Abstract
The persistent organic pollutants (POPs) due to their physicochemical properties can be widely spread all over the globe; as such they represent a serious threat to both humans and wildlife. According to Stockholm convention out of 24 officially recognized POPs, 16 are pesticides. The atmospheric life times of pesticides, up to now were estimated based on their gas-phase reactivity. It has been only speculated that sorption to aerosol particles may increase significantly the half‐lives of pesticides in the atmosphere. The results presented here challenge the current view of the half-lives of pesticides in the lower boundary layer of the atmosphere and their impact on air quality and human health. We demonstrate that semivolatile pesticides which are mostly adsorbed on atmospheric aerosol particles are very persistent with respect to the highly reactive hydroxyl radicals (OH) that is the self-cleaning agent of the atmosphere. The half-lives in particulate phase of difenoconazole, tetraconazole, fipronil, oxadiazon, deltamethrin, cyprodinil, permethrin, and pendimethalin are in order of several days and even higher than one month, implying that these pesticides can be transported over long distances, reaching the remote regions all over the world; hence these pesticides shall be further evaluated prior to be confirmed as POPs.
Aerosol particles are omnipresent in the lower boundary layer of the atmosphere and exert an important influence on the global climate and human health. Aerosol particles have typical atmospheric lifetimes of about 3 to 10 days. Considering the fact that air masses can be transported over several thousand kilometers in period of two weeks, there are really no places where we can expect to find truly remote conditions, especially in the Northern Hemisphere. Lelieveld et al.1 have shown that outdoor air pollution, mostly by particulate matter (PM) 2.5, leads to 3.3 million premature deaths per year worldwide, predominantly in Asia, a figure that could double by 2050 if emissions continue to rise at the current rate. Atmospheric aerosols may affect the human health especially the particles with diameter smaller than 100 nm exhibit more adverse health effects compared to the larger particles due to the higher probability to penetrate in the human lung and even in the blood2.
During applications, a significant fraction of applied pesticides, about 15 to 40%, is dispersed in the atmosphere by volatilization or spray drift processes3,4. Pesticides travel in the atmosphere with long range atmospheric transport and deposition from their emission area5,6. The fate of pesticides is influenced by their partition between the gas phase and particulate phase. Considering the low volatility of majority of the commonly used pesticides, they are often adsorbed on the surface of atmospheric particles7,8. They may undergo different transport and transformation processes resulting in the generation of secondary products that could be even more hazardous than the primary emitted pesticides.
These aspects are central to atmospheric composition changes, air quality and associated climate change. Hence, a better chemical characterization of the processes associated with the adsorbed pesticides on atmospheric aerosols is highly desirable to understand the variable chemical and physical controlling factors allowing assessment of the contributions and consequences of global environmental change.
According to Stockholm convention9 (a treaty to protect human health and the environment from POPs), the organic compounds with half-life time (t½) greater than 2 days in air are considered as persistent organic pollutants (POPs). The Stockholm convention considers the gas-phase reactions toward hydroxyl radicals (OH) as a major degradation pathway of pesticides in the atmosphere10. Therefore, the atmospheric half-lives of pesticides are calculated from the gas-phase reactivity with respect to the OH radicals, using structure–activity relationships (SAR) used by U.S. Environmental Protection Agency, software AOPWIN (Atmospheric Oxidation Program)11.
However, the heterogeneous reactions of pesticides which occur on the surface of atmospheric aerosols may proceed at different rates than the gas-phase reactions. Indeed, Socorro et al.12 have shown that half-lives of 8 commonly used pesticides span from 9 to >24 days for an atmospheric ozone level of 9.8 · 1011 cm−3, demonstrating that these species are very persistent regarding the ozone (O3) reactivity on atmospheric particulate phase13. In this context, it is essential to investigate the fate of pesticides adsorbed on aerosol particles to determine their persistence in the atmosphere with respect to the highly reactive hydroxyl radicals. Indeed, OH radicals are considered as the dominant oxidizing and cleansing agent determining the oxidative capacity of the atmosphere.
In the present study we investigated the heterogeneous reactivity of eight pesticides, difenoconazole, tetraconazole, fipronil, oxadiazon, deltamethrin, cyprodinil, permethrin, and pendimethalin enriched in atmospheric particulate phase toward gas phase OH radicals. The emerged results strongly indicate that these commonly used pesticides once adsorbed on the atmospheric aerosols can be transported thousands of kilometres far away from the place where have been applied.
From the environmental pollution point of view these results are extremely important and it should be considered while developing appropriate environmental strategies which in turn will contribute to better describe and understand the atmospheric behavior of pesticides and their persistence in the environment.
Results and Discussion
Silica is commonly a major constituent of mineral dust14 on which surface the organic compounds are adsorbed and (photo)oxidized during their transport15. To study the OH heterogeneous kinetic reactions the pesticides were coated on silica particles (AEROSIL R812). Silica particles are characterised by a high surface to volume ratio which makes them an ideal proxy for investigation of heterogeneous reactions. The pesticides adsorbed on silica particles were simultaneously oxidized by gas-phase OH radicals and gas-phase O3 which is necessary for the production of OH radicals as explained in the supplementary information.
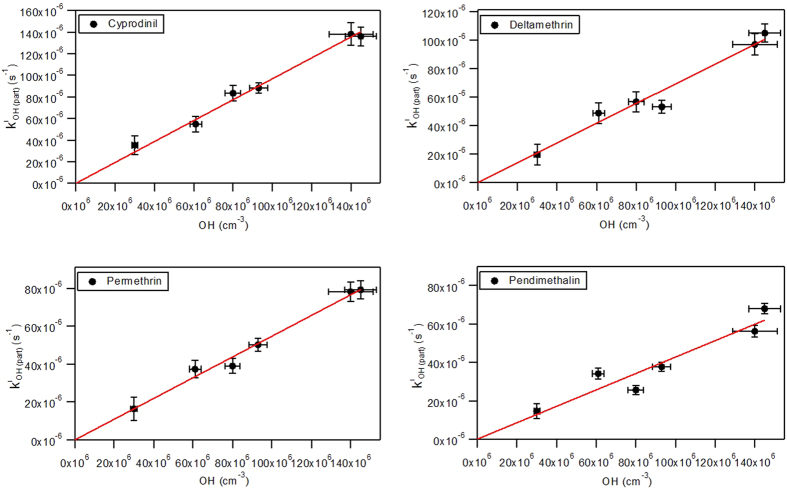
The pseudo-first-order rate constants for the heterogeneous oxidation of the particle-phase pesticides induced by OH radicals (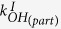 ) were determined by analysing the temporal profiles from 0 to 6 h obtained for six different OH radicals concentrations (3 · 107; 6.1 · 107; 8 · 107; 9.3 · 107; 1.4 · 108 and 1.5 · 108 cm−3).
) were determined by analysing the temporal profiles from 0 to 6 h obtained for six different OH radicals concentrations (3 · 107; 6.1 · 107; 8 · 107; 9.3 · 107; 1.4 · 108 and 1.5 · 108 cm−3).
Figure 1 shows, as an example, the exponential decays of the pesticides applying the highest OH and O3 concentrations, 1.5 · 108 cm−3 and 1.7 · 1014 cm−3, respectively.
Figure 1. Temporal profile of the normalized concentrations of eight studied pesticides to both OH and O3 reactivity for an OH concentration of 1.5 · 108 cm−3 and 1.7 · 1014 cm−3 of ozone for a period of 6 hours.
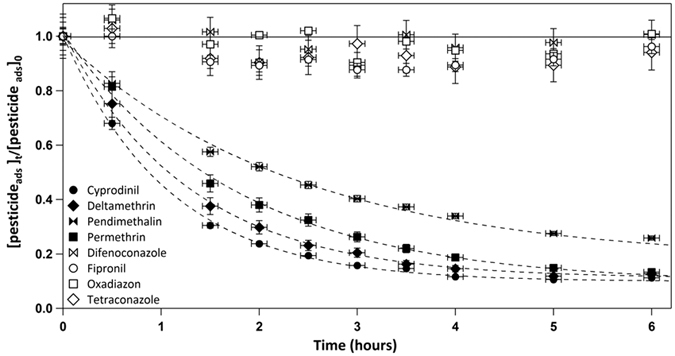
Dotted curves represent the exponential fit of the experimental points. The horizontal error bars corresponds to the sampling time of 10 min of particles during the experiment and the error bars of the pesticide concentrations are the standard deviations calculated for three injections of the sample t = 0 h in GC-(QqQ)-MS/MS.
One experimental limitation in the laboratory kinetic experiments is to reproduce realistic OH concentrations in order of 106–107 cm−3. The first two applied OH concentrations, i.e., 3 · 107 and 6.1 · 107 cm−3, correspond to the highly polluted regions16. Although the other applied OH concentrations, i.e., 8 · 107; 9.3 · 107; 1.4 · 108 and 1.5 · 108 cm−3, were 1–2 orders of magnitude higher than the global average OH concentrations16, four of the considered pesticides (difenoconazole, tetraconazole, fipronil, and oxadiazon) were almost not oxidized at all (Fig. 1). The other four pesticides (deltamethrin, cyprodinil, permethrin, and pendimethalin) were slightly degraded in the following manner from the fastest to the slowest: cyprodinil > deltamethrin > permethrin > pendimethalin.
A detailed procedure describing the calculations of the second order rate constants 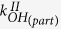 for the heterogeneous reactions of OH radical with cyprodinil, deltamethrin, permethrin, and pendimethalin is given in the SI Appendix.
for the heterogeneous reactions of OH radical with cyprodinil, deltamethrin, permethrin, and pendimethalin is given in the SI Appendix.
In order to determine the second order rate constant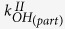 , the experimental pseudo first order reaction rate constants
, the experimental pseudo first order reaction rate constants 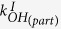 were plotted as a function of the OH radical concentrations (Fig. 2).
were plotted as a function of the OH radical concentrations (Fig. 2).
Figure 2. The observed pseudo-first order rate constants 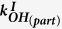 in function of OH radical concentrations.
in function of OH radical concentrations.
The errors for kIOH (part) were obtained from the statistical errors of the linear fit. The uncertainties are standard deviations estimated by the Igor Pro software (version 6.3.5.5). The relative OH radical concentration uncertainties were obtained by the sum of the relative uncertainties of mean m-xylene concentrations and rate constant of the reaction between m-xylene and OH radicals (see Eq. S8).
For the heterogeneous reactions of O3 with of cyprodinil, deltamethrin, permethrin, and pendimethalin, the following second order rate constants (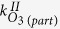 ), (8.8 ± 0.2) · 10−19, (8.0 ± 0.2) · 10−19, (6.0 ± 0.2) · 10−19 and (3.4 ± 0.1)·10−19 cm3 molecule−1 s−1 12, were used in Eq. S12. The intercept of the plot depicted in Fig. 2 corresponds to (
), (8.8 ± 0.2) · 10−19, (8.0 ± 0.2) · 10−19, (6.0 ± 0.2) · 10−19 and (3.4 ± 0.1)·10−19 cm3 molecule−1 s−1 12, were used in Eq. S12. The intercept of the plot depicted in Fig. 2 corresponds to (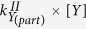 ) which was then subtracted, following the Eq. S12, to finally obtain the
) which was then subtracted, following the Eq. S12, to finally obtain the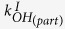 .
.
The linearity of the kinetic data depicted in Fig. 2 corresponds to a Langmuir-Rideal mechanism17,18,19. In Fig. 2 we assume that the linearity will continue down to ambient OH concentrations. Considering the extremely short life time of OH radicals comprised between 0.01 s and 1 s16, it is logical to expect direct bimolecular collision between the gas-phase OH radicals and the particulate pesticides without the adsorption step of OH radical on the surface of particles which would correspond to Langmuir-Hinshelwood mechanism12.
These results are in contrast with recent studies20,21,22,23 related to the heterogeneous OH reactions with liquid surfaces which proceed through Langmuir-Hinshelwood mechanism. The different behavior of the kinetic data could be ascribed to the different surfaces i.e. solid versus liquid film.
A study on the dependence of the first order rate coefficients of the deposited sample mass on the particles surface would be important to distinguish between the both mechanisms. Also, the experimental set-up is unable to generate low OH concentrations; hence, the parameter space of OH concentrations is not sufficient. However, even if the slope changes in Fig. 2 and kinetics behave more like Langmuir-Hinshelwood, the rates at ambient OH concentrations will be lower implying even longer half-lives of the pesticides.
The obtained 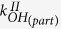 for the four pesticides span in the same order of magnitude, i.e., (4.3 ± 1.1)·10 −13 and (9.7 ± 0.7)·10−13 cm3 molecules−1 s−1 (Table 1).
for the four pesticides span in the same order of magnitude, i.e., (4.3 ± 1.1)·10 −13 and (9.7 ± 0.7)·10−13 cm3 molecules−1 s−1 (Table 1).
Table 1. Comparison of pesticides reactivity towards OH radicals in gas phase and in partficle phase (rate constants and half-lives) and partitioning of pesticides in particle phase with the effective rate constants.
| Pesticide |
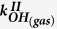 estimated in gas-phasea (cm3 molecule−1 s−1) estimated in gas-phasea (cm3 molecule−1 s−1) |
Estimated half-lives in gas phaseb (days) |
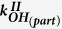 in particle phases (cm3 molecule−1 s−1) in particle phases (cm3 molecule−1 s−1) |
Partitioning in particle phasecΦpesticide |
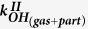 in gas and particle phases (cm3 molecule−1 s−1) in gas and particle phases (cm3 molecule−1 s−1) |
Atmospheric half-lives in gas and particle phasesb (days) |
|---|---|---|---|---|---|---|
| Pendimethalin | 3.0 · 10−11 | 0.4 | (4.3 ± 2.2) · 10−13 | 0.01 | 3.0 · 10−11 | 0.4 |
| Cyprodinil | 2.0 · 10−10 | 0.1 | (9.7 ± 2.9) · 10−13 | 0.07 | 1.9 · 10−10 | 0.1 |
| Tetraconazole | 1.1 · 10−11 | 1.0 | <<(4.3 ± 2.2) · 10−13 | 0.38 | <<7.0 · 10−12 | >>1.5 |
| Oxadiazon | 2.4 · 10−11 | 0.5 | <<(4.3 ± 2.2) · 10−13 | 0.62 | <<9.4 · 10−12 | >>1.1 |
| Fipronil | 9.6 · 10−11 | 0.1 | <<(4.3 ± 2.2) · 10−13 | 0.84 | <<1.6 · 10−11 | >>0.7 |
| Deltamethrin | 2.3 · 10−11 | 0.5 | (6.9 ± 2.8) · 10−13 | 0.91 | 2.7 · 10−12 | 4.0 |
| Permethrin | 2.3 · 10−11 | 0.5 | (5.5 ± 2.2) · 10−13 | 0.97 | 1.2 · 10−12 | 8.9 |
| Difenoconazole | 2.2 · 10−11 | 0.5 | <<(4.3 ± 2.2) · 10−13 | 0.99 | <<6.5 · 10−13 | >>16.5 |
aEstimated rate constants by modeling using AOPWIN program.
bHalf-lives for an average concentration [OH(g)] = 1.5 · 106 cm−3 and for an exposure of 12 h by day.
cPartitioning in the particle phase estimated by AEROWIN software using the Junge-Pankow adsorption mode.
According to the Stockholm convention9, the classification of POPs is made by use of AOPWIN software. Basically, the program calculates the first order rate constants (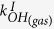
 by multiplying the second order rate constants (
by multiplying the second order rate constants (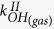 ) for the reaction of OH radicals with the organic compounds in the gas phase by an average OH radical concentration of 1.5 · 106 cm−3 and for an exposure of 12 h by day which corresponds to 7.5 · 105 cm−3 day−1 24,25. It follows from Eq. 1 the half-life (
) for the reaction of OH radicals with the organic compounds in the gas phase by an average OH radical concentration of 1.5 · 106 cm−3 and for an exposure of 12 h by day which corresponds to 7.5 · 105 cm−3 day−1 24,25. It follows from Eq. 1 the half-life (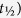 :
:
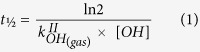 |
However, most of the pesticides represent the compounds with low vapor pressure (below 1 Pa) and thus a significant fraction of pesticides is enriched into atmospheric particulate phase. It was already speculated by Scheringer26 that the adsorption of semivolatile compounds on the atmospheric particles may significantly increase their half‐lives. This implies that in reality the half‐lives of the pesticides can be much longer than the current estimates by AOPWIN. In addition, the estimations made by AOPWIN gave unrealistically high rate constants for complex and highly chlorinated chemicals27,28.
Since the oxidation by OH radicals was considered as the most effective removal pathway of the pesticides, Scheringer et al.29 suggested a model to describe the reactivity of pesticides with respect to the OH radicals in both the gas phase and the particulate phase (Eq. 2).
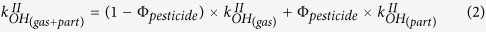 |
where 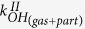 is the effective OH rate constant, Φpesticide represents the fraction of the adsorbed pesticides,
is the effective OH rate constant, Φpesticide represents the fraction of the adsorbed pesticides, 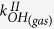 is the rate constant for the reaction between the OH radical and the pesticides dispersed in the gas phase,
is the rate constant for the reaction between the OH radical and the pesticides dispersed in the gas phase, 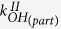 is the rate constant describing the reactions between OH radical and the pesticides adsorbed on the surface of atmospheric particles.
is the rate constant describing the reactions between OH radical and the pesticides adsorbed on the surface of atmospheric particles.
The fraction of pesticides adsorbed on the surface of particles, Φpesticide, can be determined by AEROWIN software based on the Junge-Pankow partitioning model30:
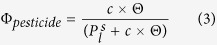 |
where c is a factor that depends on the excess heat of desorption from the particle surface (17.2 Pa cm), Θ is the surface of particles per unit of air volume (6.3 · 10−6 cm2 m−3) and 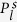 (Pa) is the vapor pressure of considered pesticide at 25 °C.
(Pa) is the vapor pressure of considered pesticide at 25 °C.
The estimated Φpesticide values, the effective rate constants and the calculated half-lives of the eight studied pesticides with respect to the OH reactivity are summarized in Table 1.
The partitioning between the gas phase and particulate phase is largely variable from 0.01 for pendimethalin up to 0.99 for difenoconazole. Therefore, Φpesticide should be taken into account while estimating the half-life of the pesticides.
Table 1 shows that pendimethalin and cyprodinil are predominantly found in the gas phase; thus the gas phase reactivity towards OH radicals contributes essentially to the half-lives of those pesticides.
Tetraconazole and oxadiazon partition more or less equally between the gas phase and the particulate phase. Considering the OH reactivity in both phases, these pesticides exhibit half-lives much higher than 1 day which implies long-range transport.
On the other hand, the estimated Φpesticide values of deltamethrin and permethrin indicate that these two pesticides are mostly enriched in the particulate phase. The experimentally measured rate constants 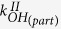 of these two pesticides are very close to the
of these two pesticides are very close to the 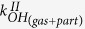 suggesting that the atmospheric half-lives are determined by the OH reactivity in the particulate phase. The half-lives of deltamethrin and permethrin are higher than 2 days strongly indicating that these two pesticides can be potentially considered as POPs according to the Stockholm convention.
suggesting that the atmospheric half-lives are determined by the OH reactivity in the particulate phase. The half-lives of deltamethrin and permethrin are higher than 2 days strongly indicating that these two pesticides can be potentially considered as POPs according to the Stockholm convention.
The example of difenoconazole that is completely enriched in the particulate phase is especially striking with half-life much higher than 16 days with respect to the OH reactivity. Such long lived pesticides will be transported far from their place of release impacting the regional and global air quality, human health and wildlife.
Figure 3 graphically shows the difference between the OH reactivity towards pesticides dispersed in the gas phase and pesticides adsorbed on the atmospheric particles.
Figure 3. Rate constants estimated by “Atmospheric Oxidation Program (AOPWIN)” of gas phase OH radical reactivity in function of experimental rate constants of heterogeneous OH radical reactivity for number of pesticides.
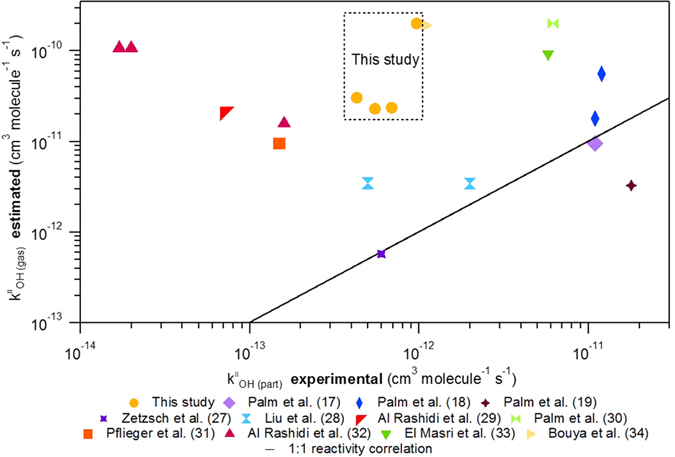
Almost all the experimental rate constants are clearly above the 1:1 correlation17,18,19,31,32,33,34,35,36,37,38 demonstrating that the experimental rate constants in the particulate phase are orders of magnitude slower than the estimated rate constants in the gas phase.
Concluding remarks
Heterogeneous reactivity of OH radical with 8 commonly used pesticides was investigated.
In the past, it has been assumed that these pesticides are predominantly degraded by the OH radical in the gas phase. However, the majority of these pesticides are adsorbed on the atmospheric particle surface; thus their reactivity in particulate phase should be considered prior to take any conclusion about their half-lives and possible hazards.
Here, we confirm that OH rate constants for difenoconazole, tetraconazole, fipronil, oxadiazon, deltamethrin, cyprodinil, permethrin, and pendimethalin are few orders of magnitude lower than the estimated rate constants in the gas phase. This implies that the heterogeneous OH oxidation of pesticides adsorbed on atmospheric particles is a very slow process suggesting that the pesticides can persist long time in the atmosphere prior to be removed and transferred to terrestrial and aquatic ecosystems.
Therefore, the estimated half-lives of these pesticides based on AOPWIN estimations are not valid and should not be used during the preparation of adequate legislation.
The emerged kinetic data from this study can be of great help for further validation of AOPWIN program with more complex organic molecules containing more functional groups in order to increase the confidence in the accuracy of the half-lives estimations for pesticides.
Materials and Methods
Chemicals
Cyprodinil (purity 99.8%), deltamethrin (99.7%), difenoconazole (97.0%), fipronil (97.5%), oxadiazon (99.9%), pendimethalin (98.8%), permethrin (98.3%), and tetraconazole (99.0%) (PESTANAL®, analytical standard), 2,3-dimethyl-2-butene (99%), m-xylene (>99.5%) were purchased from Sigma-Aldrich and were used as received (Table S1).
Silica particles coating
Commercial hydrophobic silica particles (AEROSIL R812, Degussa, purity SiO2 content ≥99.8%, average primary particle size of 7 nm and specific surface area (BET) of 260 ± 30 m2 g−1) were used as proxy of atmospheric mineral aerosols. Silica particles were coated with the pesticides according to a liquid/solid adsorption. 5 mL of pesticides solution at concentration 20 mg L−1 in dichloromethane (for HPLC, ≥99.8%, Sigma-Aldrich) was mixed with 500 mg of silica particles in a Pyrex bulb wrapped with aluminum foil. This bulb was ultrasonicated for 15 min. Then, dichloromethane was evaporated by a rotary evaporator (Rotavapor R-114, Büchi) at 40 °C and 850 ± 85 mbar. The load of pesticides on silica particles was about 0.02% by weight and the percentage of the coated aerosol surface was between 0.2 and 0.4%, less than a monolayer, assuming a uniform particle surface coverage as was detailed by Socorro et al.12.
Production and measurements of OH radical
A HS-PTR-MS (High Sensitivity – Proton Transfer Reaction – Mass Spectrometer, Ionicon Analytik) was used to follow the concentrations of m-xylene and 2,3-dimethyl-2-butene (DMB). DMB is an alkene which produces OH radicals with a yield near unity through the reaction with ozone. m-xylene was used as a tracer to determine the OH radical concentrations.
The HS-PTR-MS allows on-line and continuous monitoring of organic compounds with detection limit of only few part par trillion (ppt). The ionization process is a soft process, meaning the energy transferred during the ionization is small (as compared to electron impact ionization) which limits the fragmentation of the initial compounds.
OH radical reactivity
The coated powders of inert AEROSIL particles with pesticides were exposed in a rotating quartz bulb to six different OH radical concentrations (3 · 107; 6.1 · 107; 8 · 107; 9.3 · 107; 1.4 · 108 and 1.5 · 108 cm−3). This simplified method is a useful means to expose the pesticides at sub-monolayer thickness to reactive species from the gas phase surrounding the particles (such as ozone and OH radicals).
Detailed descriptions of materials and methods, silica particles coating, description of OH radical production and kinetic experiments, extraction and pesticide quantification, measurements of OH radicals and other procedures used are given in SI Appendix.
Additional Information
How to cite this article: Socorro, J. et al. The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 6, 33456; doi: 10.1038/srep33456 (2016).
Supplementary Material
Acknowledgments
We sincerely thank Dr. Yael Dubowski (Technion - Israel Institute of Technology) for carrying out a revision prior to submission of our work. Her comments and suggestions were really helpful and greatly improved the quality of the manuscript. This work has been carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the «Investissements d’Avenir» French Government program, managed by the French National Research Agency (ANR).
Footnotes
Author Contributions H.W., S.G. and E.Q. conceived and designed the experiments. J.S. and A.D. performed the experiments. J.S., A.D. and B.T.-R. analyzed the data. S.G. and E.Q. co-wrote the paper. J.S. prepared figures. All authors reviewed the manuscript.
References
- Lelieveld J., Evans J. S., Fnais M., Giannadaki D. & Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367–371 (2015). [DOI] [PubMed] [Google Scholar]
- Pope C. A. & Dockery D. W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 56, 709–742 (2006). [DOI] [PubMed] [Google Scholar]
- Sinfort C. et al. Influence des conditions et matériels de pulvérisation sur les pertes de pesticides au sol et dans l’air en viticulture Languedocienne. XXXIX congrès du Groupe Français des Pesticides (2009). [Google Scholar]
- Yates S. R. et al. Emissions of 1,3-Dichloropropene and Chloropicrin after Soil Fumigation under Field Conditions. J. Agric. Food Chem. 63, 5354–5363 (2015). [DOI] [PubMed] [Google Scholar]
- Jakobi G. et al. Atmospheric bulk deposition measurements of organochlorine pesticides at three alpine summits. Atmos. Environ. 101, 158–165 (2015). [Google Scholar]
- Ma Y. et al. The spatial distribution of organochlorine pesticides and halogenated flame retardants in the surface sediments of an Arctic fjord: The influence of ocean currents vs. glacial runoff. Chemosphere 119, 953–960 (2015). [DOI] [PubMed] [Google Scholar]
- Coscollà C. et al. Particle size distributions of currently used pesticides in a rural atmosphere of France. Atmos. Environ. 81, 32–38 (2013). [Google Scholar]
- Coscollà C. et al. Particle size distributions of currently used pesticides in ambient air of an agricultural Mediterranean area. Atmos. Environ. 95, 29–35 (2014). [Google Scholar]
- United Nations Environment Programme (UNEP) Stockholm Convention on Persistent Organic Pollutants. (2001) Available at: www.pops.int/documents/convtext/convtext_en.pdf. (Accessed : February 11, 2016). [Google Scholar]
- Atkinson R. et al. Transforamtions of pesticides in the atmosphere: a state of the art. Water Air Soil Pollut. 115, 219–243 (1999). [Google Scholar]
- Meylan W. M. & Howard P. H. Computer estimation of the atmospheric gas-phase reaction rate of organic compounds with hydroxyl radicals and ozone. Chemosphere 26, 2293–2299 (1993). [Google Scholar]
- Socorro J., Gligorovski S., Wortham H. & Quivet E. Heterogeneous reactions of ozone with commonly used pesticides adsorbed on silica particles. Atmos. Environ. 100, 66–73 (2015). [Google Scholar]
- Vingarzan R. A review of surface ozone background levels and trends. Atmos. Environ. 38, 3431–3442 (2004). [Google Scholar]
- Usher C. R., Michel A. E. & Grassian V. H. Reactions on Mineral Dust. Chem. Rev. 103, 4883–4939 (2003). [DOI] [PubMed] [Google Scholar]
- Falkovich A. H., Schkolnik G., Ganor E. & Rudich Y. J. Adsorption of organic compounds pertinent to urban environments onto mineral dust particles. J. Geophys. Res. 109, D02208 (2004). [Google Scholar]
- Gligorovski S., Strekowski R., Barbati S. & Vione D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 115, 13051–13092 (2015). [DOI] [PubMed] [Google Scholar]
- Palm W. U., Elend M., Krueger H. U. & Zetzsch C. OH radical reactivity of airborne terbuthylazine adsorbed on inert aerosol. Environ. Sci. Technol. 31, 3389–3396 (1997). [Google Scholar]
- Palm W. U., Millet M. & Zetzsch C. OH radical reactivity of pesticides adsorbed on aerosol materials: first results of experiments with filter samples. Ecotoxicol. Environ. Saf. 41, 36–43 (1998). [DOI] [PubMed] [Google Scholar]
- Palm W. U., Elend M., Krüger H. U. & Zetzsch C. Atmospheric degradation of a semivolatile aerosol-borne pesticide: Reaction of OH with pyrifenox (an oxime-ether), adsorbed on SiO2. Chemosphere 38, 1241–1252 (1999). [Google Scholar]
- Bagot P. A. J., Waring C., Costen M. L. & McKendrick K. G. Dynamics of inelastic scattering of OH radicals from reactive and inert liquid surfaces. J. Phys. Chem. C 112, 10868–10877 (2008). [Google Scholar]
- Vlasenko A., George I. J. & Abbatt J. P. D. Formation of Volatile Organic Compounds in the Heterogeneous Oxidation of Condensed-Phase Organic Films by Gas-Phase OH. J. Phys. Chem. A 112, 1552–1560 (2008). [DOI] [PubMed] [Google Scholar]
- Enami S., Hoffmann M. R. & Colussi A. J. In situ mass spectrometric detection of interfacial intermediates in the oxidation of RCOOH(aq) by gas-phase OH-radicals. J. Phys. Chem. A 118, 4130–4137 (2014). [DOI] [PubMed] [Google Scholar]
- Enami S., Hoffmann M. R. & Colussi A. J. Stepwise oxidation of aqueous dicarboxylic acids by gas-phase OH-radical. J. Phys. Chem. Lett. 6, 527–534 (2015). [DOI] [PubMed] [Google Scholar]
- Prinn R. et al. Atmospheric trends in methylchloroform and the global average for the hydroxyl radical. Science 238, 945–950 (1987). [DOI] [PubMed] [Google Scholar]
- Kanaya Y. G. et al. Chemistry of OH and HO2 radicals observed at Rishiri Island, Japan, in September 2003: Missing daytime sink of HO2 and positive nighttime correlations with monoterpenes. J. Geophys. Res.-Atmospheres 112, D11308 (2007). [Google Scholar]
- Scheringer M. Characterization of the environmental distribution behavior of organic chemicals by means of persistence and spatial range. Environ. Sci. Technol. 31, 2891–2897 (1997). [Google Scholar]
- Franklin J. et al. Evaluation of Persistence and Long-Range Transport of Organic Chemicals in the Environment. Chapter 2: Quantitative determination of persistence in air. (Society of Environmental Toxicology and Chemistry Press) (2000).
- Krüger H. U. & Zetzsch C. Development of a Test for Screening of Tropospheric Degradation of Semivolatile Pesticides by OH Radicals. Report UBA-FB-000378. (Bundesministerium fuer Umwelt, Naturschutz und Reaktorsicherheit, Berlin). 59p. (2001).
- Scheringer M. et al. Long-Range Transport and Global Fractionation of POPs: Insights from Multimedia Modeling Studies. Environ. Pollut. 128, 177–188 (2004). [DOI] [PubMed] [Google Scholar]
- Boethling R. S., Howard P. H. & Meylan W. M. Finding and estimating chemical property data for environmental assessment. Environ. Toxicol. Chem. 23, 2290–2308 (2004). [DOI] [PubMed] [Google Scholar]
- Zetzsch C. Photochemischer Abbau in Aerosolphasen. Umweltwiss Schadst-Forsch 3, 59–64 (1991). [Google Scholar]
- Liu Q., Krüger H. & Zetzsch C. Degradation study of the aerosol-borne insecticides Dicofol and DDT in an aerosol smog chamber facility by OH radicals in relation to the POPs convention. Geophys. Res. Abstr. 7, 05760 (2005). [Google Scholar]
- Al Rashidi M., El Mouden O., Chakir A., Roth E. & Salghi R. The heterogeneous photo-oxidation of difenoconazole in the atmosphere. Atmos. Environ. 45, 5997–6003 (2011). [Google Scholar]
- Palm W., Krüger H. U., Elend M. & Zetzsch C. Degradation of the pesticide primisulfuron-methyl in the aerosol-borne state by OH radicals. Proceedings of the 3rd International CEMEPE and SECOTOX Conference. pp. 253–258 (2011). [Google Scholar]
- Pflieger M., Monod A. & Wortham H. Heterogeneous oxidation of terbuthylazine by “dark” OH radicals under simulated atmospheric conditions in a flow tube. Environ. Sci. Technol. 47, 6239–6246 (2013). [DOI] [PubMed] [Google Scholar]
- Al Rashidi M., Chakir A. & Roth E. Heterogeneous oxidation of folpet and dimethomorph by OH radicals: A kinetic and mechanistic study. Atmos. Environ. 82, 164–171 (2014). [Google Scholar]
- El Masri A., Al Rashidi M., Laversin H., Chakir A. & Roth E. A mechanistic and kinetic study of the heterogeneous degradation of chlorpyrifos and chlorpyrifos oxon under the influence of atmospheric oxidants: ozone and OH-radicals. RSC Adv. 4, 24786–24795 (2014). [Google Scholar]
- Bouya H., Errami M., Chakir A. & Roth E. Kinetics of the heterogeneous photo oxidation of the pesticide bupirimate by OH-radicals and ozone under atmospheric conditions. Chemosphere 134, 301–306 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.