Abstract
This study elucidates the life-cycle of the reptile inhabiting nematode Abbreviata hastaspicula (Spirurida: Physalopteridae: Physalopterinae) in Australia. Eight Varanus gouldii (Lacertilia: Varanidae), and two Christinus marmoratus (Reptilia: Gekkonidae) lizards were captured in the wild. Two V. gouldii were used as controls and no experimental procedures were carried out on them. Another six V. gouldii (final host) and the two C. marmoratus (paratenic host) were treated with oral anthelmintics to remove all parasitic worms and were fed with infected live arthropods containing third stage larvae of Abbreviata hastaspicula. Faeces of V. gouldii were examined under the microscope weekly to determine whether the third stage larvae had developed into adults. Two months later, a total of 30 larvae and adults of A. hastaspicula were found in the stomachs of four experimentally-infected V. gouldii lizards. No cysts or larva were found in the C. marmoratus. This is the first study to demonstrate the life-cycle of this genus of nematode in their definitive reptile hosts.
Keywords: Lifecycle, Abbreviata spp., Abbreviata hastaspicula, Nematodes, Lizards, Physaloptera, Insects, Host-parasites
Graphical abstract

Highlights
-
•
The first study to show the life-cycle of A. hastaspicula in their definitive hosts.
-
•
Opens the way to a greater understanding of the biology of genus Abbreviata.
-
•
Our findings may have relevance for human contact and hence possible infection.
1. Introduction
The nematode Abbreviata hastaspicula (Spirurida: Physalopteridae) occurs predominantly in Varanus gouldii lizards, principally in the arid interior of Australia. It requires an arthropod intermediate host to complete its life-cycle (Mönnig, 1934, Alicata, 1937).
Distribution of the nematode genus Abbreviata is worldwide (Bain et al., 2015), in Australia, nematodes in this genus are widespread. They are most common in the lizard fauna (Jones, 2014), and physalopterid nematodes also occur in birds (Berger, 2010). The arid Australian landscapes support the richest and the most diverse lizard fauna in the world, due to the dry hot climate and the dominant, hummock grasslands (Triodia spp.), which provide various habitat niches for several species of lizard (Pianka, 1986). In addition, a range of shrubs and sparse trees provide niches for a great variety of both diurnal and nocturnal lizards (Rich and Talent, 2008). The Varanidae contain the world’s largest lizards, with at least 25 endemic described species in Australia (Bush et al., 2000). The species of lizard in this study, Varanus gouldii is found in all areas of Western Australia except the coolest and wettest parts.
Factors affecting the geographical pattern of Abbreviata are probably firstly, the distribution of suitable arthropod intermediate hosts; secondly, the ability of the eggs to survive and remain viable outside the final hosts (Jones, 2014); thirdly, the availability of prey for the hosts e.g. small lizards. A fuller understanding of the biology of species of Physalopterinae would clarify the relative importance of these factors.
The life-cycle of spirurid nematodes consists of six stages involving an egg, four larval stages (or juvenile stages) and lastly, adult. For parasitic nematodes found in vertebrates, the infective stage is always the third larval stage, L3 (Anderson, 2006). Similar to other spirurid nematodes, species of Abbreviata exhibits a heteroxeous life cycle. However, in paratenic hosts, the infective stage of a parasite persists without essential development and usually lack of growth (Anderson, 2006, Anderson et al., 2009). Its lifecycle can only be completed by the final host (larger lizards) feeding on insects (intermediate hosts) or paratenic hosts (smaller lizards) containing infective third stage larvae (Anderson, 2006, Preston and Johnson, 2010). Larvae ingested by possible paratenic hosts generally encyst in the abdominal tissues, where they can persist until they are eaten by a predaceous final host. The ingested larvae then attach to the stomach wall and, depending on the amount of food in the stomach, grow to adult (Lee, 1955). Abbreviata requires the tropical cricket, Teleogryllus oceanicus to act as its intermediate host is the only partial life cycle of Abbreviata spp. that is known from Australia (King et al., 2013). This study was undertaken to elucidate the life-cycle of Abbreviata hastaspicula in Australia by infecting wild caughtVaranus gouldii (final host) and Christinus marmoratus (paratenic host) with live arthropods that had been infected with the larvae of Abbreviata hastaspicula.
2. Materials and methods
2.1. Infecting the lizards
Eight Varanus gouldii were caught from arid Paynes Find (latitude:−29° 43′ 21.5436″, longitude: 117° 10′ 24.3912″) and the two Christinus marmoratus were caught at Wooroloo (latitude:−31° 48′ 7.3218″, longitude: 116° 18′ 51.6384″), Western Australia with the permission of Department of Environment and Conservation Australia (Licence no. SF009524). The average weight of the varanids was 4.15 ± 0.52 kg and that of the geckos was 17.1 ± 3.20 g. The Varnaus were marked from no. 1 to 8.
The faeces of the wild-caught V. gouldii were collected from the floor of their individual housing cage and were checked weekly for eggs of the nematode Abbreviata hastapicula Two V. gouldii (no. 1 and 2) were used as controls and were euthanized by sodium pentobarbital injection (dose: ≥ 100 mg/kg) as soon as embryonated eggs were found in the faeces to ascertain that nematodes in the experimental lizards were the same as the species of nematodes that occurred in these lizards in the wild.
The remaining lizards (six V. gouldii and two C. marmoratus) were treated with Panacur 25 (0.4 ml/100 g body weight orally once and repeat in 14 days). One week after the two doses of Panacur 25 were administered to all the lizards directly with an oral syringe, no eggs were found in the V. gouldii (no. 3−8) , thus ascertaining that all six V. gouldii and the two geckoes were free of parasites.
After ascertaining that all the six V. gouldii were free from nematodes, two Varanus (no. 3 and 4) were kept uninfected and parasite free, they were fed the two C. marmoratus three months later in this experiment. Four uninfected Varanus (no. 5, 6, 7 and 8) and two C. marmoratus were fed with infected live Teleogryllus oceanicus (Orthoptera: Ensifera: Gryllidae) crickets that ingested the embryonated eggs of A. hastaspicula 28 days earlier following the methodology previously described (King et al., 2013) (feeding of arthropods was a one-off event). Each Varanus consumed 19 T. oceanicus crickets that had contained third stage larvae of Abbreviata hastaspicula,and five T. oceanicus were given to each C. marmoratus. The V. gouldii had ingested all the T. oceanicus crickets as soon as they were given to them. The two C. marmoratus had broken apart and ingested some shattered parts of the crickets, but it was not certain whether they had ingested the infected cysts.
Faeces of Varanus were examined microscopically every week to determine whether the third stage larvae had developed into adults.
Two months later, in order to confirm that Abbreviata hastaspicula larvae can only develop into adults in a final host, the four infected V. gouldii (no. 5, 6, 7 and 8) were euthanized by sodium pentobarbital injection ,when the eggs of A. hastaspicula were found in their faeces.The two C. marmoratus (possible paratenic host) were euthanized by carbon dioxide inhalation and thier stomachs and gastrointestinal tracts were opened to check whether they contained larval cysts of A. hastaspicula.
After another month, the two V. gouldii (no. 3 and 4) that had consumed the two C. marmoratus were euthanized by injection of sodium pentobarbital and were dissected (UWA Animal Ethics Ref. RA/3/100/1248).
2.2. Dissecting the lizards
The lizard was laid on its back, and a vertical ventral incision was made from the sternum to the pubis. The connective tissue was peeled from skin and turned back so that viscera were exposed. The lower oesophagus, stomach and intestine were released from connective tissues and examined for adult nematodes and larvae. The stomach was opened by vertical incision, food was noted and collected. Stomach, gastrointestinal tracts, cysts and worms were removed with forceps and fixed in formalin and preserved in 70% alcohol.
Adult nematodes of A. hastaspicula found in V. gouldii (no. 5, 6, 7 and 8) were observed under light microscopy under X4, X10 and X20 objectives after clearing in chlorolactophenol, and lengths of the larvae and adult nematodes (in mm) were measured. Their stage of development was assessed by the differentiation of their sexual organs (Cawthorn and Anderson, 1977).
2.3. Histology staining
Nematodes and small segments of stomach and gastrointestinal tracts of infected Varanus were dehydrated, embedded in paraffin, and serially sectioned at 5 μm. Tissue samples from animals were stained with hematoxylin and eosin (Gabe and Blackith, 1976).
3. Results
3.1. Infection prevalence and intensity rate of A. hastaspicula in V. gouldii and C. marmoratus before the experiment
Embryonated eggs of A. hastaspicula were present in the faeces of all wild-caught V. gouldii, indicating that all the V. gouldii were infected with this nematode in the wild. Embryonated eggs of A. hastaspicula and A. antarctica were found in one of the controls V. gouldii. The density of eggs in the faeces was 4 eggs/2 mg of faeces. For the C. marmoratus, because this species of Gekkonidae could be a possible paratenic host that might contain infective third stage larvae but not adult A. hastaspicula, and eggs could only be produced by adult A. hasataspicula, we therefore were not able to tell whether the C. marmoratus have infection as we could not observe this in the faeces.
3.2. Infecting the experimental lizards
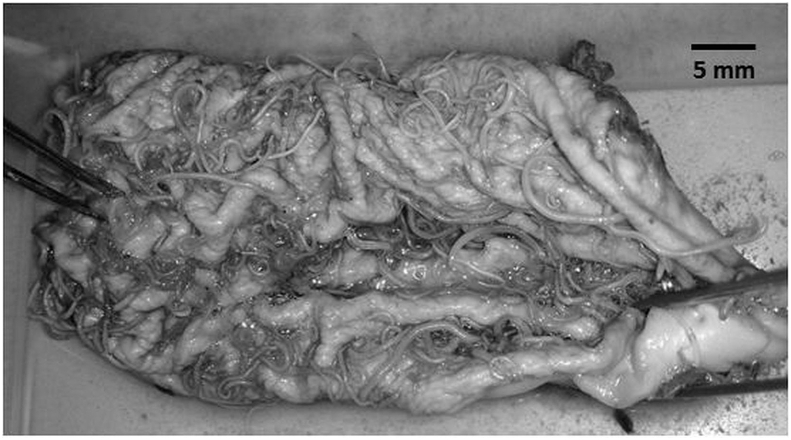
Two months after the lizards had ingested infected T. oceanicus, eggs of A. hastaspicula were being passed in the faeces of the experimentally infected V. gouldii (no. 5, 6, 7 and 8). The infection rate for the final host was 100%. The numbers of A. hastaspicula found in the experimental V. gouldii no. 5, 6, 7 and 8 were 5, 3, 8 and 14 respectively. No larva or adult nematodes of A. hastapicula were found in C. marmoratus. Concurrent infection with A. antarctica occurred in one of the two controls. Five hundred and eight and 834 nematodes were recovered respectively from the two controls V. gouldii (Fig. 1).
Fig. 1.
A large number of Abbreviata hastapicula were found in the stomach of a control Varanus gouldii.
3.3. Adult Abbreviata hastaspicula
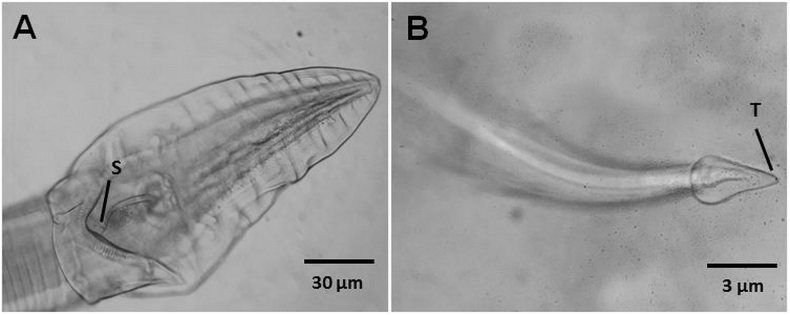
Seventeen females and 13 males were recovered. Males were 8.24–11.58 mm long, and females 13.22–19.03 mm long. Females were longer and stouter than males; the average width of males (0.57 mm) was about half of that of females (1.10 mm). Female A. hastaspicula contained eggs (Fig. 2A and 2B). The spearhead-like tip of the male right spicule was diagnostic, Fig. 3A and 3B (Jones, 1979). Diagnostic morphological features in the female include the thin-walled eggs compared with those of potential concurrent species (A. antarctica and A. bancrofti) and the tubular extension to the vulva (Fig. 2, Fig. 4). Food residues were noted. The nematodes were attached together in a mass. A. antarctica specimens were cleaned in sterile water and stored in 70% ethanol.
Fig. 2.
Female Abbreviata hastaspicula. (A) tubular vulva, laying eggs, dorsal view. (B) Embryonated eggs in the uterus.
Fig. 3.
Male Abbreviata hastaspicula. (A) Posterior end, dorsoventral view, where S is the spicule. (B) T is the tip of right spicule, lateral view.
Fig. 4.
Adult female Abbreviata hastaspicula. AE, anterior end; ME, muscular oesophagus; GE, glandular oesophagus; TV, tubular vulva; UB, eggs in the 4 uterine branches; A, anus; T, tail.
3.4. Histology staining
Histological sections showed little inflammatory cells and there was no evidence that A. hastaspicula affects the health of their final host, V. gouldi (Fig. 5). No difference of host response to infection across the species was noted in any lizards.
Fig. 5.
Section of stomach of Varanus Gouldii with an adult Abbreviata hastapicula. AT, apical tooth of A. hastaspicula.
4. Discussion
Adult Abbreviata are widespread in the stomachs of large lizards in the genus Varanus in Australia, with an infection prevalence close to 100% (Jones, 1995a, Jones, 2005). In the present study, adults or immature adults of A. hastaspicula were present in all the four experimental Varanus gouldii (no. 5, 6, 7 and 8) that had ingested the infected tropical crickets, confirming that this lizard is a definitive host of A. hastaspicula (Fig. 6). Previous studies by Jones (1983) have shown a positive correlation between the numbers of Abbreviata sp. larvae and A.hastaspicula . Lee (1957) elucidated the lifecycle of Skrjabinoptera phrynosoma by infecting 27 horned toads with larvae from intermediate host Texas agricultural ants. Five out of the 27 infected toads carried the exact number of adult nematodes they were fed. Compared with our study, although all 19 T.oceanicus crickets were ingested by the V. gouldii, we only found 5, 3, 8 and 14 A. hastaspicula in V. gouldii no.5–8 respectively during dissection. No eggs were found in the faeces of V. gouldii after the first dosage of anthelmintic Panacur, but to ascertain the Varanus were fully clear of parasites, a second dose of drug was administered to them 14 days later. In addition, no larvae or adult A. hastaspicula was observed in no. 3 and 4 V. gouldii. It is therefore concluded that the A. hastaspicula recovered from V. gouldii no. 5–8 did not resulted from natural infection but experimental feeding.
Fig. 6.
The postulated complete life cycle of Abbreviata hastapicula. Thick arrows indicated the life cycle in the final host (life cycle elucidated in this paper): Firstly, eggs passed from the faeces of the larger lizards. Secondly, eggs containing first stage lava (e) are ingested by suitable arthropod intermediate hosts (e.g. Teleogryllus oceanicus), and the 1st stage larva developed into 3rd stage larva/larvae then encysted on the guts of arthropod intermediate hosts. Lastly, arthropod intermediate hosts are consumed by final/definitive host (larger lizard). Or alternatively, as shown by the dashed arrows (this is not yet confirmed), if the life cycle is achieved through paratenic hosts, the arthropod intermediate hosts would be consumed by the paratenic host (smaller lizards), and the infective larva(e) persist without essential development or growth until it is consumed by the final host (larger lizards).
The same species of parasites are more likely to infect the host species that are taxonomically related (Freeland, 1983). In the case of Abbreviata spp., consistent with Jones, 2005, Jones, 2007, Jones, 2014 which indicated that A. hastaspicula predominates in Varanidae, all of the eight wild caught V. gouldii in our experiment were naturally infected with A. hastaspicula. Although A. antarctica was recovered at highest prevalence and intensity in V. rosenbergi (Jones, 2005, Jones, 2007, Jones, 2014), concurrent infection of both A. hastaspicula and A. antarctica occurred in one of our controls V. gouldii. In the dry inland of the continent with an annual precipitation below 400 mm, A. hastaspicula replaces A. antarctica though the two coexist over wide areas (Jones, 1983), which might explain why seven out of the eight wild captured Varanids in our study were only naturally infected by A. hastaspicula. The morphologically primitive physalopteran nematode Kreisiella chrysocampa occurs as adults in several species of smaller skinks lizards, in which physalopterid cysts occur but no adult Abbreviata suggest that Abbreviata spp.may have arisen in smaller lizards, and that their ancestor may have been species related to of Kreisiella (Jones, 1995a). Hence, it is possible that the definitive host of A. hastaspicula may have been smaller lizards and which, on being ingested by larger lizards, developed in them. Environmental changes could theoretically expose lizards to different suites of parasites over time (Poulin, 2007, Poulin and Keeney, 2008). Findings from the Australian lizard fauna show that host-specificity in the subfamily Physalopterinae is usually at the family rather than species level (Jones, 2004, Jones, 2007, Jones, 2014).
Gecko C. marmoratus was used in the present study to ascertain whether it is a potential paratenic host for V. gouldii; this was the only species of gecko we caught in the wild. We could not confirm the two C. marmoratus were uninfected before they were fed with the infected T. oceanicus as only adult A. hastaspicula is able to pass its eggs in the faeces of the lizards. As the anthelmintic drug has dewormed the Varanids, we therefore expected that the geckos were free of parasites. However, further experimentations could perhaps use X-ray to confirm the infection status of the C. marmoratus or other potential paratenic Gekkonidae. The two C. marmoratus that had ingested the crickets and the two V. gouldii that had consumed the C. marmoratus were not infected with adult Abbreviata species; however, we were unable to ascertain that the geckoes had ingested nematodes from the offered crickets, and thus we cannot confirm that C. marmoratus are potential paratenic hosts for A.hastaspicula. Many species of smaller lizards, mainly skinks and geckoes, are paratenic hosts for physalopterid larvae (Jones, 1995a), in which there is a lack of inflammatory response (Jones, 1995b). No further development in these paratenic hosts occurs unless they are consumed by a larger species of lizard (Jones, 1995a). More studies are required to ascertain the postulated life cycle in paratenic hosts (dashed arrows in Fig. 6) and we recommend the inoculation of third stage infective larvae to the geckos to be performed using a stomach tube or oral syringe.
The absence of pathological changes produced by larval Abbreviata spp. infection is probably the result of a long evolutionary association between this species of nematode and their reptile final host. Varanus gouldii can live for at least seven years in captivity (King and Green, 1999). The lifespan of A. hastaspicula within the host is unknown, but the reptile hosts outlive the third-stage physalopterid larvae (Jones, 1995b).
To conclude, our study has elucidated the complete life-cycle of A. hastaspicula in its definite V. gouldii. The findings of the present study may have relevance for human contact and hence possible infection. As early as 1902, there are records of Physalopterinae nematodes infecting humans in Caucasus, Central Africa, South America (Ortlepp, 1926, Morgan, 1945), Panama (Faust and Martinez, 1935), India (Singh and Rao, 1954) and Congo (Vandepitte et al., 1964). In Australia, physalopteran larvae have caused life-threatening eosinophilic granulomata in an 11-month-old male infant, but the species causing the infection could not be identified (Nicolaides et al., 1977). This subfamily of nematodes may perhaps be underreported in man because they are insufficiently known. Further studies of nematodes in the genus of Abbreviata in Australian lizards should provide considerably more information for the understanding of their biology, and thus the risk of humans acquiring physalopterid infection.
Acknowledgements
We are very grateful to the School of Animal Biology, University of Western Australia for providing housing for the lizards, Maxine Beveridge and Leigh Simmons who made the T. oceanicus and laboratory facility available, Rick Roberts, Husnan Ziadi and Nicolas Nagloo for catching the lizards from the wild, Alfred Tay for collaborating with us, Jacob Kenny for the histology. I would like to particularly thank Rick Roberts for his technical support along the way, without his help; this experiment would not be able to carry out. Vice- Chancellor of the University of Western Australia partially funded this project.
References
- Alicata J.E. Papers on Helminthology Published in Commemoration of the 30 Year Jubileum of KJ Skrjabin and of 15th Anniversary of the All-Union Institute of Helminthology. 1937. Larval development of the spirurid nematode, Physaloptera turgida, in the cockroach, Blattella germanica; pp. 11–14. [Google Scholar]
- Anderson R. second ed. CABI Publishing; New York: 2006. Nematodes Parasites of Vertebrates: Their Development and Transmission. [Google Scholar]
- Anderson R., Chabaud A., Willmott S. CABI Publishing; New York: 2009. Keys to the Nematode Parasites of Vertebrates: Archival Volume. [Google Scholar]
- Bain O., Mutafchiev Y., Junker K., Guerrero R., Martin C., Lefoulon E., Uni S. Review of the genus Mansonella Faust, 1929 sensu lato (Nematoda: Onchocercidae), with descriptions of a new subgenus and a new subspecies. Zootaxa. 2015;3918:151–193. doi: 10.11646/zootaxa.3918.2.1. [DOI] [PubMed] [Google Scholar]
- Berger S. GIDEON Informatics Inc; San Francisco: 2010. Less-common Nematodes: Global Status 2010. [Google Scholar]
- Bush B., Maryan B., Cooper R., Robinson D. University of Western Australia Press; Perth: 2000. A Guide to the Reptiles and Frogs of the Perth Region. [Google Scholar]
- Cawthorn R., Anderson R. Cellular reactions of field crickets (Acheta pennsylvanicus Burmeister) and German cockroaches (Blatella germanica L.) to Physaloptera maxillaris Molin (Nematoda: Physalopteroidea) Can. J. Zool. 1977;54:442–448. doi: 10.1139/z76-051. [DOI] [PubMed] [Google Scholar]
- Faust E.C., Martinez W.H. Notes on helminths from Panama. II. Rare human nematode eggs in the feces of individuals from the Chagres River, Panama. J. Parasitol. 1935;21:332–336. [Google Scholar]
- Freeland W.J. Parasites and the coexistence of animal host species. Am. Nat. 1983;121:223–236. [Google Scholar]
- Gabe M., Blackith R.E. Masson; Paris: 1976. Histological Techniques. [Google Scholar]
- Jones H. Gastrointestinal nematodes, including three new species, from Australian and Papua New Guinean pythons. Proc. Helminthol. Soc. Wash. 1979;46:1–14. [Google Scholar]
- Jones H. Abbreviata (Nematoda: Physalopteroidea) in lizards of the Varanus gouldii complex (Varanidae) in Western Australia. Aust. J. Zool. 1983;31:285–298. [Google Scholar]
- Jones H. Gastric nematode communities in lizards from the Great Victoria Desert, and an hypothesis for their evolution. Aust. J. Zool. 1995;43:141–164. [Google Scholar]
- Jones H. Pathology associated with physalopterid larvae (Nematoda: Spirurida) in the gastric tissues of Australian reptiles. J. Wildl. Dis. 1995;31:299–306. doi: 10.7589/0090-3558-31.3.299. [DOI] [PubMed] [Google Scholar]
- Jones H. Gastric nematodes, including a new species of Abbreviata (Nematoda: Physalopteridae) from the mangrove monitor Varanus indicus (Reptilia: Varanidae) Trans. R. Soc. S Aust. 2004;128:53–59. [Google Scholar]
- Jones H. The gastrointestinal nematodes of Varanus rosenbergi (Reptilia: Varanidae) and the effects of habitat change in southern Australia, with particular reference to the genus Abbreviata (Physalopteroidea) Rec. West Aust. Mus. 2005;22:259–263. [Google Scholar]
- Jones H. Helminth parasites of the lace monitor Varanus varius (Reptilia: Varanidae) in Eastern Australia. Trans. R. Soc. S Aust. 2007;131:160–166. [Google Scholar]
- Jones H. Physalopterine nematodes in Australian reptiles: interactions and patterns of infection. Aust. J. Zool. 2014;62:180–194. [Google Scholar]
- King C., Jones H.I., Tay A. Arthropod intermediate hosts of Abbreviata Antarctica (Nematoda: Physalopteridae) in Australia. J. Parasitol. 2013;99:708–711. doi: 10.1645/12-47.1. [DOI] [PubMed] [Google Scholar]
- King D., Green B. University of New South Wales Press; Sydney: 1999. Goannas. The Biology of Varanid Lizards. [Google Scholar]
- Lee S.H. The mode of egg dispersal in Physaloptera phrynosoma Ortlepp (Nematoda: Spiruroidea), a gastric nematode of Texas horned toads, Phrynosoma cornutum. J. Parasitol. 1955;41:70–74. [PubMed] [Google Scholar]
- Lee S.H. The life cycle of Skrjabinoptera phrynosoma (Ortlepp) Schulz, 1927 (Nematoda: Spiruroidea), a gastric nematode of Texas horned toads. Phrynosoma cornutum. J. Parasitol. 1957;43:66–75. [PubMed] [Google Scholar]
- Mönnig H.O. Baillière; London: 1934. Veterinary Helminthology and Entomology. [Google Scholar]
- Morgan B.B. The nematode genus Abbreviata (Travassos, 1920) Schulz, 1927. Am. Mus. Novit. 1945;34:485–490. [Google Scholar]
- Nicolaides N.J., Musgrave J., McGuckin D., Moorhouse D.E. Nematode larvae (Spirurida: Physalopteridae) causing infarction of the bowel in an infant. Pathology. 1977;9:129–135. doi: 10.3109/00313027709085251. [DOI] [PubMed] [Google Scholar]
- Ortlepp R.J. On the identity of Physaloptera caucasica v. Linstow, 1902, and Physaloptera mordens Leiper, 1908. J. Helminthol. 1926;4:199–202. [Google Scholar]
- Pianka E. Princeton University Press Princeton; New Jersey: 1986. Ecology and Natural History of Desert Lizards: Analyses of the Ecological Niche and Community Structure. [Google Scholar]
- Poulin R. Princeton University Press; New Jersery: 2007. Evolutionary Ecology of Parasites. [Google Scholar]
- Poulin R., Keeney D.B. Host specificity under molecular and experimental scrutiny. Trends Parasitol. 2008;24:24–28. doi: 10.1016/j.pt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Preston D., Johnson P. Ecological consequences of parasitism. Nat. Educ. Knowl. 2010;1:39. [Google Scholar]
- Rich C.N., Talent L.G. The effects of prey species on food conversion efficiency and growth of an insectivorous lizard. Zoo. Biol. 2008;27:181–187. doi: 10.1002/zoo.20175. [DOI] [PubMed] [Google Scholar]
- Singh S., Rao V. On a species of Physaloptera causing a subcutaneous abscess in the neck of an Indian. J. Helminthol. 1954;28:155–158. doi: 10.1017/s0022149x00032818. [DOI] [PubMed] [Google Scholar]
- Vandepitte J., Michaux J., Fain A., Gatti F. Premières observations congolaises de physaloptérose humaine. Ann. Soc. Belg. Méd. Trop. 1964;44:1067–1076. [PubMed] [Google Scholar]