ± 0.30 | 1.42 ± 0.32 § | 0.260 |
| Free fatty acid, mmol/l | 0.68 ± 0.27 | 0.58 ± 0.25 § | 0.66 ± 0.26 | 0.57 ± 0.27 § | 0.977 |
| Apo‐B, g/l | 0.72 ± 0.21 | 0.79 ± 0.26 § | 0.76 ± 0.23 | 0.84 ± 0.28 § | 0.628 |
| Apo‐A1, g/l | 1.51 ± 0.27 | 1.49 ± 0.27 | 1.48 ± 0.22 | 1.48 ± 0.21 | 0.688 |
| Adiponectin, ng/dl | 5824.54 ± 3054.51 | 13 321.71 ± 7856.63 § | 6137.73 ± 2863.21 | 14 253.96 ± 9260.51 § | 0.817 |
Apo‐A1, apolipoprotein A1; Apo‐B, apolipoprotein B; HbA1c, glycated haemoglobin; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance.
Data are expressed as mean ± standard deviation.
Significant changes from baseline to week 24 are indicated as follows:
p < 0.05;
p> < 0.01;
p < 0.001 as determined by a paired t‐test.
p values are for analysis of covariance, after adjusting for baseline values. The Bonferroni method was applied when multiple comparisons were made.
Safety
Among the safety population set (n = 253), 130 (51.4%) patients experienced one or more adverse events (AEs; lobeglitazone group, n = 66; pioglitazone group, n = 64). Severe AEs were reported in 13 patients (5.1%); all of these AEs were considered to be unrelated to the study drug by the investigator. Drug‐related AEs were reported in 14 patients (5.5%). No differences were observed between the two groups regarding the incidence of AEs, severe AEs, or drug‐related AEs (Table S3, File S1). Details of the safety variables and the results of the extension study are available in File S1.
Discussion
In the present study, lobeglitazone (0.5 mg/day) was not inferior to pioglitazone (15 mg/day) as an add‐on therapy to metformin in terms of the reduction in HbA1c concentration after 24 weeks. Changes in glycometabolic and lipid variables, and the overall safety profiles in the lobeglitazone group were similar to those observed in the pioglitazone group.
In the present study, lobeglitazone shared some safety issues with pioglitazone, such as oedema and increased body weight; however, it is reassuring that lobeglitazone, a pure PPAR‐γ agonist rather than a dual PPAR agonist, did not show any significant difference in its effects on lipid variables when compared with pioglitazone. Moreover, no severe, drug‐related AEs (including heart failure requiring admission) were observed during the study period or in the additional 28‐week extension study.
A clinically important limitation of the present study was that the dose of pioglitazone was only 15 mg/day, the highest dose covered by the Korea national health insurance system during the study period. Although up to 30 mg/day of pioglitazone is approved for clinical use in Korea and is allowed for patients who have a poor response with a dose of 15 mg/day, the use of higher dose incurs an additional cost for patients (without insurance reimbursement). For this reason, the efficacy and safety of higher doses of lobeglitazone and pioglitazone could not be compared in this study. Because lobeglitazone has been shown to be well tolerated in doses of up to 4 mg for 7 days in a short‐term study conducted in healthy volunteers 9, the results of the present study should encourage future international studies comparing the efficacy and safety of higher doses of lobeglitazone and maximum dose of pioglitazone.
In conclusion, the efficacy of lobeglitazone (0.5 mg/day) was not inferior to pioglitazone (15 mg/day) as an add‐on to metformin in terms of the change in HbA1c concentration from baseline. Additionally, the effects on lipid variables, adiponectin secretion and AEs were similar to those observed with pioglitazone.
Supporting information
File S1. Supplementary Methods and Results, Acknowledgments.
Table S1. Patient demographics and baseline characteristics.
Table S2. Demographics and baseline characteristics of patients in extension study.
Table S3. Safety profiles in the safety population over the 24‐week study period.
Table S4. Adverse events additionally reported during the extension period (Week 25 to 52).
Figure S1. Patient dispositions.
Figure S2. Mean changes in fasting plasma glucose (FPG) levels from baseline.
Acknowledgements
This study was supported by the Chong Kun Dang Pharmaceutical Corp., Seoul, Republic of Korea. Other funding sources and the full list of investigators are available in File S1.
References
- 1. Lewis JD, Ferrara A, Peng T et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34: 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Home PD, Pocock SJ, Beck‐Nielsen H et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009; 373: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 3. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- 4. Corzo C, Griffin PR. Targeting the peroxisome proliferator‐activated receptor‐gamma to counter the inflammatory milieu in obesity. Diabetes Metab J 2013; 37: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colca JR, Tanis SP, McDonald WG, Kletzien RF. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin Investig Drugs 2014; 23: 1–7. [DOI] [PubMed] [Google Scholar]
- 6. Kim SG, Kim DM, Woo JT et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24‐weeks: a multicenter, randomized, double‐blind, parallel‐group, placebo controlled trial. PLoS One 2014; 9: e92843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim BY, Ahn JB, Lee HW et al. Synthesis and biological activity of novel substituted pyridines and purines containing 2,4‐thiazolidinedione. Eur J Med Chem 2004; 39: 433–447. [DOI] [PubMed] [Google Scholar]
- 8. Lee HW, Kim BY, Ahn JB et al. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem 2005; 40: 862–874. [DOI] [PubMed] [Google Scholar]
- 9. Kim JW, Kim JR, Yi S et al. Tolerability and pharmacokinetics of lobeglitazone (CKD‐501), a peroxisome proliferator‐activated receptor‐gamma agonist: a single‐ and multiple‐dose, double‐blind, randomized control study in healthy male Korean subjects. Clin Ther 2011; 33: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 10. Moon KS, Lee JE, Lee HS et al. CKD‐501, a novel selective PPARgamma agonist, shows no carcinogenic potential in ICR mice following oral administration for 104 weeks. J Appl Toxicol 2014; 34: 1271–1284. [DOI] [PubMed] [Google Scholar]
- 11. Lee HS, Chang M, Lee JE et al. Carcinogenicity study of CKD‐501, a novel dual peroxisome proliferator‐activated receptors alpha and gamma agonist, following oral administration to Sprague Dawley rats for 94–101weeks. Regul Toxicol Pharmacol 2014; 69: 207–216. [DOI] [PubMed] [Google Scholar]
- 12. Shin D, Kim TE, Yoon SH et al. Assessment of the pharmacokinetics of co‐administered metformin and lobeglitazone, a thiazolidinedione antihyperglycemic agent, in healthy subjects. Curr Med Res Opin 2012; 28: 1213–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supplementary Methods and Results, Acknowledgments.
Table S1. Patient demographics and baseline characteristics.
Table S2. Demographics and baseline characteristics of patients in extension study.
Table S3. Safety profiles in the safety population over the 24‐week study period.
Table S4. Adverse events additionally reported during the extension period (Week 25 to 52).
Figure S1. Patient dispositions.
Figure S2. Mean changes in fasting plasma glucose (FPG) levels from baseline.


