Abstract
Aim
This study evaluated the effects of a topical herbal patch (PerioPatch®) for gingival wound healing in a rat model.
Materials and Methods
A mid‐crestal incision was performed on each side of the edentulous anterior maxilla in 48, 6‐month‐old, Wistar rats. Full‐thickness flaps were raised, repositioned and sutured. Four experimental groups were established: herbal patch, placebo patch, no patch and no patch and no surgery. Patches were placed immediately after surgery and replaced every 12 h for the following 3 days. Half of the animals were killed after 5 and the remaining ones after 12 days. Tissue blocks were retrieved and processed for histological and immunohistochemical evaluation. Epithelial gap, collagen contents, amount of macrophages, cellular proliferation and vascular contents were evaluated in the central incision area. Statistical analysis consisted of two‐way anova.
Results
The herbal patch group presented the smallest epithelial gap at 12 days, the highest collagen content both at 5 and 12 days, a larger number of proliferating cells at day 5 and more numerous blood vessels at day 12. Macrophage number was similar in all groups.
Conclusion
Herbal patch improved wound healing in this animal model.
Keywords: animal study, collagen, epithelium, herbal, histology, immunohistochemistry, inflammation, topical, wound healing
Inflammation is necessary for the effective defence against pathogens and to set in motion tissue repair following injury (Romero‐Cerecero et al. 2014). However, exaggerated inflammatory mechanisms may compromise results of bone and tissue regeneration procedures, especially where soft tissue dehiscence, membrane exposure or both occur (Carlson & Monteleone 2004, Polimeni et al. 2004a,b).
A controlled inflammatory process with rapid healing of the soft tissue wound created during the surgical procedures is critical for their success. Effective wound healing requires a highly organized series of events that comprise inflammation, re‐epithelialization, keratinocyte and fibroblast proliferation, matrix deposition and angiogenesis.
PerioPatch® ([PP], Izun Pharmaceuticals Ltd. Jerusalem, Israel) has been applied to enhance healing of various types of oral wounds, injuries and ulcerations of the gingival and oral mucosa, including stomatitis, minor chaffing and traumatic ulcers and lesions associated with oral surgery. According to the manufacturer specifications, it is composed of two layers, an outer layer composed of a non‐absorptive non‐dissolving matrix that allows for slow dissolution of the inner layer, composed of a gel containing natural plant extracts. The inner layer of the patch is self‐adhesive and protects the underlying tissues by forming a protective seal over inflamed gingiva and oral mucosa while at the same time, due to its herbal components, reducing the signs and symptoms of inflammation. At some interval after application, the outer layer loosens and detaches. The gel layer may remain in place for up to 6 h.
PP contains extracts of three herbs: Centella asiatica, Echinacea purpurea and Sambucus nigra which have been shown to present healing effects, especially wound healing and collagen synthesis in skin (Kim et al. 2011), to positively affect the expression of genes involved in angiogenesis and the remodelling of extracellular matrix (Coldren et al. 2003) and be effective in reducing gingival inflammation (Sastravaha et al. 2003, 2005, Harokopakis et al. 2006). Centella Asiatica extract presents a multiplicity of actions associated to six important mechanisms: oedema and capillary filtration control; strong antioxidant power, effective on several forms of oxidative stress associated to inflammation or infections and synergic with other antioxidant products; an anti‐inflammatory action; a modulation of the collagen production avoiding slower scarring or faster, hyperthrophic scarring and cheloids; a modulating action of local growth factors; a modulation of angiogenesis (Belcaro et al. 2011).
PP has been evaluated in previous clinical trials following non‐surgical periodontal treatment with positive results (Grbic et al. 2011, Samuels et al. 2012, Levine et al. 2013) and no adverse effects. In these studies, PP was found to provide beneficial outcome by reducing signs of gingival inflammation when applied in conjunction with scaling and root planing. Clinical signs of inflammation and crevicular fluid β‐glucuronidase levels at 24 h were reduced following PP placement, significantly more than in control sites. Individuals with gingivitis who received PP had a greater reduction in mean gingival index scores compared to those receiving placebo patches (Grbic et al. 2011). Also, percentage of participants whose gingival index scores decreased by one or more was higher among those receiving PP than among those receiving the placebo patches. The average decrease in gingival crevicular fluid ß‐glucuronidase levels for participants receiving PP were significantly greater than for those receiving the placebo patches. It was concluded that topical treatment by means of the topical herbal patch may be effective and safe in reducing gingival inflammation.
However, while these controlled clinical studies suggest that PP application reduces gingival inflammation following periodontal non‐surgical treatment, its effect on the outcomes of oral surgical procedures has not been evaluated. The effect of PP on the various processes essential for wound healing: re‐epithelialization, inflammation, angiogenesis cellular proliferation and matrix deposition, may provide an answer to its potential role in improving soft tissue wound healing.
The aim of this study was to evaluate the effects of a topical herbal patch (PP) on healing of a surgical gingival wound in the rat model.
Our hypothesis was that PP has the potential to improve healing of a surgical gingival wound such as the one performed during routine periodontal/implant procedures.
Materials and methods
Forty‐eight 6‐month‐old Wistar rats weighting 427–577 g. were included in this study. The study protocol was approved by the Tel Aviv University Animal care committee. Animals were housed in wire cages in temperature and humidity‐controlled rooms with a 12‐h light/dark cycle, with food and water ad‐libitum.
General anaesthesia was achieved by an intra‐peritoneal injection of 0.1% Xylazine (13 mg/kg) and 0.1% ketamine (87 mg/kg). Local anaesthesia was performed using 0.5 cc Lidocaine 2% with adrenaline 1:100,000.
A mid‐crestal incision was made on the maxillary alveolar ridge in the existing edentulous area between the 1st molar and the incisors. A full‐thickness flap was raised on each side of the incision and then repositioned and sutured with resorbable vycril 4‐0 sutures (Ethicon Inc., Johnson & Johnson, NJ, USA).
Eight experimental groups were established:
Study groups: PerioPatch® was placed on the incision line immediately following surgery and was replaced every 12 h for the following 3 days.
Placebo patch control groups: Protocol was similar as in the study groups only that a placebo patch (similar in composition to the PerioPatch®, but without the active herbal ingredients) was applied. Neither study nor placebo patches were present at the time of the new patch application.
Negative control groups: No patch was placed on the incision line following surgery.
Intact control groups: No surgery was performed and no patch was applied.
The type of patch was coded (A or B) and was not available to the researchers until the results were available, therefore, the study was designed as blind.
To minimize the number of animals without compromising results, each hemi‐maxilla served as a sample in a group. A single type of patch was applied only on one side of each animal while in half of them, the contra‐lateral side served as negative control and in the remaining as intact group. Half of the animals were killed after 5 days and the remaining ones after 12 days.
Initially, each one of the eight groups consisted of 12 sites, therefore, altogether, a total of 96 samples were available. Two animals died during the experiment. As a result, two groups were left with 10 samples each.
Tissue harvesting
Animals were killed under anaesthesia using CO2 and the maxillae were excised and fixed in 4% paraformaldehyde for 7 days at room temperature. After decalcification in a 10% EDTA solution for 30 days, the specimens were embedded in paraffin and cut into serial sections of 4 μm thickness in a coronal plane, perpendicular to the line of incision.
Histological evaluation
Conventional histology (Haematoxylin & Eosin) for measurement of epithelial gap distance: Slides were stained with Haematoxylin & Eosin with the standard methods. Epithelial gap was measured under a 40× magnification using an Olympus BH‐2 (Olympus America Inc, New York, USA) light microscope. A gap zone was defined by lack of wound tissue and its width was measured at three epithelial heights within the incision line: a. deepest (close to the connective tissue), b. midway between the epithelial surfaces and c. most superficial portion of the epithelial wound. A mean of all measurements in two sections was computed. The mean ± standard deviation (SD) for each of the eight groups was then calculated.
Quantitation of collagen fibre content with picrosirius red (PSR) stain under polarized light microscopy: Adjacent sections were stained with picrosirius red solution (ScyTek kit SRS250X (ScyTek Laboratories Inc., Logan, USA). Using polarized light microscopy and a ×200 objective, the wound site was photographed and the area of the wound occupied by collagen fibres was determined as percent of the total area of the connective tissue.
Total connective tissue pixels adjacent to incision site were calculated as:
The percentage of collagen in connective tissue was calculated as:
Connective tissue collagen fibres pixels (red, yellow, green)*100/total connective tissue pixels.
Immunnohistochemistry: Antibodies used in this study are summarized in Table S1. Macrophages stained with CD68 were counted and expressed as number of cells/mm2 of gingival connective tissue adjacent to the incision site. The number of Ki‐67 positive cells was determined in the connective tissue below the epithelial area of interest for all eight groups. The general degree of proliferation with no differentiation between cells types was assessed. Cells stained for Ki‐67 were counted and expressed as number of cells/mm2 of gingival connective tissue adjacent to the incision site.
Endothelial cells positive to transglutaminase II with a lumen were recognized as blood vessels. The number of blood vessels was expressed per mm2 of gingival connective tissue adjacent to the incision site.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (Armonk, New York, U.S.). A two‐way anova analysis was performed to examine changes between groups for each dependent parameter. The statistical analysis was conducted for the 5 and 12 days data separately. Level of significance for all hypotheses was 5%.
Results
Clinical healing was not evaluated, however, in most animals; partial soft tissue healing could be appreciated by 5 days, whereas it was complete and uneventful by the end of the study at day 12.
Five days after the surgical procedure, the smallest epithelial gap was found in the study group. However, the difference between the three groups where surgery was performed, was not statistically significant (p = 0.701). In contrast, on day 12, the epithelial gap was significantly smaller in the study group compared to the placebo group (p = 0.073) and to the negative control group (p = 0.056) (Figs 1, 2, 3).
Figure 1.
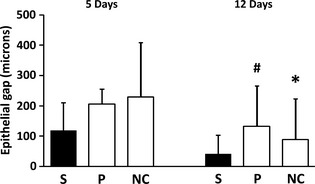
Epithelial gap (mean and SD) in microns in the various experimental groups. S=study group (PP); P, placebo patch group; NC, negative control (no patch) group. #, p = 0.07 (versus the respective study group). *, p = 0.05 (versus the respective study group).
Figure 2.
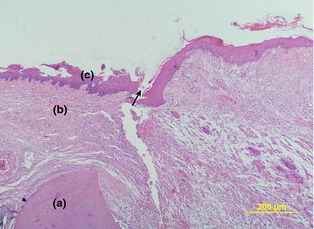
Epithelial healing in the study group 12 days after surgery. (H&E). (a) alveolar crest, (b) gingival connective tissue,(c) epithelium and incision site (arrow). (×110).
Figure 3.
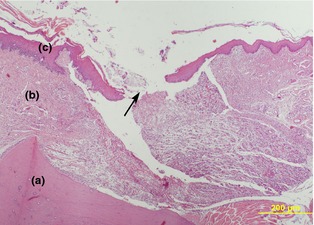
Epithelial healing in the placebo patch group 12 days after surgery. (H&E). (a) alveolar crest, (b) gingival connective tissue, (c) epithelium and incision site (arrow). (×110).
A statistically significant higher collagen content was found in the study group both at 5 and 12 days after the surgical procedure (p < 0.001) (Fig. 4 + Figures S2a, S2b, S3a, S3b).
Figure 4.
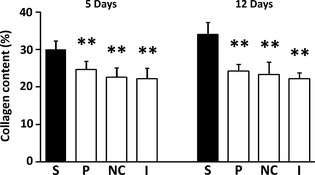
Connective tissue collagen content (%, mean and SD) in the various experimental groups. S, study group (PP); P, Placebo patch group; NC, negative control (no patch) group. I, Intact (no surgery) group. **, p < 0.001 (versus the respective study group).
Although macrophage counts were highest in the study group both at 5 and 12 days, differences between groups were not statistically significant (Fig. 5).
Figure 5.
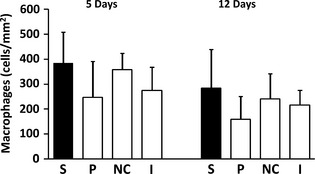
Number of connective tissue macrophages (CD68 positive cells) (/mm2, mean and SD) in the various experimental groups. S, study group (PP); P, placebo patch group; NC, negative control (no patch) group. I, Intact (no surgery) group.
A larger number of proliferating cells was found in the study group. At 5 days, the effects of the type of patch approached statistical significance (p = 0.054) (Fig. 6 + Figures S4a, S4b). On day 12, although the mean number of proliferating cells was highest in the study group, differences between groups were not statistically significant.
Figure 6.
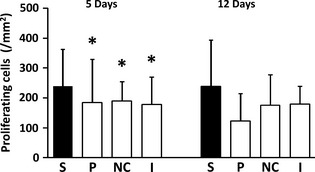
Number of proliferating (Ki67 positive) cells (/mm2, mean and SD) in the various experimental groups. S, study group (PP); P, placebo patch group; NC, negative control (no patch) group. I, Intact (no surgery) group. *, p = 0.05 (versus the respective study group).
The mean number of blood vessels was highest in the study group, however, at 5 days, differences were not statistically significant. (p = 0.354), reaching significance only at 12 days (p = 0.014) (Fig. 7 + Figures S5a, S5b).
Figure 7.
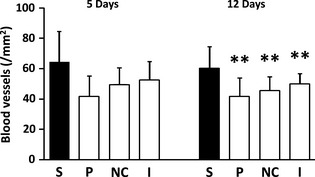
Number of blood vessels (TGII positive) (/mm2, mean and SD) in the various experimental groups. S, study group (PP); P, placebo patch group; NC, negative control (no patch) group. I, Intact (no surgery) group. **, p = 0.01 (versus the respective study group).
Discussion
Our results showed that application of the topical herbal patch improved gingival healing compared to control and the placebo patch, thus, suggesting that the active herbal ingredients (Centella asiatica, Echinacea purpurea and Sambucus nigra) play an important role in this effect. Asiaticoside and Madecassoside, found in Centella asiatica, promote fibroblast proliferation and extracellular matrix synthesis in wound healing (Lu et al. 2004), alleviate infiltration of inflammatory cells, enhance epithelialization resulting from fibroblasts proliferation and promote angiogenesis (Liu et al. 2008). Sambucus nigra extract was found to potently inhibit proinflammatory activities (Harokopakis et al. 2006). Certain components in Echinacea species display remarkable wound healing and anti‐inflammatory activities (Tumen et al. 2011).
Previous studies evaluating soft tissue healing (Sumitra et al. 2005, Firat et al. 2013, Ganjali et al. 2013, Kulac et al. 2013, Kant et al. 2014) applied a similar methodology as the present one. Degree of epithelialization, number of blood vessels, proliferating cells and collagen deposition are the most generally evaluated parameters to demonstrate positive effects on wound healing. An animal study (Ganjali et al. 2013) investigated if the methanolic extract of the Otostegia persica can accelerate the healing process of burn wound because of its anti‐inflammatory and antioxidant effects. It was concluded that methanolic extract of Otostegia persica exhibited significant healing activity when topically applied on rats. In another example, β‐glucan exerted various positive effects in burn wound healing, including immunomodulatory effects, antioxidant effects (free‐radical scavenging activity) and effects associated with the reduction in the inflammatory response (Firat et al. 2013). β‐glucan application led to higher fibroblast proliferation, angiogenesis and re‐epithelialization on the 7th day. In particular, re‐epithelialization on the 21st day was significantly better where β‐glucan was administered. In yet another study, topical treatment with curcumin (Cur) on burn wound healing in rats showed positive effects (Kulac et al. 2013). Other studies have also reported that Cur‐treated wounds were found to heal much faster as indicated by improved rates of inflammatory cells, collagen deposition, angiogenesis, granulation tissue formation and epithelialization which were also confirmed by histopathological and biochemical examinations (Akbik et al. 2014).
Previous controlled clinical studies have proven that PerioPatch® application is useful to reduce gingival inflammation following periodontal non‐surgical treatment (Grbic et al. 2011, Samuels et al. 2012) and that its application, following scaling and root planing and oral hygiene instructions, together with a mouth rinse containing the herbal active ingredients may decrease gingival recession and gingival index scores with increased gingival thickness (Levine et al. 2013). This study evaluated, for the first time, the effects of this device on the various processes essential for soft tissue surgical wound healing: re‐epithelialization, inflammation, angiogenesis cellular proliferation and matrix deposition.
Our results showed that the topical herbal patch application enhanced the epithelial bridging and improved healing. Although, the effect was noted already at day 5, it only approached statistical significance on day 12. However, the placebo patch, which provided only mechanical protection, did not exert a positive healing effect.
The results concerning inflammatory cells were not significantly different between groups. One may speculate that the study model was based on a surgical wound which is clean, therefore, resulting mainly in a reparative process with minimal inflammation.
PerioPatch® application resulted in an improved healing as evaluated by an increased number of proliferating cells. This effect only approached statistical significance by day 5; however, by day 12 results were statistically similar in the different groups, coinciding with the pattern of increased cellular proliferation during the healing process.
Increased collagen fibre quantity was appreciated in the study groups which was statistically significant both at 5 and 12 days. The placebo patch did not affect this parameter positively.
Blood vessels were significantly more numerous in the study group compared to placebo patch group at day 12. This coincides with the timing of angiogenesis during the healing process.
Effective wound healing requires a highly organized series of events that comprise inflammation, re‐epithelialization, keratinocyte and fibroblast proliferation, matrix deposition, angiogenesis and wound contraction. Together, these processes result in the restoration of tissue integrity and functional healing. The clinical investigation in search for therapeutic tools to improve the wound healing process has led to the development of novel treatment strategies (Hackam & Ford 2002) among which this herbal topical patch may be suitable for some clinical applications. Further clinical human controlled studies are mandatory to corroborate our results.
Conclusions
In this preclinical, animal study, application of the PerioPatch® and not the placebo patch improved wound healing in the described model. The topical herbal patch significantly decreased the epithelial gap following surgery. The positive effects on soft tissue wound healing were also appreciated by increased number of proliferating cells, collagen production and number of blood vessels.
Clinical Relevance.
Scientific rationale for the study: Rapid healing of the soft tissue wound is critical for the success of surgical procedures in the oral cavity. The evaluated topical herbal patch may have the potential to improve gingival healing following routine periodontal/implant procedures.
Principal findings: Application of the herbal patch resulted in smaller epithelial gap after surgery, an effect which was accentuated with time. Other patch positive effects on wound healing were increased number of proliferating cells, increased collagen production and increased number of blood vessels.
Practical implications : PerioPatch® could offer clinical benefits for enhanced soft tissue healing following oral surgical procedures.
Supporting information
Figure S1. Epithelial healing in the negative control group 12 days after surgery.
Figure S2. Study group. 5 days after surgery.
Figure S3. Study group. 12 days after surgery.
Figure S4. Study group 5 days after surgery.
Figure S5. Study group. 12 days after surgery.
Figure SA. Mid‐crestal incision on the maxillary alveolar ridge in the existing edentulous area between the 1st molar and the incisors.
Figure SB. Following full‐thickness flaps raised and repositioned on each side of the incision, resorbable sutures were performed to replace the soft tissues.
Figure SC. PerioPatch® or placebo patch was placed on the incision line immediately following surgery and replaced every 12 h for the following 3 days.
Table S1. Antibodies applied in this study.
Chaushu L, Weinreb M, Beitlitum I, Moses O, Nemcovsky CE. Evaluation of a topical herbal patch for soft tissue wound healing: an animal study. J Clin Periodontol 2015; 42: 288–293. doi: 10.1111/jcpe.12372.
Conflict of interest and source of funding statement
This study was funded by a grant from Izun Pharmaceuticals Ltd. Jerusalem, Israel. The authors report no conflict of interest.
References
- Akbik, D. , Ghadiri, M. , Chrzanowski, W. & Rohanizadeh, R . (2014) Curcumin as a wound healing agent. Life Sciences 116, 11–7 [DOI] [PubMed] [Google Scholar]
- Belcaro, G. , Maquart, F. X. , Scoccianti, M. , Dugall, M. , Hosoi, M. , Cesarone, M. R. , Luzzi, R. , Cornelli, U. , Ledda, A. & Feragalli, B. (2011) TECA (Titrated Extract of Centella Asiatica): new microcirculatory, biomolecular, and vascular application in preventive and clinical medicine. A status paper. Panminerva Medica 53 (3 Suppl. 1), 105–118. [PubMed] [Google Scholar]
- Carlson, E. R. & Monteleone, K. (2004) An analysis of inadvertent perforations of mucosa and skin concurrent with mandibular reconstruction. Journal of Oral Maxillofacial Surgery 62, 1103–1107. [DOI] [PubMed] [Google Scholar]
- Coldren, C. D. , Hashim, P. , Ali, J. M. , Oh, S. K. , Sinskey, A. J. & Rha, C. (2003) Gene expression changes in the human fibroblast induced by Centella asiatica triterpenoids. Planta Medica 69, 725–732. [DOI] [PubMed] [Google Scholar]
- Firat, C. , Samdanci, E. , Erbatur, S. , Aytekin, A. H. , Ak, M. , Turtay, M. G. & Coban, Y. K. (2013) β‐Glucan treatment prevents progressive burn ischaemia in the zone of stasis and improves burn healing: an experimental study in rats. Burns 39, 105–112. [DOI] [PubMed] [Google Scholar]
- Ganjali, A. , Sotoudeh, A. , Jahanshahi, A. , Takhtfooladi, M. A. , Bazzazan, A. , Roodbari, N. & Harati, M. P. (2013) Otostegia persica extraction on healing process of burn wounds. Acta Cirúrgica Brasileira 28, 407–411. [DOI] [PubMed] [Google Scholar]
- Grbic, J. , Wexler, I. , Celenti, R. , Altman, J. & Saffer, A. (2011) A Phase II Trial of a Transmucosal Herbal Patch for the Treatment of Gingivitis. The Journal of the American Dental Association 142, 1168–1175. [DOI] [PubMed] [Google Scholar]
- Hackam, D. J. & Ford, H. R. (2002) Cellular, biochemical, and clinical aspects of wound healing. Surgical Infections 3 (Suppl 1), S23–S35. [DOI] [PubMed] [Google Scholar]
- Harokopakis, E. , Albzreh, M. H. , Haase, E. M. , Scannapieco, F. A. & Hajishengallis, G. (2006) Inhibition of proinflammatory activities of major periodontal pathogens by aqueous extracts from elder flower (Sambucus nigra). Journal of Periodontology 77, 271–279. [DOI] [PubMed] [Google Scholar]
- Kant, V. , Gopal, A. , Pathak, N. N. , Kumar, P. , Tandan, S. K. & Kumar, D. (2014) Antioxidant and anti‐inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin‐induced diabetic rats. International Immunopharmacology 20, 322–330. [DOI] [PubMed] [Google Scholar]
- Kim, Y. J. , Cha, H. J. , Nam, K. H. , Yoon, Y. , Lee, H. & An, S . (2011) Centella asiatica extracts modulate hydrogen peroxide‐induced senescence in human dermal fibroblasts. Experimental Dermatology 20, 998–1003. [DOI] [PubMed] [Google Scholar]
- Kulac, M. , Aktas, C. , Tulubas, F. , Uygur, R. , Kanter, M. , Erboga, M. , Ceber, M. , Topcu, B. & Ozen, O. A. (2013) The effects of topical treatment with curcumin on burn wound healing in rats. Journal of Molecular Histology 44, 83–90. [DOI] [PubMed] [Google Scholar]
- Levine, W. Z. , Samuels, N. , Bar Sheshet, M. E. & Grbic, J. T. (2013) A novel treatment of gingival recession using a herbal topical gingival patch and mouthrinse. The Journal of Contemporary Dental Practice 14, 948–953. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Dai, Y. , Li, Y. , Luo, Y. , Huang, F. , Gong, Z. & Meng, Q. (2008) Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Medica 74, 809–815. [DOI] [PubMed] [Google Scholar]
- Lu, L. , Ying, K. , Wei, S. , Fang, Y. , Liu, Y. , Lin, H. , Ma, L. & Mao, Y. (2004) Asiaticoside induction for cell‐cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. International Journal of Dermatology 43, 801–807. [DOI] [PubMed] [Google Scholar]
- Polimeni, G. , Albandar, J. M. & Wikesjö, U. M. E. (2004a) Prognostic factors for alveolar regeneration: osteogenic potential of resident bone. Journal of Clinical Periodontolology 31, 840–844. [DOI] [PubMed] [Google Scholar]
- Polimeni, G. , Koo, K. T. , Qahash, M. , Xiropaidis, A. V. , Albandar, J. M. & Wikesjö, U. M. E. (2004b) Prognostic factors for alveolar regeneration: effect of tissue occlusion on alveolar bone regeneration with guided tissue regenration. Journal of Clinical Periodontolology 31, 730–735. [DOI] [PubMed] [Google Scholar]
- Romero‐Cerecero, O. , Zamilpa‐Álvarez, A. , Díaz‐García, E. R. & Tortoriello, J . (2014) Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. Journal of Ethnopharmacology 156, 222–227. [DOI] [PubMed] [Google Scholar]
- Samuels, N. , Saffer, A. , Wexler, I. D. & Oberbaum, M. (2012) Localized reduction of gingival inflammation using site‐specific therapy with a topical gingival patch. The Journal of Clinical Dentistry 23, 64–67. [PubMed] [Google Scholar]
- Sastravaha, G. , Gassmann, G. , Sangtherapitikul, P. & Grimm, W. D. (2005) Adjunctive periodontal treatment with Centella asiatica and Punica granatumextracts in supportive periodontal therapy. Journal of the International Academy of Periodontology 7, 70–79. [PubMed] [Google Scholar]
- Sastravaha, G. , Yotnuengnit, P. , Booncong, P. & Sangtherapitikul, P. (2003) Adjunctive periodontal treatment with Centella asiatica and Punica granatumextracts. A preliminary study. Journal of the International Academy of Periodontology 5, 106–115. [PubMed] [Google Scholar]
- Sumitra, M. , Manikandan, P. & Suguna, L. (2005) Efficacy of Butea monosperma on dermal wound healing in rats. The International Journal of Biochemistry & Cell Biology 37, 566–573. [DOI] [PubMed] [Google Scholar]
- Tumen, I. , Akkol, E. K. , Süntar, I. & Keleş, H. (2011) Wound repair and anti‐inflammatory potential of essential oils from cones of Pinaceae: preclinical experimental research in animal models. Journal of Ethnopharmacology 137, 1215–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Epithelial healing in the negative control group 12 days after surgery.
Figure S2. Study group. 5 days after surgery.
Figure S3. Study group. 12 days after surgery.
Figure S4. Study group 5 days after surgery.
Figure S5. Study group. 12 days after surgery.
Figure SA. Mid‐crestal incision on the maxillary alveolar ridge in the existing edentulous area between the 1st molar and the incisors.
Figure SB. Following full‐thickness flaps raised and repositioned on each side of the incision, resorbable sutures were performed to replace the soft tissues.
Figure SC. PerioPatch® or placebo patch was placed on the incision line immediately following surgery and replaced every 12 h for the following 3 days.
Table S1. Antibodies applied in this study.


