Abstract
West Syndrome is characterized by infantile spasms, a hypsarrhythmic electroencephalogram (EEG) pattern, and a poor neurodevelopmental prognosis. First-line treatments include adrenocorticotrophic hormone (ACTH) and vigabatrin, but adverse effects often limit their use. CPP-115 is a high-affinity vigabatrin analogue developed to increase therapeutic potency and to limit retinal toxicity. Here, we present a child treated with CPP-115 through an investigational new drug protocol who experienced a marked reduction of seizures with no evidence of retinal dysfunction. Given the potential consequences of ongoing infantile spasms and the limitations of available treatments, further assessment of CPP-115 is warranted.
Keywords: West Syndrome, Infantile spasms, Epileptic spasms, Epilepsy, CPP-115
1. Introduction
West Syndrome is an infantile-onset epileptic encephalopathy characterized by the following: 1) clusters of flexor, extensor, or mixed spasms (infantile spasms); 2) cognitive and/or psychomotor disability; and 3) hypsarrhythmia on electroencephalogram (EEG). West Syndrome can result from diverse genetic and acquired causes, and in many cases, the etiology remains unknown [1], [2]. Patients typically develop spasms in the first year of life which often resolve by four years of age. Spasms are often accompanied or followed by other seizure types. Further, the combination of the underlying disorder, abundant interictal epileptiform activity, and seizures often causes severe intellectual and motor deficits [3], [4].
Early and effective treatment intervention may reduce or prevent developmental delays associated with West Syndrome [5], [6]. The FDA-approved treatments are limited by tolerability and efficacy [4]. Adrenocorticotropic hormone (ACTH) and (RS)-4-aminohex-5-enoic acid (vigabatrin) are effective in many cases but have potentially serious adverse effects [3], [4]. The ACTH can lead to immunosuppression and infections, hypertension, metabolic abnormalities, and renal impairment and is rarely fatal [7]. Vigabatrin can cause progressive and potentially irreversible visual field loss from retinal toxicity. Despite the risks associated with vigabatrin, its efficacy makes it the first line treatment for some etiologies of infantile spasms, especially tuberous sclerosis [8]. There is a need for safer and more effective therapies.
Vigabatrin inactivates GABA aminotransferase (GABA-AT), an intracellular enzyme that degrades GABA, increasing synaptic GABA concentrations [9]. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115) is a high-affinity vigabatrin analogue. CPP-115 was developed to produce greater therapeutic potency than vigabatrin without retinal toxicity [10], [11]. The molecular structure prevents formation of the reactive metabolite implicated in retinal toxicity [10]. Mechanism-based inhibition assays demonstrated that CPP-115 design increased its ability to inactivate GABA-AT to 187 times that of vigabatrin [11].
Animal studies support increased efficacy and decreased retinal toxicity associated with CPP-115. Administration of CPP-115 in a multiple-hit rat model of infantile spasms significantly reduced spasms and normalized the EEG. Results were significant at a dose 400 times lower than that of vigabatrin, with effects lasting two to three times as long [12]. Rats treated with CPP-115 had reduced retinal toxicity compared with those treated with vigabatrin [13]. In humans treated with CPP-115 for one week, there were significantly increased GABA concentrations on 1H magnetic resonance spectroscopy (MRS) in the supplementary motor area and parietal–occipital cortex [14].
The FDA has granted CPP-115 orphan drug designation for the treatment of infantile spasms. The potential safety and efficacy of CPP-115 led us to treat a child with pharmacologically resistant infantile spasms in an open-label investigational new drug protocol.
2. Case report
The boy presented at one year of age with episodes of head-nodding and shoulder/arm shrugs upon waking. After an EEG recorded episodic electrodecremental responses associated with motor paroxysms and hypsarrhythmia, infantile spasms were diagnosed. Magnetic resonance imaging (MRI) showed a mild decreased cerebral volume, dysmeylination, and an atrophic corpus callosum. Genetic testing, including whole exome sequencing of the child and parents, was negative. The patient also had cortical blindness, bilateral sensory hearing loss, hypotonia, and global developmental delay. Over the next two years, spasms continued despite various treatment combinations of ACTH, clonazepam, zonisamide, vigabatrin, valproic acid, ketogenic diet, topiramate, clobazam, felbatol, intravenous solumedrol, and lamotrigine. Seizure cessation was achieved only during the first month of treatment with vigabatrin. An EEG five months prior to CPP-115 initiation recorded several tonic spasms seizures on one day and 71 parental pushbuttons within a separate 16-hour period, of which ~ 50% were myoclonic jerks without EEG correlates and ~ 50% were spasms with ictal correlates.
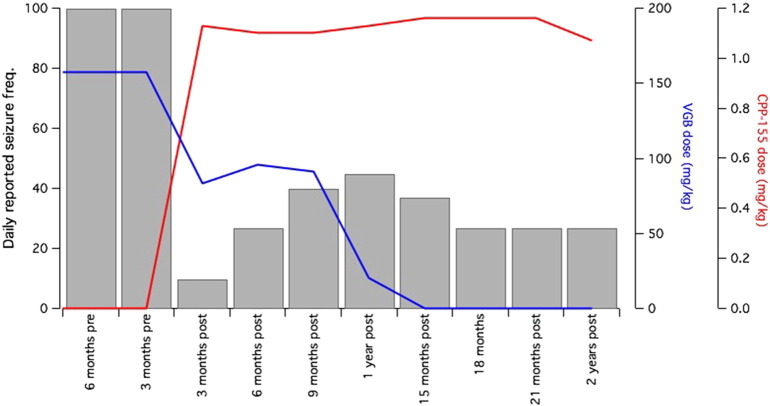
This patient began taking CPP-115 at age 3 years as an FDA-authorized investigational drug with institutional review board approval. His current antiseizure drugs were clobazam 1.8 mg/kg daily and vigabatrin 157.5 mg/kg, and he was on the ketogenic diet. Vigabatrin was reduced to 118.1 mg/kg with CPP-115 treatment initiation. CPP-115 is a powder prepared in 5 mL of sterile water and administered as a liquid via syringe. It was mixed with a noncarbonated, artificially sweetened beverage. CPP-115 titrations followed a dosing protocol provided by Catalyst Pharmaceutical Partners, the drug manufacturer. CPP-115 titrations started at 0.2 mg/kg with planned increases of 0.2 mg/kg per week as tolerated up to a maximum daily dose of 1.2 mg/kg. CPP-115 reached maximal dosing in 6 weeks as per protocol. However, the dose was reduced to 1.1 mg/kg ~ 2 months later because of concerns over decreased appetite and fatigue, both of which resolved within 1 year of CPP-115 initiation. His seizure frequency increased with the reduction of the CPP-115 dose. At the time of the dose reduction, the patient was also taking 83.3 mg/kg vigabatrin. Vigabatrin was completely weaned within 1 year of CPP-115 initiation (Fig. 1). Mood and cognitive improvement were noted with the cessation of vigabatrin, but an increase in seizure frequency persisted for ~ 5 months. During the vigabatrin weaning period, CPP-115 was increased by 0.03 mg/kg. Clobazam was also reduced to 0.83 mg/kg daily within the same year.
Fig. 1.
Patient's estimated daily seizure frequency as reported by his parents and concurrent vigabatrin (VGB) and CPP-115 dosages (mg/kg).
The patient's seizure frequency went from ~ 100 seizures per day before CPP-115 to ~ 25–30 seizures per day after 1.5 years on CPP-115 (Fig. 1). The greatest reduction in seizures was reported during the two months that he was on ~ 83.3 mg/kg vigabatrin and the maximal dose of CPP-115 (1.2 mg/kg or 14.4 mg). During this time, his seizures were initially reduced to 5–10/day, and he went four weeks without observed seizures. After the weaning of the vigabatrin, tiagabine was introduced to control a relative increase in reported seizure frequency and demonstrated some seizure control, but gagging, insomnia, and decreased appetite led to discontinuation of tiagabine. During treatment with CPP-115, electroretinograms (ERGs), complete blood count, and comprehensive metabolic panel were obtained every 3–6 months with no abnormalities. A recent 18-hour, 24-channel EEG study showed the absence of normal wake and sleep state features, moderate generalized slowing, increased beta activity, bilateral multifocal epileptiform discharges, and generalized discharges. The report noted improvement because of a complete lack of electrodecremental responses or seizures. The events classified as myoclonic seizures by the parents, were milder in intensity than before CPP-115, and were not associated with EEG changes. Currently, the patient continues to experience reduced myoclonic seizures, ~ 25–30 per day according to parental report, on CPP-115 at 1.2 mg/kg or 13.9 mg per day, clobazam 0.4 mg/kg daily, and the ketogenic diet.
Clinically, with the addition of CPP-115, his parents and therapists observed improved environmental interaction, attention, and motor function.
3. Discussion
This case study suggests sustained efficacy and tolerability of CPP-115 in treating epileptic spasms. Prior to CPP-115 treatment, this patient's seizures had failed ten drugs and the ketogenic diet, and this patient had approximately 100 seizures per day. One year after starting CPP-115 and tapering off of clobazam and vigabatrin, his reported seizure frequency decreased by more than 50%, and his cognition and behavior improved. An 18-hour EEG at age five revealed resolution of electrographic seizures and reduction of interictal discharges by more than 50%. The patient had no evidence of retinal damage seen on regular ERGs, which is consistent with a potentially increased safety profile with CPP-115 versus vigabatrin [10], [11], [13]. The cognitive and behavioral improvement observed by parents and therapists could have been due to reduced seizure frequency and interictal epileptiform discharges or other factors.
As an open-label, single subject case report, this evidence is limited by several factors. The discrepancy between the seizure-free 18-hour EEG and parental report of 25–30 seizures per day suggests some inaccuracies in parental reporting of seizure frequency. Many of his ‘myoclonic seizures’ may be nonepileptic or seizures that are not associated with an EEG change. Notably, the baseline parental reports of ~ 100 seizures per day were associated with > 35 electrographic seizures per day on an EEG, and an additional equal number of events reported by the family were myoclonic jerks lacking ictal correlates. Parental reports of reduced seizure frequency and duration were concordant with the improvement observed in the EEG. While the reduction in reported seizure frequency and improvement in EEG could represent the natural clinical progression of his disorder, his seizure frequency had remained constant except for a one-month cessation during initial vigabatrin therapy. Despite ten antiepileptic drugs and dietary therapy, his seizures remained drug-resistant. Another limitation was that CPP-115 was not administered in a blinded setting but in tandem with other antiseizure medications. The patient was treated simultaneously with vigabatrin during the first year of treatment, during which time the therapy exhibited the most marked apparent reduction in seizure frequency. This could reflect a synergistic effect that warrants further investigation. However, vigabatrin was weaned and has not been reinitiated in the last year, during which time the patient has continued to have improved seizure control. Finally, we cannot exclude long-term retinal toxicity in this child. Never the less, regular ERGs are considered sensitive to detect vigabatrin-associated retinal toxicity and meet current clinical safety guidelines to detect retinal dysfunction in infants and other patients who cannot perform perimetry [15].
4. Conclusion
Despite the appreciable limitations of the available data, the reported reduction in seizure frequency and documented improvements in the interictal and ictal EEG temporally associated with CPP-115 initiation in this patient are noteworthy. In the context of infantile spasms and associated morbidity, mortality, and poor neurodevelopmental outcomes, CPP-115 presents a promising alternative to vigabatrin therapy, albeit one requiring further comprehensive analysis in a controlled setting.
Conflict of interest
None of the authors has any conflict of interest to disclose.
References
- 1.Pellock J.M., Hrachovy R., Shinnar S., Baram T., Bettis D., Dlugos D. Infantile spasms: a US consensus report. Epilepsia. 2010;51:2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 2.Pavone P., Striano P., Falsaperla R., Pavone L., Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36:739–751. doi: 10.1016/j.braindev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Nelson G.R. Management of infantile spasms. Transl Pediatr. 2015;4:260–270. doi: 10.3978/j.issn.2224-4336.2015.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go C.Y., Mackay M.T., Weiss S.K., Stephens D., Adams-Webber T., Ashwal S. Evidence-based guideline update: medical treatment of infantile spasms. Report of the guideline development subcommittee of the American Academy of Neurology and the practice committee of the Child Neurology Society. Neurology. 2012;78:1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riikonen Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol. 2010;14:13–18. doi: 10.1016/j.ejpn.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Bombardieri R., Pinci M., Moavero R., Cerminara C., Curatolo Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–149. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Shumiloff N.A., Lam W.M., Manasco K.B. Adrenocorticotropic hormone for the treatment of West Syndrome in children. Ann Pharmacother. 2013;47:744–754. doi: 10.1345/aph.1R535. [DOI] [PubMed] [Google Scholar]
- 8.Faulkner M.A., Tolman J.A. Safety and efficacy of vigabatrin for the treatment of infantile spasms. J Cent Nerv Syst Dis. 2011;3:199–207. doi: 10.4137/JCNSD.S6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesaturo K.A., Spooner L.M., Belliveau P. Vigabatrin for infantile spasms. Pharmacotherapy. 2011;31:298–311. doi: 10.1592/phco.31.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Lee H., Doud E.H., Wu R., Sanishvili R., Juncosa J.I., Liu D. Mechanism of inactivation of γ-aminobutyric acid aminotransferase by (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115) J Am Chem Soc. 2015;137:2628–2640. doi: 10.1021/ja512299n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman R.B. The 2011 E. B. Hershberg Award for important discoveries in medicinally active substances: (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms. J Med Chem. 2012;55:567–575. doi: 10.1021/jm201650r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briggs S.W., Mowrey W., Hall C.B., Galanopoulou A.S. CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spams. Epilepsia. 2014;55:94–102. doi: 10.1111/epi.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y., Gerasimov M.R., Kvist T., Wellendorph P., Madsen K.K., Pera E. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a potent gamma-aminobutyric acid aminotransferase inactivator for the treatment of cocaine addiction. J Med Chem. 2012;55:357–366. doi: 10.1021/jm201231w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Data provided by Catalyst Pharmaceuticals, Inc. Catalyst Study CPP-115-00002 2016.
- 15.Sergott R.C., Wheless J.W., Smith M.C., Westall C.A., Kardon R.H., Arnold A. Evidence-based review of recommendations for visual function testing in patients treated with vigabatrin. J Neuroophthalmol. 2010;34:20–35. [Google Scholar]