Abstract
Background
Oral solution N-acetylcysteine (NAC) is an antidote for acetaminophen overdose, but its unpleasant taste and aroma can impede delivery even after the coadministration of antiemetic medications. Flavored effervescent NAC tablets dissolved in water might be a more palatable formulation than oral solution NAC diluted with soft drink.
Objectives
To evaluate the relative bioavailability of these 2 formulations and assess subjective preferences between them.
Methods
Thirty healthy adult volunteers (mean [SD] = 35.2 [9.14] years) were enrolled in this open-label, randomized, single-dose, crossover study, with a 7-day washout period. Volunteers were randomized to receive 11 g effervescent test formulation or the reference product under fasting conditions, after which 19 serial blood samples were collected over 48 hours. Total plasma NAC concentrations were evaluated by LC-MS, and pharmacokinetic parameters were calculated. The 2 formulations were considered bioequivalent if the 90% CIs of log-transformed ratios of pharmacokinetic parameters were within the predetermined bioequivalence range (80%–125%) established by the US Food and Drug Administration. Within 15 minutes of dosing, subjects were also asked to rank formulation attributes on a 5-point hedonic scale, with mean group differences analyzed by Wilcoxon signed rank test. Safety-profile assessment included treatment-emergent adverse events, physical examination, chemistry, and hematology parameters.
Results
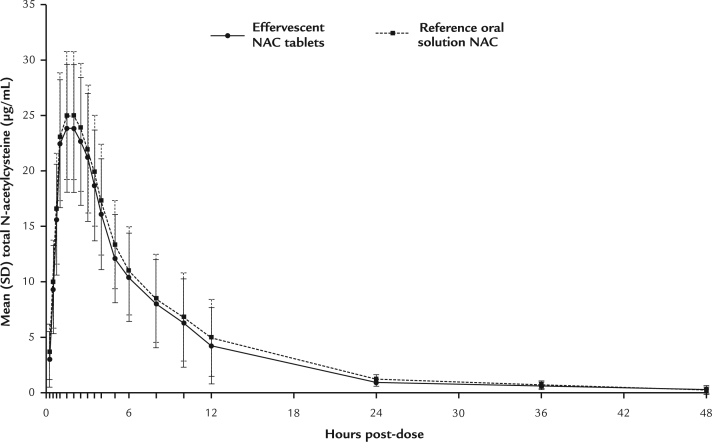
The concentration-versus-time profiles were similar for the 2 formulations, with mean Cmax of 26.5 μg/mL for effervescent NAC tablets and 28.4 μg/mL for oral solution NAC. The 90% CIs for the pharmacokinetic parameters met the criteria for concluding bioequivalence, and subjects preferred effervescent NAC tablets in terms of taste (P = 0.0247), flavor (P = 0.0082), texture (P = 0.009), and overall likeability (P = 0.0012), but there was no difference for smell (P = 0.0533). All treatment-emergent adverse events were mild, with no differences between the treatment groups.
Conclusions
Data from this study of a single dose of 11 g oral NAC demonstrated that effervescent NAC tablets and oral solution NAC met the regulatory criteria for bioequivalence in fasting healthy adult subjects. Effervescent NAC tablets appear to be a more palatable alternative for treatment of acetaminophen overdose. ClinicalTrials.gov identifier: NCT02723669.
Key words: acetaminophen, bioavailability, effervescent tablets, N-acetylcysteine, pharmacokinetics
Introduction
In the United States, acetaminophen is the medicine that is most commonly associated with overdose and the leading cause of overdose-related hepatotoxicity leading to acute liver failure.1 Approximately half of overdose-related deaths are due to products containing acetaminophen in combination with other drugs. According to the 2015 report of the American Association of Poison Control Centers,2 overuse of either acetaminophen alone or in combinations with other drugs accounted for the highest percentage of fatalities (16.9%) associated with single-substance exposures alone (10.70%) and in combinations (6.23%). In a 2011 epidemiology study,3 acetaminophen-associated overdoses were responsible for an average of 78,414 annual emergency department visits and an average of 33,520 annual hospitalizations.
Oral solution N-acetylcysteine (NAC) was approved by the Food and Drug Administration (FDA) for treatment of acetaminophen overdose in 1978. Single large doses or repeated subtherapeutic doses of acetaminophen can deplete hepatic glutathione, which detoxifies N-acetyl-p-benzoquinone imine, a metabolite of acetaminophen that is extremely toxic to the liver. NAC prevents hepatic injury primarily by restoring hepatic glutathione.1 The FDA-approved treatment protocol for use of oral NAC as an acetaminophen-overdose antidote requires a loading dose followed by 17 additional doses over 72 hours. Shortened courses of oral NAC (≤48 hours), guided by laboratory parameters for patient-tailored discontinuation of treatment, have also been studied and are sometimes used.[4], [5] Intravenous (IV) NAC was approved by the FDA for treatment of acetaminophen overdose in 2004. The FDA-approved treatment protocol for use of IV NAC requires a loading dose followed by 2 additional doses over 21 hours. Both oral solution NAC and IV NAC are highly effective in preventing hepatotoxicity from acetaminophen overdose, with comparable efficacy.[6], [7] In the United States, institutional preference for either oral solution NAC or IV NAC for treatment of acetaminophen overdose depends on such factors as manpower and utilization costs associated with delivery of these different formulations.7
Because of its sulfur moiety, NAC has a putrid rotten-egg smell and taste, which can cause patients to experience nausea and vomiting and become intolerant of therapy. For patients receiving oral solution NAC, vomiting can be sufficient to impede medication delivery. Gastrointestinal adverse events occur not only with oral NAC solution, which is commonly diluted with diet caffeine-free soft drink to mask the smell and taste, but with IV NAC as well. These symptoms are often treated with antiemetic agents. In a retrospective, 503-patient multicenter comparison of the safety of oral versus IV NAC for treatment of acetaminophen overdose, the rate of nausea and vomiting was higher with oral NAC than with IV NAC (23% vs 9%), but the same percentage of patients in each group required antiemetic medication (25.5% vs 26.5%).8
Flavored effervescent tablets are a novel formulation of NAC intended for oral treatment of acetaminophen overdose. When effervescent NAC tablets are dissolved in water, the flavored taste and smell of the solution might be preferred to the combination of oral solution NAC diluted with diet caffeine-free soft drink. Our purpose was to compare the pharmacokinetic (PK) parameters and relative bioavailability of effervescent NAC tablets and oral solution NAC given as a single 11-g dose under fasting conditions to healthy adult subjects. This study was conducted in accordance with FDA regulatory criteria for assuming bioequivalence of orally administered drugs.9 A secondary objective of this study was to assess subject preferences for attributes of effervescent NAC tablets compared with those of oral solution NAC.
Materials and Methods
Study design and subjects
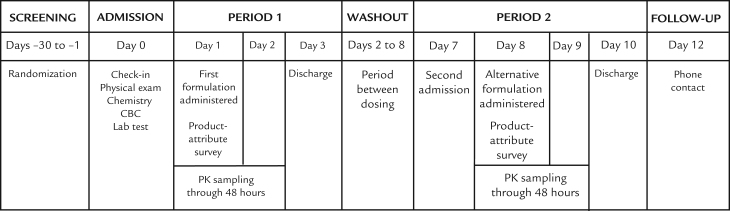
To evaluate the relative bioavailability of effervescent NAC tablets versus oral solution NAC, we performed an open-label crossover study in 30 male and female subjects with a body mass index < 30 and who were between the ages of 18 and 50 years. Conducted at a single research center, which recruited and paid volunteers to participate, the study consisted of a screening period, 2 crossover dosing periods (Period 1 and Period 2), with the actual dosing separated by a 7-day washout period, and a follow-up telephone call (Figure 1). Because of the obvious differences between the effervescent formulation and the standard solution, subjects and investigators were aware of treatment assignment.
Figure 1.
Study design of the open-label, randomized-sequence, single-dose, crossover, relative bioavailability and attribute-preference study of effervescent N-acetylcysteine (NAC) tablets and oral solution NAC in fasting healthy adult subjects.
The study was approved by the local institutional review board and was conducted in accordance with the principles in the World Medical Association Declaration of Helsinki. The ClinicalTrials.gov registration number for this trial was NCT02723669.
After providing informed consent, subjects underwent baseline testing, which included medical history, clinical examination, laboratory tests, and 12-lead ECG within the 30 days before starting the study. Inclusion required being a nonsmoker and a negative ß-human chorionic gonadotropin test in reproduction-capable women. Subjects were not allowed prescription or over-the-counter drugs (including vitamins and natural supplements) throughout the study duration.
On study Day 0, a basic metabolic profile with liver-function tests, complete blood count, and urinalysis were administered. Subjects were then provided a standard meal and fasted overnight for at least 10 hours. Subjects then underwent randomization using a balanced block randomization schedule, generated before the start of dosing, to ensure alternating NAC formulations for the crossover investigation.
Subjects received the first assigned formulation on Day 1 (Period 1), with samples for PK analysis collected predose and at scheduled postdose time points through 48 hours. Subjects completed a formulation-attribute survey within 15 minutes after completing dosing activities. Period 1 ended when subjects were discharged on Day 3. They then were readmitted on Day 7 for the beginning of Period 2. The crossover washout was 7 days—from the administration of the first dose on Day 1 to the administration of the assigned crossover NAC formulation on Day 8, again under fasting conditions. During Period 2, subjects underwent the same PK and safety-profile assessments and completed the same formulation-attribute survey as during Period 1. Patients were discharged on Day 10 after a final evaluation.
Study drugs and study drug administration
The effervescent tablet formulation of NAC (Cetylev, Arbor Pharmaceuticals, Atlanta, Georgia) was available in strengths of 500 mg and 2.5 g. Each tablet was white, round, flat, and debossed to signify the dose strength. When a tablet was dissolved in water, the mixture had a lemon-mint flavor. The tablets were packaged in silver 2-count peelable foil blister packs, which were not opened until the time of use. The reference oral solution NAC, manufactured by Gland Pharma Ltd for APP Pharmaceuticals, LLC (Schaumburg, Illinois), had a dose strength of 20% (200 mg/mL).
A dose of 11 g NAC was selected for evaluation because it approximated the therapeutic loading dose for an adult weighing 70 to 79 kg who was receiving treatment for acetaminophen overdose (a loading dose of 140 mg/kg).10 The doses of effervescent NAC tablets, dissolved in water, and of oral solution NAC, diluted with soft drink, were used within 10 minutes of preparation and were administered to subjects while in the sitting position.
Subjects received 11 g (four 2.5-g and two 0.5-g tablets) effervescent NAC dissolved in 300 mL room-temperature bottled water. After the complete dose was consumed, the dosing glass was rinsed with 100 mL water that was swallowed. Subjects received 55 mL 20% NAC solution (a total of 11 g NAC) diluted with 165 mL diet caffeine-free lemon-lime soft drink (a volume of 220 mL) (Shasta Diet Lemon Lime Twist, Shasta Food Service, Gainesville, Georgia). The prescribing information for generic oral solution NAC specifies that dilution should occur with diet cola or with other diet soft drinks. A soft drink with a lemon-lime flavor was chosen for dilution because that flavor seemed most similar to the lemon-mint flavor of effervescent NAC tablets. After the dose of oral solution NAC was consumed, 80 mL room-temperature water was poured into the same container and swallowed, for a total volume of 300 mL. After the complete dose was consumed, the dosing glass was rinsed with 100 mL water that was swallowed. A visual mouth check was performed after drug administration to confirm that each study subject had completely swallowed the full dose. Subjects remained in a sitting posture for the first 4 hours after dose administration in each period, except in cases of study or procedural requirements. All subjects maintained a fasting state for at least 4 hours after dosing in each period.
PK evaluation
Blood samples were collected from all subjects on Day 1 and Day 8 for the measurement of NAC. For PK analysis, samples were collected predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 24, 36, and 48 hours postdose in each period. The predose sample was obtained within 60 minutes before dosing. An indwelling venous catheter was placed for PK blood sample collection through 12 hours, and direct venipuncture or an indwelling catheter could be used for PK sample collection at 24, 36, and 48 hours. The start time of each PK sampling was recorded electronically.
Plasma samples of all subjects were assayed for total NAC using LC-MS/MS carried out at AIT Bioscience, LLC (Indianapolis, Indiana). The bioanalytical method was developed in compliance with AIT Bioscience standard operating procedures and validated with procedures and documentation consistent with FDA and European Medicines Agency requirements[11], [12] and with a 2007 industry consensus white paper.13 Covering the measurement range of 0.05 to 50.0 μg/mL NAC, with N-acetyl-L-cysteine-D3 used as the respective internal standard, the bioanalytical method was based on a simultaneous reduction/thioether formation reaction to form a stable and easily detected derivative, followed by protein precipitation extraction and LC-MS/MS instrumental analysis. No samples were reassayed because of aberrant PK values.
Concentration-time data for total NAC for individual subjects were analyzed by noncompartmental methods using actual elapsed blood draw times in WinNonlin (version 5.3) (Pharsight, Cary, North Carolina). Plasma concentration–time data were summarized by descriptive statistics at each scheduled time point. Individual and mean concentration–time profiles were provided for each treatment arm. Plots were presented for the mean (SD) plasma concentration versus time on linear and semilogarithmic scales. Descriptive statistics for the PK parameters were tabulated by treatment.
The natural log transformed values for the PK parameters Cmax, AUC0–last, and AUC0–∞ were analyzed for statistical differences between effervescent NAC tablets and oral solution NAC using a linear mixed model with fixed effects for sequence, treatment, and period, and a random effect for subject nested in sequence. The 90% CIs were calculated for the ratio of test and reference products using the natural log transformed values for the PK parameters Cmax, AUC0–last, and AUC0–∞. Tmax was analyzed using nonparametric analysis for the same comparisons shown for 90% CIs. Bioequivalence could be concluded if the 90% CIs of the estimated mean ratios (expressed as a percentage) fell entirely within the 80% to 125% limits established by the FDA.9
Analysis populations
The safety population, used for all safety-profile and preference survey analyses, included all subjects who received any amount of study drug. The safety profile was assessed by evaluation of adverse events, hematology, chemistry, and urinalysis laboratory test results, vital signs, physical examination, and ECG findings. Subjects were monitored throughout the study and asked about how they felt at the time of each clinical examination and during the recording of vital signs. The PK population, used for all PK analyses, included all subjects who completed both treatment periods and had sufficient quantifiable plasma concentration data to provide Cmax and AUC data. No subjects were excluded due to aberrant PK values.
Formulation attribute assessment
Within 15 minutes after completing dosing activities in each period, subjects were asked to evaluate the taste, smell, flavor, texture, and overall likeability for the assigned product using the adapted British Nutrition Foundation Sensory Evaluation 5-point hedonic scale, ranging from “dislike very much” to “like very much.”14 Definitions of the evaluated attributes were not provided to subjects before the study began. Subjects were instructed not to discuss their opinions with others and were housed in private rooms to minimize communication and influence from other subjects. The health care providers were also instructed not to discuss the formulation attributes with the subjects or with one another.
Statistical analysis
Continuous variables were summarized with means (SD), medians, and ranges. Categorical variables were summarized by counts and by percentage of subjects in corresponding categories. For the formulation-attribute survey, the Wilcoxon signed rank test was used to assess for each attribute whether the mean difference in scale between the 2 treatment groups was equal to zero.
Results
A total of 30 healthy subjects (25 male and 5 female) were enrolled (age = 35.2 [9.14] years; body mass index = 26.97 [1.99]). One adult male subject was withdrawn from the study before the dosing of Period 2 because of a nonserious, mild, and unrelated treatment-emergent adverse event (TEAE) more than 24 hours after receipt of oral solution NAC in Period 1. Consequently, 29 subjects completed both treatment periods and were included in the PK population. All subjects received some study drug and were thus included in the safety population, with 29 of the subjects receiving effervescent NAC tablets and all 30 subjects receiving oral solution NAC.
Concentrations of NAC
All predose concentrations were less than the limit of quantitation (<0.050 µg/mL). The mean Cmax of NAC in subjects receiving effervescent NAC tablets was 26.5 µg/mL, and the mean Cmax of NAC in subjects receiving oral solution NAC was 28.4 µg/mL. The concentration-versus-time profiles were similar for the 2 products. Total NAC concentration-versus-time profiles, showing mean (SD) concentration values for the PK population, are presented in linear scale in Figure 2.
Figure 2.
Mean (SD) concentrations of total N-acetylcysteine (NAC) in a linear scale for effervescent NAC and oral solution NAC in the pharmacokinetics population (N = 29).
PK and bioequivalence analysis
PK parameters were generated from the plasma total NAC concentrations and actual elapsed blood sampling times. Summary statistics for the total NAC parameters are provided in Table I. The mean (SD) relative bioavailability (calculated with AUC0–∞) of effervescent NAC tablets versus oral solution NAC was 94.0% (18.5%).
Table I.
Total N-acetylcysteine (NAC) pharmacokinetic parameters and reference bioavailability of effervescent NAC tablets versus oral solution NAC.*
| Parameter | Effervescent NAC tablets (n = 29) | Reference oral solution NAC (n = 29) |
|---|---|---|
| Tlag (h)† | 0.00 (0.00) | 0.00 (0.00) |
| Cmax (μg/mL) | 26.5 (7.58) | 28.4 (7.86) |
| Tmax (h) | 2.12 (0.677) | 1.89 (0.80) |
| AUC0–last (h × μg/mL) | 179 (52.3) | 195 (62.6) |
| AUC0–∞ (h × μg/mL) | 186 (54.3) | 202 (64.4) |
| AUCextrap (%)‡ | 3.61 (0.939) | 3.43 (0.842) |
| λz (L/h)§ | 0.0404 (0.0112) | 0.0407 (0.00703) |
| t1/2 (h) | 18.1 (3.96) | 17.5 (2.98) |
| CL/F (L/h)|| | 65.1 (22.8) | 59.3 (16.3) |
| Vz/F (L)¶ | 1720 (731) | 1510 (503) |
| Fr (%)# | 94.0 (18.5) |
Values are presented as mean (SD). One subject discontinued during Period 2 without receiving effervescent NAC tablets and is not included in these data.
Lag time (time before first quantifiable concentration).
AUC extrapolated from the time of last non-zero concentration (Tlast) to infinity.
Apparent first-order terminal rate constant.
Apparent clearance, calculated as dose/AUC∞.
Apparent volume of distribution, calculated as dose/(AUC∞ × λz).
Bioavailability relative to reference product calculated as the test/reference ratio of AUC∞ values × 100.
Results for the analysis of bioequivalence of effervescent NAC tablets and reference oral solution NAC with respect to Cmax, AUC0–last, and AUC0–∞ are provided in Table II. Because the 90% CIs for the effervescent NAC tablets and the reference oral solution NAC were within the accepted range for bioequivalence (80%–125%),9 the 2 products were bioequivalent with respect to Cmax, AUC0–last, and AUC0–∞.
Table II.
Bioequivalence of effervescent N-acetylcysteine (NAC) tablets and oral solution NAC for prespecified pharmacokinetic parameters.
| Parameter | Effervescent NAC tablets (LSM) | Reference oral solution NAC (LSM) | Ratio (% reference product) | 90% CI | ANOVA %CV |
|---|---|---|---|---|---|
| AUC0–∞ (h × μg/mL) | 177.31 | 192.15 | 92.28 | 86.39–98.56 | 14.81 |
| AUC0–last (h × μg/mL) | 170.90 | 185.54 | 92.11 | 86.18–98.44 | 14.94 |
| Cmax (μg/mL) | 25.44 | 27.46 | 92.64 | 86.84–98.84 | 14.54 |
LSM = least squares mean.
TEAEs
A total of 52 TEAEs were reported during the study. Fourteen of 29 subjects (48.3%) experienced 23 TEAEs while receiving effervescent NAC tablets; 15 of 30 subjects (50.0%) experienced 29 TEAEs while receiving the reference product (Table III). The most common TEAEs reported by more than 1 subject, regardless of the product, were diarrhea, flatulence, nausea, dysgeusia, upper abdominal pain, headache, abdominal discomfort, vomiting, and dizziness. After administration of effervescent NAC tablets and oral solution NAC, a similar number of subjects reported TEAEs of nausea (4 [13.8%] and 3 [10.0%], respectively) or vomiting (1 [3.4%] and 1 [3.3%], respectively).
Table III.
Frequency of subjects experiencing treatment-emergent adverse events in the safety population.*
| Incident | Effervescent NAC tablets (n = 29) | Reference oral solution NAC (n = 30) |
|---|---|---|
| Subjects reporting at least 1 TEAE† | 14 (48.3) | 15 (50.0) |
| Subjects reporting at least 1 nausea or vomiting TEAE | 4 (13.8) | 3 (10.0) |
| Subjects reporting at least 1 serious TEAE | 0 | 0 |
| Gastrointestinal disorders | 11 (37.9) | 9 (30.0) |
| Diarrhea | 6 (20.7) | 6 (20.0) |
| Flatulence | 2 (6.9) | 5 (16.7) |
| Nausea | 4 (13.8) | 3 (10.0) |
| Abdominal pain upper | 3 (10.3) | 1 (3.3) |
| Abdominal discomfort | 1 (3.4) | 1 (3.3) |
| Vomiting | 1 (3.4) | 1 (3.3) |
| Nervous system disorders | 4 (13.8) | 7 (23.3) |
| Dysgeusia | 3 (10.3) | 3 (10.0) |
| Dizziness | 1 (3.4) | 1 (3.3) |
| Headache | 0 | 2 (6.7) |
| Convulsion | 0 | 1 (3.3) |
| Syncope | 0 | 1 (3.3) |
| Closest relationship to study drug | ||
| Related‡ | 13 (44.8) | 14 (46.7) |
| Not related§ | 1 (3.4) | 1 (3.3) |
NAC = N-acetylcysteine; TEAE = treatment-emergent adverse event.
Values are presented as number (%).
Subjects reporting more than 1 adverse event are counted only once using the closest relationship to study drug.
Includes all events reported as “possible,” “probable,” “definitely,” or missing relationship to study drug.
Includes all events reported as “unlikely” or “unrelated” relationship to study drug.
All TEAEs were considered to be mild in intensity. Of the subjects who experienced TEAEs, events considered related to study drug were reported for 13 of 14 subjects receiving effervescent NAC tablets and 14 of 15 subjects receiving oral solution NAC. All TEAEs reported by more than 1 subject overall were considered related to study drug. There were no apparent differences in time to onset or duration for the TEAEs of diarrhea, flatulence, and nausea after administration of effervescent NAC tablets or reference product. For the other TEAEs, the numbers of subjects experiencing events was too small (1–3 subjects per treatment group), to allow a meaningful comparison. No deaths or other serious adverse events were reported during this study.
One 38-year-old male subject did not complete both treatment periods of the study. He experienced a nonserious, mild, and unrelated TEAE of syncope and seizure-like activity (ie, convulsion) without a postictal period before dosing during Period 2 and was discontinued from the study. The event occurred more than 24 hours after the subject received the reference oral solution NAC during Period 1, with the total event lasting 2 to 4 minutes.
Because there were no meaningful differences between effervescent NAC tablets and oral solution NAC in terms of TEAEs, vital signs, ECG readings, or laboratory values, the safety profile for effervescent NAC tablets was found to be comparable to that for oral solution NAC.
Subject preference
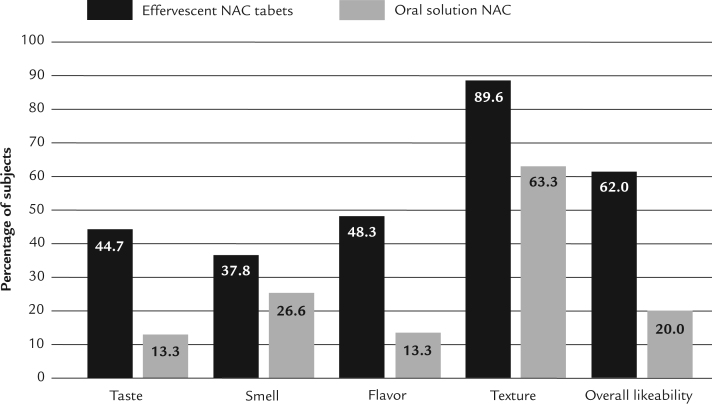
The results of the preference survey indicated that subjects preferred effervescent NAC tablets over reference oral solution NAC for all 5 surveyed formulation attributes (ie, taste, smell, flavor, texture, and overall likeability). For 4 of these 5 attributes (ie, taste, flavor, texture, and overall likeability), the preference for effervescent NAC tablets was statistically significant (P values of 0.0247, 0.0082, 0.0090, and 0.0012, respectively, by Wilcoxon signed rank test) (Table IV). For the attribute of smell, effervescent NAC tablets were numerically preferred over the reference product, but this preference did not reach statistical significance (P = 0.0533 by Wilcoxon signed rank test). More subjects “liked,” “liked very much,” or ranked effervescent NAC tablets as neutral for taste (Figure 3).
Table IV.
Subject preference survey results for formulation attributes.*
| Attribute | Effervescent NAC tablets (n = 29) | Reference oral solution NAC (n = 30) | P value† |
|---|---|---|---|
| Taste | 0.0247 | ||
| Like very much | 1 (3.4) | 0 | |
| Like | 7 (24.1) | 0 | |
| Neither like nor dislike | 5 (17.2) | 4 (13.3) | |
| Dislike | 7 (24.1) | 20 (66.7) | |
| Dislike very much | 9 (31.0) | 6 (20.0) | |
| Smell | 0.0533 | ||
| Like very much | 1 (3.4) | 0 | |
| Like | 5 (17.2) | 1 (3.3) | |
| Neither like nor dislike | 5 (17.2) | 7 (23.3) | |
| Dislike | 12 (41.4) | 14 (46.7) | |
| Dislike very much | 6 (20.7) | 8 (26.7) | |
| Flavor | 0.0082 | ||
| Like very much | 0 | 0 | |
| Like | 8 (27.61) | 0 | |
| Neither like nor dislike | 6 (20.7) | 4 (13.3) | |
| Dislike | 7 (24.1) | 19 (63.3) | |
| Dislike very much | 8 (27.6) | 7 (23.3) | |
| Texture | 0.0090 | ||
| Like very much | 1 (3.4) | 0 | |
| Like | 7 (24.1) | 4 (13.3) | |
| Neither like nor dislike | 18 (62.1) | 15 (50.0) | |
| Dislike | 2 (6.9) | 8 (26.7) | |
| Dislike very much | 1 (3.4) | 3 (10.0) | |
| Overall likeability | 0.0012 | ||
| Like very much | 2 (6.9) | 0 | |
| Like | 7 (24.1) | 0 | |
| Neither like nor dislike | 9 (31.0) | 6 (20.0) | |
| Dislike | 5 (17.2) | 18 (60.0) | |
| Dislike very much | 6 (20.7) | 6 (20.0) |
NAC = N-acetylcysteine.
Values are presented as number (%). One subject discontinued during Period 2 without receiving the Period 2 treatment (effervescent NAC tablets).
Wilcoxon signed rank test.
Figure 3.
Percent of subjects ranking formulation attributes of effervescent N-acetylcysteine (NAC) tablets and oral solution NAC as “like very much,” “like,” or “neither like nor dislike.”
Regarding overall likeability, during the first dosing period, when all subjects were naive to both treatments, preference was higher for effervescent NAC tablets. Of 15 subjects receiving effervescent NAC tablets on the first day, 47% marked “liked” or “liked very much,” whereas none of the 15 subjects who received the reference product marked “liked” or “liked very much” on the survey. Approximately 3 times as many subjects rated effervescent NAC tablets (compared with the reference product) as positive (“liked” or “liked very much”) or neutral in the first dosing period (73.3% vs 20%). During the second dosing period, although the products were generally assessed as less “liked” compared with the first week, fewer subjects disliked (“disliked” or “disliked very much”) effervescent NAC tablets compared with the reference product (50.0% vs 80.0%).
Discussion
In this single-center, open-label, randomized, single-dose, crossover, relative-bioavailability study, the bioavailability of effervescent NAC tablets was not different from that of the reference product, oral solution NAC, in healthy adult subjects under fasting conditions. The single 11-g doses of either formulation approximated the loading dose of oral NAC treatment for acetaminophen overdose. For the 2 formulations, the 90% CIs for the prespecified PK parameters of Cmax, AUC0–last, and AUC0–∞ were within the accepted bioequivalence interval of 80% to 125%, meeting the regulatory criteria for assuming bioequivalence in fasting healthy adult subjects.9 Both products were well tolerated, with no serious adverse events during the study, and with similar numbers of patients in each group experiencing at least 1 TEAE: 14 of 29 subjects (48.3%) after receiving effervescent NAC tablets and 15 of 30 subjects (50.0%) after receiving oral solution NAC. There were no meaningful treatment-related differences between effervescent NAC tablets and oral solution NAC during the study.
Subjects preferred effervescent NAC tablets (with lemon-mint flavor) dissolved in water to oral solution NAC diluted in diet caffeine-free lemon-lime soft drink in terms of taste, flavor, texture, and overall likability. For the formulation attribute of overall likeability, 62.0% of subjects ranked effervescent NAC tablets “like very much,” “like,” or “neither like nor dislike,” whereas only 20.0% of patients ranked oral solution NAC “like very much,” “like,” or “neither like nor dislike.”
The absorption time of NAC is critical because of the narrow 8- to 10-hour window in which the drug is most effective in preventing acetaminophen-induced hepatotoxicity. Thus an advantage of the availability of a more palatable oral formulation of NAC for treatment of acetaminophen overdose—a formulation that better mitigates the putrid smell and taste of NAC—might be that NAC administration would be delayed for fewer patients. Unlike IV NAC, orally administered NAC undergoes an extensive first-pass metabolism so that the entire dose passes through the liver, the potential site of damage, with resulting high NAC concentrations.15
The availability of a more palatable oral NAC formulation might also reduce the number of patients requiring antiemetic agents or several doses of such agents to tolerate treatment. In a small, retrospective study comparing antiemetic use between patients receiving oral solution NAC (n = 20) and IV NAC (n = 17) for treatment of acetaminophen overdose, Miller et al16 found that subjects receiving oral solution NAC required 2.8 (0.7) doses of antiemetic medication, whereas subjects receiving IV NAC required 1.1 (0.2) doses of an antiemetic medication (P = 0.04). Altogether, 75.0% of patients treated with oral solution NAC received antiemetic therapy in contrast to 58.8% of the patients treated with IV NAC (P = 0.48).
Another advantage of effervescent NAC tablets might be preparation convenience for practitioners and patients outside the emergency department in selected cases. For example, after administration of the loading dose of NAC in the emergency department for acetaminophen overdose, if the patient is well and clearly not intending self-harm, the patient could be discharged from the emergency department with additional doses of oral NAC (70 mg/kg) to be taken every 4 hours, with scheduled returns to an outpatient clinic for repeat measurements of alanine aminotransferase activity.17
There were limitations to the present study. Data were obtained from a small number of healthy adult subjects, under fasting conditions, after administration of a single 11-g dose of NAC. The PK profiles of effervescent NAC tablets and oral solution NAC might be different in other populations, although that is unlikely because both products were administered as oral solutions. Because this was a single-dose PK study, meaningful safety-profile conclusions cannot be drawn from small differences between the treatments. The lemon-mint flavor of the effervescent NAC tablets dissolved in water was not exactly the same as the lemon-lime flavor of the diet caffeine-free soft drink in which oral solution NAC was diluted, a difference that could have affected the preference of formulation attributes. Because the formulation attributes were not defined for the subjects before the study began, their meaning was subjectively interpreted, and that could have affected preference choices. Finally, the study was open label, with subjects and investigators both aware of formulation assignment, which might have affected the evaluation of tolerability and influenced subject preference of formulation attributes.
Conclusions
The data from this study of a single 11-g dose of oral NAC demonstrated that effervescent NAC tablets and oral solution NAC met the regulatory criteria for assuming bioequivalence in fasting healthy adult subjects. Both formulations were well tolerated, but subjects preferred the formulation attributes of effervescent NAC tablets to those of oral solution NAC.
Conflicts of Interest
This research was funded by Arbor Pharmaceuticals, Atlanta, Georgia. The study sponsor was actively involved in the study design, assurance of the accuracy of the data collection and analysis, and interpretation of data. The authors retained full control of the manuscript content. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
Overall responsibility for the manuscript belonged to Spencer C. Greene and W. Frank Peacock. Carlos Sanabria was the study principal investigator. Patrick K. Noonan was involved with the conception and design of the study and provided pharmacokinetic analysis. All authors were involved in analysis, interpretation, and critical revision of the manuscript. Editorial support for the manuscript was provided by representatives of Galen Press, Inc, and was paid for by the study sponsor.
References
- 1.Heard K., Green J. Acetylcysteine therapy for acetaminophen poisoning. Curr Pharm Biotechnol. 2012;13:1917–1923. doi: 10.2174/138920112802273146. [DOI] [PubMed] [Google Scholar]
- 2.Mowry J.B., Spyker D.A., Brooks D.E., McMillan N., Schauben J.L. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol. 2015;53:962–1147. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 3.Manthripragada A.D., Zhou E.H., Budnitz D.S., Lovegrove M.C., Willy M.E. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf. 2011;20:819–826. doi: 10.1002/pds.2090. [DOI] [PubMed] [Google Scholar]
- 4.Betten D.P., Cantrell F.L., Thomas S.C., Williams S.R., Clark R.F. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50:272–279. doi: 10.1016/j.annemergmed.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Betten D.P., Burner E.E., Thomas S.C., Tomaszewski C., Clark R.F. A retrospective evaluation of shortened-duration oral N-acetylcysteine for the treatment of acetaminophen poisoning. J Med Toxicol. 2009;5:183–190. doi: 10.1007/BF03178264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackford M.G., Felter T., Gothard M.D., Reed M.D. Assessment of the clinical use of intravenous and oral N-acetylcysteine in the treatment of acute acetaminophen poisoning in children: a retrospective review. Clin Ther. 2011;33:1322–1330. doi: 10.1016/j.clinthera.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Green J.L., Heard K.J., Reynolds K.M., Albert D. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: a systematic review and meta-analysis. West J Emerg Med. 2013;14:218–226. doi: 10.5811/westjem.2012.4.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bebarta V.S., Kao L., Froberg B. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol. 2010;48:424–430. doi: 10.3109/15563650.2010.486381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration . Rockville, MD; US Department of Health and Human Services: March 2003. Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products – general considerations.http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf Accessed February 20, 2016. [Google Scholar]
- 10.Smilkstein M.J., Knapp G.L., Kulig K.W., Rumack B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration . Rockville, MD; US Deparment of Heath and Human Services: May 2001. Bioanalytical method validation.http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf Accessed February 20, 2016. [Google Scholar]
- 12.Committee for Medicinal Products for Human Use . London, United Kingdom; European Medicines Agency: July 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf Accessed February 20, 2016. [Google Scholar]
- 13.Viswanthan C.T., Bansal S., Booth B. Workshop/conference report – quantitative bioanalytical methods validation and implementation: best practices for chromoatographic and ligand binding assays. The APPS Journal. 2007;9:E30–E42. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 14.British Nutrition Foundation. Food a fact of life: Sensory evaluation. http://www.foodafactoflife.org.uk/sheet.aspx?siteId=20§ionId=85&contentId=329. Accessed February 20, 2016.
- 15.Klein-Schwartz W., Doyon S. Intravenous acetylcysteine for the treatment of acetaminophen overdose. Expert Opin Pharmacother. 2011;12:119–130. doi: 10.1517/14656566.2011.537261. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.A., Navarro M., Bird S.B., Donovan J.L. Antiemetic use in acetaminophen poisoning: how does the route of N-acetylcysteine administration affect utilization? J Med Toxicol. 2007;3:152–156. doi: 10.1007/BF03160931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heard K.J. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]