Abstract
Aim
This study sought to investigate dimensional changes to the alveolar bone following extraction and application of novel devices used for obturation of socket orifice (socket cap) and space maintenance in sockets with facial dehiscence (socket cage).
Material and methods
Six Macaca fascicularis had six teeth each removed according to the following intervention groups (groups A–C intact alveolar bone; D–E facial dehiscence): negative control (A); socket obturated with cap (B); filled with anorganic bovine bone mineral (ABBM) + socket cap (C); dehiscence negative control (D); socket cap + socket cage (E); ABBM + socket cap + socket cage (F). Serial CBCT scans at preoperatively, 6 and 12 weeks following intervention were compared to quantify linear alveolar bone alterations.
Results
Without therapeutic intervention, intact sockets exhibited significant reduction in width at the crestal 2 mm of the ridge crest within 6 weeks. Compared with the negative control sites which lost up to 52% of crestal bone width, sites treated with socket cap + ABBM lost at most 4% of bone width at the crestal 2 mm. Similar results were seen in the dehiscence groups, with the combination of socket cap + socket cage + ABBM maintaining the greatest socket width and height dimensions.
Conclusions
Results from the current non‐human primate study suggest that the socket cap and socket cage devices, when used in conjunction with xenograft proved effective in minimizing post‐extraction socket width loss and height seen in both intact sockets and sockets with facial dehiscence defects.
Keywords: alveolar bone, bone substitutes, CT imaging, extraction socket, wound healing
The alveolar process is a dynamic structure whose integrity and function are to a large extent tooth dependent. Loss of teeth predictably leads to resorption of the tooth‐bearing alveolus, a process that has been extensively studied in both animal models (Lekovic et al. 1997; Camargo et al. 2000; Botticelli et al. 2004; Elian et al. 2007; Hammerle et al. 2012) and humans (Schropp et al. 2003a,b; Chen et al. 2005; Kan et al. 2007; Valentini et al. 2010). These studies have shown that most of the resorption occurs during the first 3 months of healing, although dimensional changes can be observed up to 1 year or more after tooth extraction. Such changes result in approximately 50% reduction of the bucco‐lingual dimension of the alveolar ridge (Schropp et al. 2003a,b), mainly due to the resorption of the buccal bone plate (Araujo & Lindhe 2005). In spite of predictable negative post‐extraction resorption, no non‐human primate split‐mouth designed studies currently exist that examine both the efficacy of ridge preservation procedures and the ongoing dynamics of alveolar ridge dimensional changes following tooth removal.
Post‐extraction ridge resorption imposes significant limitations on subsequent efforts to restore lost dentition, both in terms of compromised implant placement, or less than ideal esthetic results obtained with traditional fixed prosthetic restorations. In response to predictable post‐extraction ridge remodeling, multiple ridge preservation procedures have evolved to preserve alveolar ridge volume and morphology. These include careful flapless tooth extraction designed to achieve undisturbed socket healing (Chen et al. 2005, 2007), immediate implant placement (Chen et al. 2005, 2007), grafting the post‐extraction socket with bone graft substitute materials, with or without barrier membranes (Fickl et al. 2008a,b), and placement of tissue engineered growth factors, that is, rhPDGF‐BB or rhBMP‐2, following tooth removal (Sigurdsson et al. 1997; Paolantonio et al. 2001; Devlin & Sloan 2002; Schropp et al. 2003a,b; Fickl et al. 2008a,b; Trombelli et al. 2008). However, to date, there has been no consensus verifying superior efficacy among these ridge preservation procedures. A systematic review by Vignoletti et al. (2012) concluded that different ridge preservation techniques can significantly reduce post‐extraction alveolar ridge resorption, but was unable to conclude superiority of one technique over another.
Multiple materials and techniques have been used in ridge preservation procedures, including xenograft (Araujo & Lindhe 2009; Mardas et al. 2010; Favero et al. 2013), allograft (Araujo & Lindhe 2011; Wallace 2013), and alloplastic materials. An important objective of ridge preservation procedures is the protection of the graft material from the oral environment during the healing process. A variety of both resorbable (Scheyer et al. 2012; Favero et al. 2013) and non‐resorbable (Lekovic et al. 1997) membranes have been used for this purpose. It remains controversial, however, whether it is best to achieve primary soft tissue closure over the grafted socket and membrane, or to leave the membrane exposed without changing the position of the gingival margin.
A common challenge in current ridge preservation procedures is the application of flat‐shaped membranes to the complex geometric configurations of extraction sockets. In an attempt to respond to limitations inherent in post‐extraction membrane placement, preformed novel devices have been fabricated as an alternative to membrane positioning in ridge preservation procedures. A dome‐shaped non‐resorbable device has been devised with different sizes to readily adapt to socket orifice in different oral sites. The purpose of this “socket cap” is to seal access to the underlying residual socket following tooth removal.
Additional post‐extraction challenge is the management of labial or buccal alveolar wall dehiscence defects. Currently, the most common technique designed for reconstruction of the missing facial plate is the “socket repair technique” (Elian et al. 2007), also known as the “ice cream cone technique”. A resorbable cage device has been designed to maintain the space within socket in the absence of such function normally played by the facial alveolar bone. The “socket cage” device functions to support and maintain normal 3‐dimensional volume of the socket in sites with facial wall dehiscence defects to prevent tissue collapse following tooth removal. The aims of this study were to (i) Investigate the dimensional changes occurring to the alveolar bone following tooth extraction in the non‐human primate animal model using serial CBCT studies and (ii) Examine the efficacy of socket cap and socket cage for ridge preservation and augmentation procedures following tooth extraction.
Material and methods
Animals
This animal study was carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Capital Medical University, Beijing, China. A total of six young adult male crab‐eating macaque (Macaca fascicularis), 8–12 years old, and weighing between 4.0 and 5.0 kg were included in this study. Before surgery, the animals were maintained in individual cages with water and food ad libitum.
Preoperatively, monkeys were sedated with subcutaneous injection of 5 mg/kg of ketamine (Jiang Su Heng Rui Medicine Co., LTD, Lianyun Gang, China). Anesthesia was achieved by veterinarian staff with i.v. propofol (8 mg/kg; Diprivan, Astra Zeneca, London, UK). Endotracheal intubation was performed using an oral‐tracheal tube with a diameter of 3.5 mm (Sheridan™, Teleflex Medical, Research Triangle Park, NC, USA). The anesthesia was maintained by i.v. propofol (2.5 mg/kg) per 30~45 min. Local anesthesia was achieved by intra‐mucosal injection of lidocaine with 1 : 100,000 epinephrine (Astra Zeneca, London, UK). Post‐operative care included analgesia by i.m. injection of carprofen (2 mg/kg; Harbin Pharmaceutical Group, Co., LTD, Harbin, China) for 3 days following surgery. Animals were maintained on a soft diet for 2 weeks post‐operatively. Oral hygiene measure consisted of spraying the teeth of animals with 0.2% chlorhexidine gluconate mouthwash (Etouch, Shandong, China) once a day. Mechanical biofilm removal was not attempted because of difficulty in performing this on conscious animals.
Extraction socket devices
Preformed socket devices were used to seal the orifice of sockets (SocketKAP™; Regenimmune, Woodland Hills, CA, USA) or to provide structural stability to sockets with loss of facial alveolar bone (SocketKAGE™; Regenimmune) (Fig. 1a,b). Socket cap was composed of polypropylene, and socket cage was produced from poly D, L‐lactic acid copolymer (PLLA; 5% D and 95% L‐lactite). In sockets with facial dehiscence, socket cage was placed to maintain normal 3‐dimensional residual socket geometry (Fig. 2). An appropriately sized socket cap was secured over the socket orifice with the aid of sutures (USP 4.0 PTFE with CS‐0618 RC needle, Cytoplast; Osteogenics Biomedical, Lubbock, TX, USA) that passed through channels provided on the top dome of the cap. When called for by the study protocol, anorganic bovine bone mineral (ABBM) (Bio‐Oss® Geistlich Pharma, AB, Wolhusen, Switzerland) was the bone graft substitute material placed within residual sockets following tooth removal. All surgical procedures were performed by four of the coauthors (H.H.Z., J.T., Y.X., and J.X.).
Figure 1.
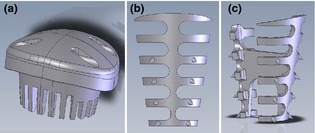
Extraction devices utilized in this study. Socket cap consists of a dome‐shaped device composed of polypropylene with channels on the superior surface for passage of suture (a). The socket cap was used for obturation of the extraction socket orifice and protection from the oral environment. Facial (b) and lateral (c) views of the socket cage illustrate a device consisting of a rigid series of inter‐connected ribs composed of PLLA utilized for support of sockets with facial dehiscence. The conical projections on ribs are intended as spacers to prevent direct contact with facial and lingual alveolar plates, to allow better blood circulation.
Figure 2.
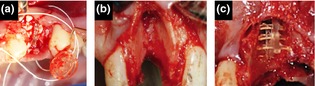
Clinical images of extraction socket devices utilized for treatment of extraction sockets. Placement of socket cap in situ (a). Teeth with intact alveolar bone were extracted with flapless approach, and the socket cap was secured to the opening of sockets with aid of PTFE sutures. Surgically induced facial dehiscence (b), A mucoperiosteal flap was elevated to expose the facial alveolar bone. Following tooth extraction, the entire facial plate was surgically removed from crest to apex, extending to interproximal line angles. Placement of socket cage in situ (c).
Defect models following extractions
To examine device effectiveness, two defect models were utilized in this study following tooth removal: (i) Intact extraction socket wall model (socket cap) or (ii) Facial dehiscence defect model (socket cap + socket cage), each with or without ABBM. Prior to surgery, each animal received a dental prophylaxis followed by a 0.12% chlorhexidine gluconate solution surgical site wash. Maxillary incisors, premolars and molars, and mandibular premolars and molars were atraumatically extracted in each monkey, followed by thorough degranulation of any soft tissue remnants. A surgical defect of the dehiscence defect model facial bony plates was removed from the alveolar crest to the tooth apex (Fig. 2).
Six intervention groups were planned by random allocation in advance of the experiments. The considerations for the allocations included the fact that 36 teeth were going to be extracted in six animals in different oral regions, so that in each group incisors, premolars and molars were included. The following intervention groups were executed:
Group A: Intact Socket Unfilled and Uncovered (Negative Control)
Group B: Intact Socket obturated with Socket cap without filler
Group C: Intact Socket Filled with ABBM and Covered with Socket cap
Group D: Facial Dehiscence Socket Unfilled and Uncovered (Negative Control)
Group E: Facial Dehiscence Sockets supported by Socket cage and Covered with Socket cap without filler
Group F: Facial Dehiscence Sockets reconstructed with Socket cage, ABBM and Covered with Socket cap
At 4 weeks, the non‐resorbable socket cap device, which had been sutured in place with PTFE sutures, was removed from all defect sites.
CBCT analysis
Live animals were scanned with CBCT scan at baseline and then at 6 and 12 weeks after surgery, followed by quantitative analysis to measure new bone formation at defined locations within the grafted sites. In addition, three‐dimensional reconstruction of bone and soft tissues was performed. Each specimen was placed in a sample holder and scanned using high resolution. After scanning, the 2D image data were stored in Digital Imaging and Communications in Medicine (DICOM) format and then transferred to a computer for 3D reconstruction and analysis. The bone tissues were segmented using a global thresholding procedure. Threshold equaled to −360 HU was used to investigate bone tissues within the defects. The proportion of bone volume occupying the defect virtual spaces was measured, allowing quantitative comparisons among Groups A – F. Bone volume within any defect area was measured using Simplant® software (Dentsply Implants, Waldham, MA, USA).
CBCT linear measurements at baseline, 6, and 12 weeks post‐surgery using Simplant® software were as follows: (i) Bone width at different levels (at 0, 1, 2, 3, and 5 mm) apical to the alveolar bone crest and (ii) Bone height at the buccal, middle, and lingual thirds of the examined alveolus relative to the bone crest and root apex at baseline. Fig. 3 illustrates the anatomic landmarks used as reference points for bone width and height at 6 and 12 weeks included the tooth apex and the marginal bone crest at adjacent teeth. The absolute measurements of the alveolar width and height are listed in Table S1a. As teeth with varying sizes from all anatomic areas were represented in each group, percentage of change from baseline was calculated and the values are presented in Table S1b. The percentage of bone remaining at each time point and location was calculated using the formula of: (Dimension of the alveolar bone at follow‐up) × 100/(Dimension of the alveolar bone at baseline). One examiner (S.M.) performed all CBCT measurements. Ten percent of the sites were randomly selected for repeated measurement. The second measurement differed <5% from first measurement.
Figure 3.
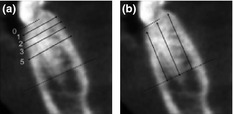
The radiographic landmarks used for measurement of bone width and bone height on CBCT images. The bone crest and tooth apex were demarcated as dotted lines. Bone width was measured at 1, 2, 3, and 5 mm relative to the alveolar bone crest (a). Bone height was measured at facial/buccal, middle, and lingual third of the alveolar bone relative to bone crest and root apex (b).
Statistical analysis
Statistical analysis was performed using SPSS v. 18, IBM, Chicago, IL, USA and R 3.0.2 Statistical Software, Institute for Statistics and Mathematics (WU, Wien, Austria) and Microsoft Excel 2013, Microsoft Co., Redmond, WA, USA. We examined the effects of treatment (A, B, C, D, E, and F), location (widths or heights), and week in analysis of variance models, using (i) absolute change from baseline or (ii) percent change from baseline as the dependent variable, with or without interaction terms. To deal with possible non‐normality of distributions, we repeated the analyses using (iii) rank of absolute change from baseline and (iv) rank of percent change from baseline (comparable to a Wilcoxon's analysis or Kruskal–Wallis analysis, but allowing use of covariates). For all these models, there were significant differences between treatments and significant differences between locations. Week was significant in some but not all models. Interaction terms were significant in some but not all models. Table 1 shows the ANOVA results for the model with the dependent variable rank of absolute change from baseline, and the independent variables treatment, location, and week, with two‐way and three‐way interaction terms. The P‐value for treatment in other models was in some cases better than and in some cases worse than in this example, but in all models, treatment was significant.
Table 1.
ANOVA table for model with dependent variable rank of absolute change from baseline, and independent variables treatment, location, and week, with two‐way and three‐way interaction terms
| df | Sum Sq | Mean Sq | F value | Pr (>F) | |
|---|---|---|---|---|---|
| Treatment | 5 | 941,354 | 188,271 | 10.6671 | 1.15E‐09 |
| Location | 6 | 1,436,590 | 239,432 | 13.5658 | 4.33E‐14 |
| Week | 1 | 204,611 | 204,611 | 11.5929 | 0.000726 |
| Treatment : Location | 30 | 475,888 | 15,863 | 0.8988 | 0.623581 |
| Treatment : Week | 5 | 59,553 | 11,911 | 0.6748 | 0.642737 |
| Location : Week | 6 | 44,996 | 7499 | 0.4249 | 0.862404 |
| Treatment : Location : Week | 30 | 92,543 | 3085 | 0.1748 | 1 |
| Residuals | 420 | 7,412,853 | 17,650 |
The hypothesis tests indicate that the treatment groups differed significantly in absolute change, percent change, and ranked change from baseline. To further examine the treatment effects, we performed separate pairwise t‐tests for all combinations of week, treatment group (pairwise for treatments {A, B, C} or {D, E, F}), and location (widths and heights). These post hoc tests have low power because of the small number of observations in each comparison. In addition, unlike the global hypothesis tests described above, these post hoc t‐tests require adjustment for multiple comparisons. Of 48 post hoc t‐tests for treatment effect on width, 21 are significant (P < 0.05) without correction for multiple comparisons, while seven are significant after Bonferroni's correction for multiple comparison. Of 36 post hoc t‐tests for treatment effect on height, six are significant (P < 0.05) without correction for multiple comparisons, while one is significant after Bonferroni's correction. These results are expected due to the large number of tests performed, the small number of observations used in each test, and the conservative nature of the Bonferroni test. The P‐values for all comparisons are listed in Table S2.
Results
Clinical observations
Throughout the study, all surgical sites healed uneventfully with minimal inflammation and no signs of infection.
Radiographic CBCT measurements
Bone width at 6 weeks: intact sockets
Group A Negative Control: Significant loss of bone width occurred, especially at 1 and 2 mm apical to the alveolar crest (Figs 4 and 5a; Table S1). At 6‐week post‐surgery, the percentage of remaining bone width were 18.9 ± 23.3%, 48.2 ± 10.6%, 84.4 ± 13.2%, and 91.5 ± 7.2% at 1, 2, 3, and 5 mm from the crest, respectively (Fig. 5a).
Figure 4.
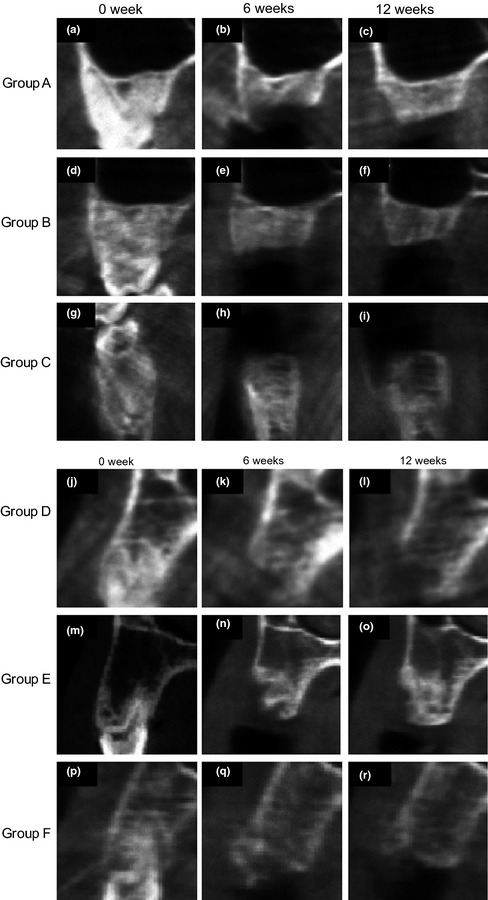
Representative CBCT images at baseline, 6 and 12 weeks after extraction. Groups A–C had intact alveolar socket walls. Facial bony dehiscence was created for groups D–F. Group A (negative control), Group B (socket cap), Group C (socket cap plus ABBM), Group D (negative control), Group E (socket cap and socket cage), and Group F (socket cap, socket cage plus ABBM).
Figure 5.
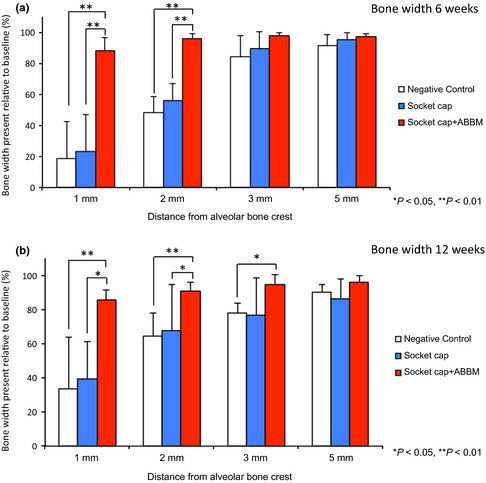
(a) The dimensional changes at 6 weeks following treatment of extraction sockets of teeth with intact alveolar bone is shown in Fig. a. Results revealed significant differences in remaining percentage of bone width at 1 and 2 mm from the crest among the treatment groups, with statistically significantly greater percentages of bone noted for socket cap + ABBM vs. socket cap alone or the unfilled negative control (**P < 0.01). (b) At 12 weeks, results revealed the percentage of bone width in intact sockets present at 1, 2, and 3 mm relative to the bone crest were significantly different among the three treatment groups. Ridge width was maintained best (~85%) for sockets filled with ABBM and covered with socket cap relative to other groups. Socket cap + ABBM group also demonstrated greater bone width at 3 mm compared to the negative control (*P < 0.05). No significant differences in bone width were seen between the negative control and socket cap alone groups.
Group B Socket cap Only: When socket cap was used to seal sockets without a bone filler, approximately 23.1 ± 24.3%, 56.1 ± 10.6%, 89.2 ± 11.3%, and 95.2 ± 4.8% remained at 1, 2, 3, and 5 mm from the crest, respectively. At 6 weeks, the bone widths of groups A and B were not statistically significantly different (Fig. 5a).
Group C Socket cap + ABBM: When sockets were filled with ABBM and covered with socket cap, approximately 87.8 ± 8.7%, 96.2 ± 3%, 98.1 ± 1.6%, and 97.0 ± 2.4% bone width remained at 1, 2, 3, and 5 mm from the crest, respectively. At 1 and 2 mm from the bone crest, the percentage of bone width remaining in Group C was statistically significantly higher than the remaining bone width in groups A or B (Fig. 5a).
Bone width at 12 weeks: intact sockets
Group A Negative Control: Significant loss of bone width persisted, especially at 1 mm apical to the alveolar crest (Figs 4 and 5b). After 12 weeks, the percentages of bone width remaining were 33.5 ± 30%, 64.4 ± 13.7%, 77.7 ± 6.3%, and 89.8 ± 5.1% at 1, 2, 3, and 5 mm from the crest, respectively (Fig. 5b).
Group B Socket cap Only: Approximately 39.2 ± 22.2%, 67.7 ± 27.2%, 76.7 ± 21.7%, and 86.4 ± 11.5% remained at 1, 2, 3, and 5 mm from the crest, respectively, at 12 weeks (Fig. 5b).
Group C Socket cap + ABBM: When sockets were filled with ABBM and covered with Socket cap, 85.3 ± 6.4%, 90.6 ± 5.2%, 94.5 ± 5.5%, and 96.0 ± 3.6% of the original bone width remained at 1, 2, 3, and 5 mm from the crest, respectively. At 1 and 2 mm from the bone crest, the percentage of bone width remaining in Group C was statistically significantly higher than the remaining bone width in groups A or B and statistically significantly greater than Group A at 3 mm from the ridge crest (Fig. 5b).
Bone height at 6 weeks: intact sockets
Group A Negative Control: Significant loss of bone height occurred, most notably at the buccal aspects of the ridge (Figs 4 and 6a). At 6‐week post‐surgery, the percentages of bone height remaining were 52.8 ± 17.9%, 77.2 ± 17.6%, and 85.6 ± 10.7% at the buccal, middle, and lingual aspects of sockets, respectively.
Figure 6.
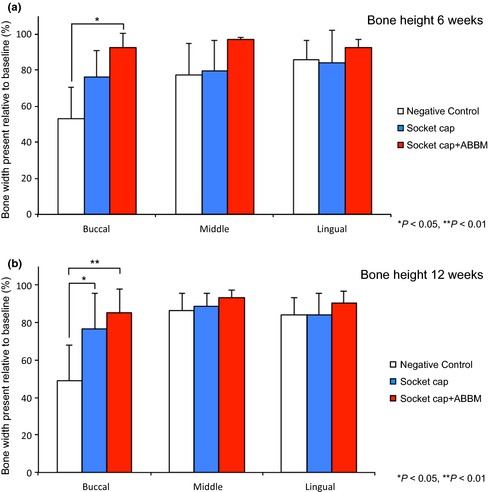
(a) The dimensional changes in height at 6 weeks following treatment of extraction sockets of teeth with intact alveolar bone are shown in Fig. a. At the buccal aspect socket, cap + ABBM exhibited significantly greater bone height compared to the negative control (*P < 0.05). (b) Dimensional bone height changes at 12 weeks following treatment of extraction sockets of teeth with intact alveolar bone are shown in Fig. b. At the buccal aspect, Group B (socket cap alone) and Group C (socket cap + ABBM) exhibited significantly greater bone height when compared to the negative control (*P < 0.05, **P < 0.01).
Group B Socket Cap Only: No statistically significant differences in height at 6 weeks were observed between Group B and Groups A and C at any measured location (Fig. 7a).
Figure 7.
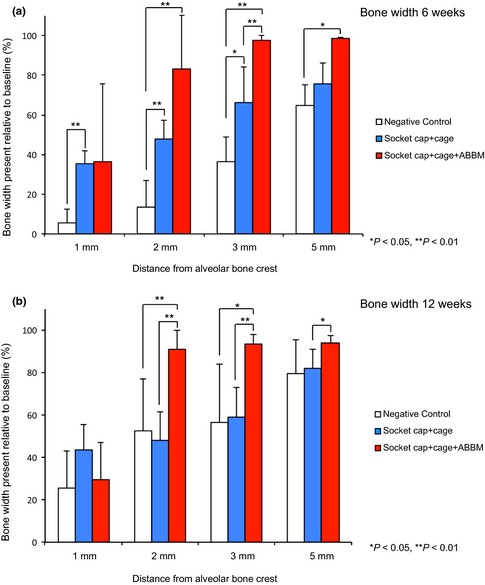
(a) The dimensional changes at 6 weeks following treatment of extraction sockets of teeth with facial dehiscence are shown in Fig. a. Group F (socket cap + socket cage + ABBM) exhibited statistically significantly greater bone width at 2, 3, and 35 mm from the alveolar bone crest compared to other groups (*P < 0.05, **P < 0.01). Group E (socket cap + socket cage) demonstrated greater bone width than the negative control group at 1, 2, and 3 mm from the crest (*P < 0.05, **P < 0.01). (b). The dimensional width changes at 12 weeks following treatment of extraction sockets of teeth with facial dehiscence are shown in Fig. b. Significant differences were seen among the three groups at 2, 3, and 5 mm from the alveolar crest. Group F (socket cap + socket cage + ABBM) exhibited statistically significantly greater bone width at 2 and 3 mm relative to the alveolar bone crest compared to Groups D and E (*P < 0.05, **P < 0.01) and significantly greater bone width at 5 mm compared to Group E (socket cap + socket cage). No significant differences were seen between Groups D and E at any distance from the alveolar crest.
Group C Socket cap + ABBM: When sockets were filled with ABBM and covered with socket cap most of the loss of vertical bone height was avoided, that is, 92.5 ± 8.1%, 97.1 ± 1.2%, and 92.4 ± 4.5% remained at buccal, middle, and lingual aspects of sockets, respectively. The difference between the loss of vertical height between groups A and C was significant in the buccal aspect (P < 0.05) (Fig. 6a).
Bone height at 12 weeks: intact sockets
Group A Negative Control: Significant loss of bone height occurred, most notably at the buccal aspects of the ridge (Figs 4 and 6b). At 12‐week post‐surgery, the percentages of bone height remaining were 49.2 ± 18.9%, 86.5 ± 9.1%, and 84.2 ± 9.2% in the buccal, middle, and lingual aspects, respectively.
Group B Socket cap Only: Vertical bone height at the buccal aspect was significantly greater compared with the negative control group (P < 0.05).
Group C Socket cap + ABBM: Most of the loss of vertical bone height was avoided with 85.0 ± 12.7%, 93.4 ± 4.1%, and 90.4 ± 6.6% bone height remaining in the buccal, middle, and lingual aspects, respectively. At 12 weeks, in intact sockets, significantly greater percentages of bone height remained in Group C compared with the negative control Group A (P < 0.05, P < 0.01).
Bone width at 6 weeks: facial dehiscence sockets
Group D Negative Control: Significant loss of width occurred at 6 weeks, with the majority of bone width loss occurring at 1, 2, and 3 mm from the crest (Figs 4 and 7a). At 6 weeks, 5.5 ± 6.9%, 13.4 ± 13.4%, 36.6 ± 12.3%, 83.2 ± 10.6% remained at 1, 2, 3, and 5 mm from crest, respectively.
Group E Socket cap + Socket cage Only: At 6 weeks, the bone width remaining was significantly greater than in negative control sites at 1, 2, and 3 mm from the crest, with 35.3 ± 6.3%, 47.8 ± 9.4%, 66.3 ± 17.9%, and 84.8 ± 10.6% remaining at 1, 2, 3, and 5 mm from crest, respectively.
Group F: Socket cap + Socket cage + ABBM: At 6 weeks, the remaining bone width was statistically significantly greater than Groups D and E at 2 and 3 mm from the alveolar crest and significantly greater than Group D negative control at 5 mm (P < 0.05, P < 0.01).
Bone width at 12 weeks: facial dehiscence sockets
Group D negative control: At 12 weeks, improvement in remaining width was seen compared with week six findings, with 25.6 ± 17.6%, 52.5 ± 24.8%, 56.3 ± 27.8%, and 79.5 ± 16.2% remaining at 1, 2, 3, and 5 mm from the crest, respectively (Figs 4 and 7b).
Group E Socket cap + Socket cage Only: At 12 weeks, 43.4 ± 12.1%, 48.0 ± 13.5%, 59.0 ± 14.2%, and 82.0 ± 8.6% bone width remained at 1, 2, 3, and 5 mm from the crest, respectively. No significant differences in bony width were seen between Group D and Group E at any level from the alveolar crest at 12 weeks.
Group F: Socket cap + Socket cage + ABBM: At 12 weeks, the bone width remaining was statistically significantly greater in Group F compared with both other groups at 2 and 3 mm from the alveolar bone crest (P < 0.05, P < 0.01). At 5 mm from the bone crest, Group F also demonstrated significantly greater bone width compared to Group E.
Bone height at 6 weeks: facial dehiscence sockets
Group D negative control: At 6 weeks, sites with facial dehiscence lost a significant degree of bone height, with 40.4 ± 12%, 63.8 ± 24%, and 87.1 ± 11.5% of bone height remaining at the buccal, middle, and lingual aspects, respectively, when no device or grafting material was placed (Figs 4 and 8a).
Figure 8.
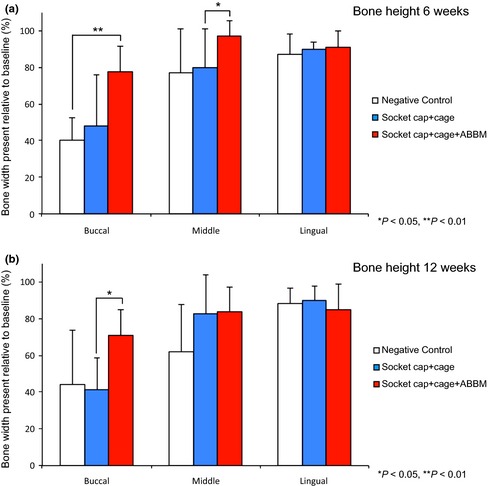
(a) The dimensional changes in height at 6 weeks following treatment of extraction sockets of teeth with facial dehiscence are shown in Fig. a. Statistically significant differences in bone height are noted between Group F (socket cap + socket cage + ABBM) and Group D negative control at the buccal aspect and between Group F and Group E (socket cap + socket cage) at the middle aspect of the treated sockets. (b) The dimensional changes in height at 12 weeks following treatment of extraction sockets of teeth with facial dehiscence are shown in Fig. b. At 12 weeks, the only statistically significant difference among the three groups was noted at the buccal aspect between Group F (socket cap + socket cage + ABBM) and Group E (socket cap + socket cage) (*P < 0.05).
Group E Socket cap + Socket cage Only: At 6 weeks, no significant differences in height were observed at any location between Group E and the negative control Group D sites.
Group F: Socket cap + Socket cage + ABBM: Significant differences in height in favor of Group F were noted at 6 weeks compared with Group D at the buccal aspect and Group E at the middle aspect of the treated sockets.
Bone height at 12 weeks: facial dehiscence sockets
Group D negative control: At 12 weeks, the remaining bone height of Group D remained similar to the height observed at 6 weeks (Figs 4 and 8b). At 12 weeks, 44.1 ± 29.7%, 62.3 ± 25.6%, and 88.3 ± 8.5% bone height in this facial dehiscence‐treated group were noted at buccal, middle, and lingual aspects, respectively.
Group E Socket cap + Socket cage Only: No statistically significant differences in height were observed at any measured location between Group E and Group D and between Group E and Group F at the middle and lingual aspects. There was, however, a statistically significant difference seen at the buccal aspect, between Group E and Group F, in favor of Group F.
Group F: Socket cap + Socket cage + ABBM: At 12 weeks, the only statistically significant finding was a greater percentage of bone height of Group F sites compared with Group E sites at the buccal aspect (P < 0.05).
Discussion
Proper management of post‐extraction ridge resorption is critical to the development of sites with adequate bone volume and shape needed for implant placement or for conventional fixed prostheses designed for optimal function and esthetics. A variety of current ridge preservation protocols attempt to reduce the magnitude of negative ridge remodeling that invariably occurs following tooth removal. This study was undertaken to better understand the magnitude and kinetics of post‐extraction ridge remodeling. The non‐human primate model was chosen as the study vehicle because of its closeness to humans, and therefore, its potential ability to extrapolate results relevant to clinical practice and as a guide for future human clinical trials. In addition, the effectiveness of newly developed extraction socket devices was also evaluated in this study using CBCT with linear measurement outcomes.
The present data demonstrated that without therapeutic intervention, post‐extraction remodeling in this non‐human primate model leads to predictable alveolar bone width reduction within 6 weeks in intact sockets within the first 2 mm apical to the ridge crest. These results are consistent with those observations noted in human clinical (Camargo et al. 2000; Schropp et al. 2003a,b) and canine (Cardaropoli et al. 2003; Araujo & Lindhe 2005) models. The socket cap device examined in this study, when used in conjunction with a xenograft material proved effective in reducing the magnitude of crestal ridge width loss. Compared with the negative control sites which lost up to 81% of crestal bone width, sites treated with socket cap + ABBM lost at most 13% of bone width within the 2 mm zone apical to the ridge crest. Similar statistically significant results were seen when comparing buccal height loss experienced in the untreated negative controls to those intact sockets treated with socket cap + ABBM at both 6 and 12 weeks. In a recent systematic review, Hammerle et al. (2012) concluded that the alveolar ridge undergoes a mean horizontal width reduction of 3.8 mm and a mean vertical height reduction of 1.24 mm within 6 months after tooth extraction. Vignoletti et al. (2012) in their meta‐analyses of data demonstrated a statistically significant greater ridge reduction in bone height of 1.4 mm and bone width of 1.8 mm for control sockets without intervention as compared to test sites with ridge preservation.
Unlike intact sockets, little data are currently available for extraction sockets with facial dehiscence, nor have the reconstruction protocols treating facial dehiscence been validated with experimental data. Therefore, the present data are an important first step in leading to a more comprehensive understanding of the trajectories of healing that occur when attempting to reconstruct the missing facial bony plate. The results in this study, both for alveolar width and height at both time periods, suggest that a protocol using Socket cap + Socket cage + ABBM following tooth removal in sockets with severe labial dehiscence defects will likely be more effective in restoring normal dimensional anatomy when compared to the other groups in this non‐human primate study. However, the facial dehiscence data exhibited larger standard deviations than seen for data derived from intact sockets. In view of the small number of sites investigated in this study, it is impossible to determine whether treatment with socket cap + socket cage, without additional bone filler, will be effective in restoration of bone height and width in the presence of significant labial dehiscence defects.
Previous canine animal model studies have demonstrated that placement of implants in sites with facial dehiscence is accompanied with only partial restoration of lost facial bone (Botticelli et al. 2004). Moreover, implants placed into human sites with facial dehiscence tend to lead to higher early failure (Valentini et al. 2010), more mucosal recession (Kan et al. 2007), fewer sites with bone fill (Schropp et al. 2003a,b), and greater peri‐implant horizontal bone resorption (Chen et al. 2005, 2007). Evidence therefore suggests that it may be clinically important to reconstruct the missing facial plate prior to implant placement.
The current study's outcome parameters looked at linear ridge dimensions using serial CBCT. Further studies examining the quality of regenerated bone through histologic and histomorphometric analysis will be required in further determining the efficacy of the devices under present examination. Such studies are currently being conducted.
Although important, the results derived from the current non‐human primate model need to be verified with comprehensive, randomized controlled human clinical trials. There is presently an ongoing human clinical trial, which seeks to investigate the efficacy of ridge preservation and reconstructive techniques using the socket cap and socket cage devices. It is anticipated that results from this human study will be useful in validating the utility of these extraction socket devices in the clinical post‐extraction management of extraction sockets.
In conclusion, results from the current non‐human primate animal model suggest that the socket cap and socket cage devices, when used in conjunction with an appropriate bone filler, may prove effective in treating post‐extraction socket width loss and height seen in both intact sockets and sockets with significant labial dehiscence defects.
Supporting information
Table S1. (a). Alveolar bone width and height (mm) of teeth in 6 intervention groups (A, B, C, D, E, F) prior to tooth extraction and at 6 and 12 weeks following tooth extraction. (b) The percentage of alveolar bone width and height of teeth in 6 intervention groups (A, B, C, D, E, F) prior to tooth extraction and at 6 and 12 weeks following tooth extraction.
Table S2. (a) Statistical significance of the absolute values of the alveolar bone width and height (mm) comparisons among the 6 intervention groups (A, B, C, D, E, F). (b) Statistical significance of the percentage change of the alveolar bone width and height relative to baseline among the 6 intervention groups (A, B, C, D, E, F).
Acknowledgements
The expert assistance of Prof. Robert Keim, University of Southern California Orthodontic department and Prof. Michael Walker, Stanford University with statistical analysis is acknowledged. This study was partly supported by Capital Medical University, School of Stomatology, Beijing, China as well as, Qassim University, College of Dentistry, Saudi Arabia. The authors report no conflicts of interest related to this study.
Min S, Liu Y, Tang J, Xie Y, Xiong J, You H‐K, Zadeh HH. Alveolar ridge dimensional changes following ridge preservation procedure with novel devices: Part 1 – CBCT linear analysis in non‐human primate model. Clin. Oral Impl. Res. 27, 2016, 97–105 doi: 10.1111/clr.12521
References
- Araujo, M.G. & Lindhe, J. (2005) Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology 32: 212–218. [DOI] [PubMed] [Google Scholar]
- Araujo, M.G. & Lindhe, J. (2009) Ridge preservation with the use of Bio‐Oss collagen: a 6‐month study in the dog. Clinical Oral Implants Research 20: 433–440. [DOI] [PubMed] [Google Scholar]
- Araujo, M.G. & Lindhe, J. (2011) Socket grafting with the use of autologous bone: an experimental study in the dog. Clinical Oral Implants Research 22: 9–13. [DOI] [PubMed] [Google Scholar]
- Botticelli, D. , Berglundh, T. & Lindhe, J. (2004) Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri‐implant bone. An experimental study in the dog. Journal of Clinical Periodontology 31: 309–317. [DOI] [PubMed] [Google Scholar]
- Camargo, P.M. , Lekovic, V. , Weinlaender, M. , Klokkevold, P.R. , Kenney, E.B. , Dimitrijevic, B. , Nedic, M. , Jancovic, S. & Orsini, M. (2000) Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 90: 581–586. [DOI] [PubMed] [Google Scholar]
- Cardaropoli, G. , Araujo, M. & Lindhe, J. (2003) Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. Journal of Clinical Periodontology 30: 809–818. [DOI] [PubMed] [Google Scholar]
- Chen, S.T. , Darby, I.B. , Adams, G.G. & Reynolds, E.C. (2005) A prospective clinical study of bone augmentation techniques at immediate implants. Clinical Oral Implants Research 16: 176–184. [DOI] [PubMed] [Google Scholar]
- Chen, S.T. , Darby, I.B. & Reynolds, E.C. (2007) A prospective clinical study of non‐submerged immediate implants: clinical outcomes and esthetic results. Clinical Oral Implants Research 18: 552–562. [DOI] [PubMed] [Google Scholar]
- Devlin, H. & Sloan, P. (2002) Early bone healing events in the human extraction socket. International Journal of Oral Maxillofacial Surgery 31: 641–645. [DOI] [PubMed] [Google Scholar]
- Elian, N. , Cho, S.C. , Froum, S. , Smith, R.B. & Tarnow, D.P. (2007) A simplified socket classification and repair technique. Practical Procedures & Aesthetic Dentistry: PPAD 19: 99–104; quiz 106. [PubMed] [Google Scholar]
- Favero, G. , Lang, N.P. , Romanelli, P. , Pantani, F. , Caneva, M. & Botticelli, D. (2013) A digital evaluation of alveolar ridge preservation at implants placed immediately into extraction sockets: an experimental study in the dog. Clinical Oral Implants Research: 1–7. [DOI] [PubMed] [Google Scholar]
- Fickl, S. , Zuhr, O. , Wachtel, H. , Bolz, W. & Huerzeler, M. (2008a) Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. Journal of Clinical Periodontology 35: 356–363. [DOI] [PubMed] [Google Scholar]
- Fickl, S. , Zuhr, O. , Wachtel, H. , Stappert, C.F. , Stein, J.M. & Hurzeler, M.B. (2008b) Dimensional changes of the alveolar ridge contour after different socket preservation techniques. Journal of Clinical Periodontology 35: 906–913. [DOI] [PubMed] [Google Scholar]
- Hammerle, C.H. , Araujo, M.G. , Simion, M. & Osteology Consensus, G. (2012) Evidence‐based knowledge on the biology and treatment of extraction sockets. Clinical Oral Implants Research 23(Suppl 5): 80–82. [DOI] [PubMed] [Google Scholar]
- Kan, J.Y. , Rungcharassaeng, K. , Sclar, A. & Lozada, J.L. (2007) Effects of the facial osseous defect morphology on gingival dynamics after immediate tooth replacement and guided bone regeneration: 1‐year results. Journal of Oral Maxillofacial Surgery 65(7 Suppl 1): 13–19. [DOI] [PubMed] [Google Scholar]
- Lekovic, V. , Kenney, E.B. , Weinlaender, M. , Han, T. , Klokkevold, P. , Nedic, M. & Orsini, M. (1997) A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. Journal of Periodontology 68: 563–570. [DOI] [PubMed] [Google Scholar]
- Mardas, N. , Chadha, V. & Donos, N. (2010) Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine‐derived xenograft: a randomized, controlled clinical trial. Clinical Oral Implants Research 21: 688–698. [DOI] [PubMed] [Google Scholar]
- Paolantonio, M. , Dolci, M. , Scarano, A. , d'Archivio, D. , di Placido, G. , Tumini, V. & Piattelli, A. (2001) Immediate implantation in fresh extraction sockets. A controlled clinical and histological study in man. Journal of Periodontology 72: 1560–1571. [DOI] [PubMed] [Google Scholar]
- Scheyer, E.T. , Schupbach, P. & McGuire, M.K. (2012) A histologic and clinical evaluation of ridge preservation following grafting with demineralized bone matrix, cancellous bone chips, and resorbable extracellular matrix membrane. The International Journal of Periodontics and Restorative Dentistry 32: 543–552. [PubMed] [Google Scholar]
- Schropp, L. , Kostopoulos, L. & Wenzel, A. (2003a) Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: a prospective clinical study. International Journal of Oral Maxillofacial Implants 18: 189–199. [PubMed] [Google Scholar]
- Schropp, L. , Wenzel, A. , Kostopoulos, L. & Karring, T. (2003b) Bone healing and soft tissue contour changes following single‐tooth extraction: a clinical and radiographic 12‐month prospective study. The International Journal of Periodontics and Restorative Dentistry 23: 313–323. [PubMed] [Google Scholar]
- Sigurdsson, T.J. , Fu, E. , Tatakis, D.N. , Rohrer, M.D. & Wikesjo, U.M. (1997) Bone morphogenetic protein‐2 for peri‐implant bone regeneration and osseointegration. Clinical Oral Implants Research 8: 367–374. [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Farina, R. , Marzola, A. , Bozzi, L. , Liljenberg, B. & Lindhe, J. (2008) Modeling and remodeling of human extraction sockets. Journal of Clinical Periodontology 35: 630–639. [DOI] [PubMed] [Google Scholar]
- Valentini, P. , Abensur, D. , Albertini, J.F. & Rocchesani, M. (2010) Immediate provisionalization of single extraction‐site implants in the esthetic zone: a clinical evaluation. The International Journal of Periodontics and Restorative Dentistry 30: 41–51. [PubMed] [Google Scholar]
- Vignoletti, F. , Matesanz, P. , Rodrigo, D. , Figuero, E. , Martin, C. & Sanz, M. (2012) Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clinical Oral Implants Research 23(Suppl 5): 22–38. [DOI] [PubMed] [Google Scholar]
- Wallace, S.C. (2013) Guided bone regeneration for socket preservation in molar extraction sites: histomorphometric and 3D computerized tomography analysis. The Journal of Oral Implantology 39: 503–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. (a). Alveolar bone width and height (mm) of teeth in 6 intervention groups (A, B, C, D, E, F) prior to tooth extraction and at 6 and 12 weeks following tooth extraction. (b) The percentage of alveolar bone width and height of teeth in 6 intervention groups (A, B, C, D, E, F) prior to tooth extraction and at 6 and 12 weeks following tooth extraction.
Table S2. (a) Statistical significance of the absolute values of the alveolar bone width and height (mm) comparisons among the 6 intervention groups (A, B, C, D, E, F). (b) Statistical significance of the percentage change of the alveolar bone width and height relative to baseline among the 6 intervention groups (A, B, C, D, E, F).


