Abstract
Objectives
This study aimed to investigate mirtazapine-induced changes in responsive neurostimulator (RNS) recordings in a patient with epilepsy.
Materials and methods
Cortical detection/stimulation counts from an RNS implanted in a patient with bitemporal epilepsy were matched to mirtazapine use to see if that drug altered hippocampal excitability.
Results
Mirtazapine decreased hippocampal stability; when mirtazapine was held after a washout period, DSC counts declined, but when it was retrialed, DSC counts increased. Responsive epilepsy neurostimulator system data helped design an optimal and individualized medication regimen for our patient with drug-resistant focal epilepsy.
Conclusions
Responsive neurostimulator systems in epilepsy may assess a medication's effect on hippocampal excitability. Mirtazapine worsened hippocampal excitability in a patient with bitemporal epilepsy.
Keywords: Neurostimulation, Epilepsy, Mirtazapine, Safety, Medication, Therapy
1. Introduction
In clinical trials, the RNS system (NeuroPace, Mountain View, CA) was shown to significantly reduce self-reported seizure frequencies in patients with drug-resistant focal epilepsy [1]. The RNS does so by surveying the electrocorticogram, specifically recognizing physician-selected patterns of neural activity associated with seizure onset, which, in turn, trigger electrical stimulation(s) or "therapies" designed to help mitigate or hopefully terminate the electrical abnormalities already underway.
Outcomes of the RNS clinical trial relied on patient diaries of seizure events [1]. Self-reported seizure frequencies remain a mediocre yardstick from which most epilepsy therapies are judged. Seizure events that corrupt memory circuits, e.g., seizures of temporal lobe origin or brief runs of asymptomatic spike-and-wave discharges, result in undercounting [2]. We need ways to objectively count seizure frequencies that do not rely on self-reporting.
If correctly programmed and electrodes are appropriately placed, detection/stimulation counts (DSCs) can be considered as potential markers of abnormal cortical excitability. An enabled RNS detects abnormal activity and delivers stimuli that records that twinned response as an event. Events can then be reviewed on an hourly, daily, or monthly basis. Contemporary RNS programming detects and delivers therapies generously. In the open-label portion of the RNS pivotal trial that meant an average of 1175 DSCs per day [1, personal communication]. Therapies may target benign interictal patterns or alternatively terminate early ictal patterns before a clinical seizure occurs. Individualizing approaches to epilepsy management where self-reported seizure counts are enhanced with DSC may suggest the need for RNS or medical therapies that are either useful or counterproductive. In one case report of a patient with an RNS system, treating physicians were able to identify weekend caffeine consumption as a potential reason for increased detections [3]. This case report sets out to document one way we were able to use DSC to alter decisions involved in medical therapy.
2. Materials and methods
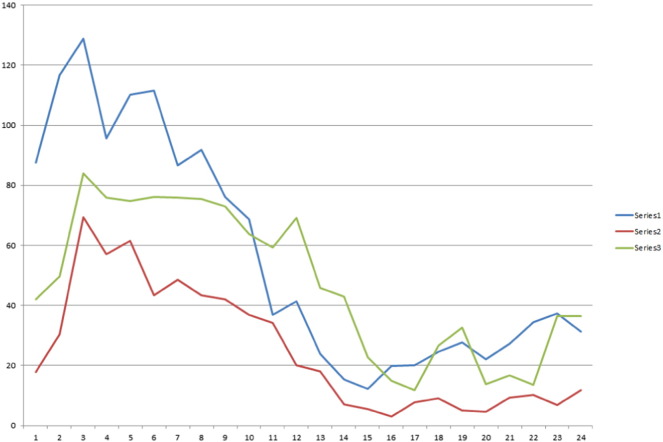
With informed patient consent, patient data were abstracted from electronic medical records including RNS logs of DSC. Average DSC counts by time of day were graphed for three different 6-day epochs:first, when mirtazapine was regularly used; second, during a postwashout period where mirtazapine was not used; and third, when mirtazapine was retrialed (Fig. 1).
Fig. 1.
Average detection/stimulation counts (DSCs) by time of day (h). Blue, series one, refers to six-day average detection (January 1–6) on full-dose mirtazapine. Red, series two, refers to six-day average off of mirtazapine and trazodone (January 11–12 and 15–18; trazodone was taken on January 13–14 prior to bedtime). Green, series three, refers to six-day average back to full-dose mirtazapine (January 19–24).
3. Case report
A 45-year-old female with drug-resistant bitemporal epilepsy underwent implantation of the RNS device in 2014 after failure of medical and VNS therapies. Seizure types include audiogenic (Johnny Cash's song Ring of Fire, for instance, would trigger focal seizures and potentially convulsions). A brother and a second cousin also have epilepsy, however her LG1 gene status is unknown. Comorbid conditions include type I diabetes mellitus, depression, and insomnia. Her RNS detections were recorded from two four-contact depth electrodes placed over the left and the right hippocampus. Those locations were chosen based on intracranial monitoring results suggesting hippocampal involvement in seizure generation. Her vagus nerve stimulator, which historically produced no effect on seizure controls, was in the off position. A functional inslumin pump was used for the duration studied.
Prior to this study period, depression and insomnia issues worsened. Trazodone was initially tried, and although depressive symptoms improved, she lacked motivation and energy. She was switched to 15-mg of mirtazapine at night because it is mildly sedating and may boost mood. However, when on mirtazapine, the patient complained of worsening nighttime anxiety. Her RNS system was interrogated; histograms of DSC by hour suggested far higher counts during sleeping hours than during wakeful hours while the patient was on mirtazapine (Fig. 1). Two therapy changes occurred, first — a small lowering of her RNS output current (from 4 mA to 3.5 mA) back to historical settings where her seizure controls were thought to be better and second — a taper of mirtazapine. Mirtazapine doses were weaned over several days, and DSCs were assessed for a six-day period a week after discontinuation of mirtazapine. Six nonconsecutive nights out of eight without mirtazapine were recorded (for two consecutive nights, the patient used trazodone as a sleep aid, those nights were not studied). Mirtazapine was then reintroduced at full strength and six additional days of DSC counts assessed to see if drug retrial had a reproducible change in DSC. All other antiseizure medication doses during this time were kept the same. Data appear in Fig. 1.
4. Discussion
Mirtazapine is an atypical antidepressant with effects on noradrenergic, serotonin, norepinephrine, and histamine neurotransmission. It reaches peak dose within 2 h and has an elimination half-life of 20–40 h and a steady state by day five [4]. Mirtazapine was recently reported to contribute to nonconvulsive status epilepticus in three patients [5]. In these patients, the use of mirtazapine corresponded to more frequent nocturnal DSC. We stopped mirtazapine with the assumption that the medication was altering seizure thresholds. This important clinical data would not have been available to us without the RNS system.
The purpose of reporting this case is not to implicate mirtazapine as a dangerous medication for patients with epilepsy. Instead, individualized and very specific DSC data show that mirtazapine may have contributed to nocturnal or sleep-related hippocampal excitability. Whether this is perhaps a mechanism by which mirtazapine works for mood or sedation is unknown. Clinically, mirtazapine use in this patient worsened anxiety. Responsive neurostimulator data can be effectively used beyond just delivering focal therapies based on recognition of abnormal cortical patterns. A concession could be the therapy changes instituted by lowering RNS output currents might also change DSC, though the retrial of mirtazapine and recurrence of higher DSC argues against this.
With time, clinical implications of DSC alterations should become clarified. The most obvious one would be in the use of conventional antiseizure drugs (ASDs). In this patient, for instance, the use of a higher nighttime and lowered daytime ASDs might decrease night events and minimize the mood blunting effects of ASD therapies during the day. Responsive neurostimulator programming teams could incorporate similar strategies in reviewing psychoactive medication problems or successes. To avoid confounding, we found that medication DSC correlations probably should be independent of periods where major RNS system therapy changes are made and that medication washout and retrials may be of use to confirm specificity of medication effects on DSC.
Responsive neurostimulator device designs and usability teams may wish to consider ways to make DSC data easy to visualize for RNS programming teams. In this case, abstracting the DSC by hour and date was not particularly straightforward and required assistance from the RNS/NeuroPace team.
Conflict of interest
This paper has no financial support. Dr. Doherty has no disclosures. Dr. Gwinn has consulted for Boston Scientific and NeuroPace. Nicole Fortier has consulted for NeuroPace.
Acknowledgment
The authors would like to thank Chad Hamilton, NeuroPace, Mountain View California for his aid in providing data.
References
- 1.Morrell M.J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [Available from: http://tinyurl.com/zvokx6a] [DOI] [PubMed] [Google Scholar]
- 2.Hoppe C., Feldmann M., Blachut B., Surges R., Elger C.E., Helmstaedter C. Novel techniques for automated seizure registration: patients' wants and needs. Epilepsy Behav. 2015;52:1–7. doi: 10.1016/j.yebeh.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Mackow M.J., Krishnan B., Bingaman W.E., Najm I.M., Alexopoulos A.V., Dileep R. Increased caffeine intake leads to worsening of electrocorticographic epileptiform discharges as recorded with a responsive neurostimulation device. Clin Neurophysiol. 2016;127:2341–2342. doi: 10.1016/j.clinph.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Kasper S., Praschak-Rieder N., Tauscher J., Wolf R. A risk–benefit assessment of mirtazapine in the treatment of depression. Drug Saf. 1997;17:251–264. doi: 10.2165/00002018-199717040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi G., Miyajima M., Watanabe M., Murata Y., Sone D., Watanabe Y. Nonconvulsive status epilepticus in the elderly associated with newer antidepressants used at therapeutic doses: a report of three cases. Epilepsy Behav Case Rep. 2014;3:8–11. doi: 10.1016/j.ebcr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]