Abstract
This study is to test the effects of Bt gene introduction on the foliar water/nitrogen use efficiency in cotton. We measured leaf stomatal conductance, photosynthetic rate, and transpiration rate under light saturation condition at different stages of a conventional cultivar (zhongmian no. 16) and its counterpart Bt cultivar (zhongmian no. 30) that were cultured on three levels of fertilization, based on which leaf instantaneous water use efficiency was derived. Leaf nitrogen concentration was measured to calculate leaf photosynthetic nitrogen use efficiency, and leaf δ13C was used to characterize long term water use efficiency. Bt cultivar was found to have lower stomatal conductance, net photosynthetic rates and transpiration rates, but higher instantaneous and long time water use efficiency. In addition, foliar nitrogen concentration was found to be higher but net photosynthetic rate was lower in the mature leaves of Bt cultivar, which led to lower photosynthetic nitrogen use efficiency. This might result from the significant decrease of photosynthetic rate due to the decrease of stomatal conductance. In conclusion, our findings show that the introduction of Bt gene should significantly increase foliar water use efficiency but decrease leaf nitrogen use efficiency in cotton under no selective pressure.
China, as the largest cotton-producing country in the world with more than 2000 years’ history of cotton cultivation, holds approximately 25% of global annual production1. Yet, China loses 10% to 15% of its cotton production by insect pest each year. This proportion is even higher in serious years2. Therefore, Chinese scientists selected, bred and commercially cultivated Bt cotton since 1990s. The popularization and application of Bt cotton in China reduced the amount of chemical pesticide usage to 20~50% as compared to conventional cotton, alleviated environmental pollution caused by pesticide, protected beneficial insects in cotton field2.
However, Bt cotton brings some safety problems. First, transgenic Bt crops have been demonstrated to affect soil biota3,4,5,6,7 and gene flow between species or populations8,9,10,11, which is mostly adverse to functioning of natural ecosystems; second, although the control of Bt toxin over target insects and the positive effect on crop production12,13 have been well documented in previous studies14,15,16, the hidden dangers of selecting more resistant individuals from target insects17,18 or resulting in the break out of secondary insects and the decrease of crop production have also been proved19,20; third, the fabricated products of Bt cotton may have adverse effect to the health of human2.
Apart from the effects on external environments, the physiology of cotton has been proven affected by many studies21,22,23. However, studies about the Bt gene’s effect on resource allocation pattern from the life history perspective are scarce. From a basic assumption of life history theory, the increased allocation of resources to one function precludes the allocation of these functions to other functions24, we speculate that the introduced Bt gene may affect cotton’s water use and nitrogen use efficiencies. First, the transcription and translation of Bt gene consume resources that could have been used for plant growth and reproduction. Second, the expression of Bt protein may change the interaction between crop plants and microbe including ectomycorrhizal and endomycorrhizal fungi which may further change plants’ water/nutrient absorptive ability25,26,27. Third, Bt gene may have uncertain effects on the expression of the genes related to plant water and nutrient use efficiency by changing their expressive intensity, quantity or even enzyme activity28,29,30.
As the most important commercial crop in China, cotton is the main consumer of agricultural water2. The main cotton growing districts in China locate in the North China plain, Loess Plateau and middle reaches of the Yangtze River, majority of which suffers from drought in most time of a year1. China is already in moderate status of water shortage31, and among national annual water consumption, agricultural water use accounts for nearly 62%. Moreover, available water for agriculture is still in severe shortage. Out of nationwide 121.72 million hm2 arable land, only 58.47 million hm2 could be effectively irrigated31,32. Considering the large area of cotton plantation and low water use efficiency as little as 30% to 40%2, if Bt cotton truly has a relatively lower water use efficiency, the serious situation of water deficiency will undoubtedly worsen.
Not only the water supply problem, but the water quality problem in China is unprecedentedly dangerous as well. The situation is primarily owing to the eutrophification of waterbodies, especially within main crop-growing area such as Changjiang and Zhujiang Deltas33. Apart from the main consumer of agricultural water, cotton is also the main consumer of chemical fertilizer and the main source of agricultural non-point source pollution in China. If the nutrient use efficiency declines due to the introduction of Bt gene, more chemical fertilizer may have to be used to maintain cotton yield, which will intensify environmental pollutions around farmlands. Therefore, it is imperative to evaluate the Bt-gene introduction effects on water and nutrient use efficiency.
Leaves are the organ completing the processes of both water transpiration and CO2 sequestration; the ratio of photosynthetic rate to transpiration rate is often used to characterize instantaneous water use efficiency (IWUE). Similarly, leaf photosynthetic nitrogen use efficiency (PNUE) can be characterized as the ratio of photosynthetic rate to leaf nutrient concentration. Because the instantaneous water efficiency is not a life-time characteristic, long term water use efficiency (LWUE) should be examined. Carbon isotope composition (δ13C) has been widely used to characterize the LWUE because it is associated with the ratio of CO2 partial pressure within the leaves (Pi) to the CO2 partial pressure outside the leaves (Pamb). Previous studies have extensively demonstrated a positive correlation between the long term efficiency and δ13C34,35.
In this study, leaf photosynthetic rate, transpiration rate, foliar total nitrogen concentration and δ13C were measured to calculate IWUE and PNUE and characterize LWUE. The objective of this study was to test whether the introduction of Bt-gene affects water use efficiency and nitrogen use efficiency in cotton.
Materials and Methods
Cotton plantation
Experiments with cotton plantation and field measurements were conducted in Ferry Village, Yudai Town, Luhe District, Nanjing (118°54′ E, 32°14′ N), eastern China in 2009. The study site is within northern subtropical monsoon climate zone. Mean annual temperature is 16.1 °C, with the monthly means in January and July being 1.5 °C and 29.0 °C, respectively. Mean annual precipitation is 975 mm, more than 70% of which occurs in summer between June and August.
The experiment was started at the middle of April and was ended in late October. Zhongmian no. 30 (Z30 for abbreviation), the genetically modified cotton expressing the Bacillus thuringiensis Cry1AA toxin and its compared isogenic conventional cultivar zhongmian no. 16 (Z16 for abbrevation) were used. In middle April, each seed was placed on the soil surface of an individual pot. The seedlings were transplanted to the farmland at the density of 30,000 individuals per hectare when majority of them reached approximately 15 cm in height. The farmland’s basal total nitrogen concentration was 2.35 g kg−1. For each cultivar, three fertilizer levels were set up as 50%, 100% and 150% of regular level according local peasant’s experience. Regular level was: base manure contained 15 tons of manure and 225 kg urea per hectare, additional manure contained 10 tons of manure and 150 kg urea per hectare. Thus, the factorial experiment consists of two factors (cultivar and fertilizer level) that had two and three levels, respectively, forming six treatments in total.
After transplantation, all managements including irrigation, pesticide and herbicide application were following the techniques of local peasants.
Physiological Measurements
Li-cor 6400XT portable photosynthesis system (Li-Cor, Lincoln, NE, USA) was used to measure and record photosynthetic parameters under saturated light conditions (photosynthetic active radiation (PAR) was set as 1250 μmol·m−2·s−1) at three stages (flowering peak stage (FPS), the beginning of boll opening stage (BBOS), boll opening peak stage (BOPS)) of the growing season. During measurements, flow rate was set as constant 500 μmol s−1 and leaves were maintained at 33 °C, which was most frequently measured during the growing season of the study area. At FPS, 3 individuals from each treatment were randomly selected and one flag leaf of each individual was used to measure photosynthetic parameters including net photosynthetic rate (Pn, μmolCO2·m−2·s−1), transpiration rate (E, μmolH2O·m−2·s−1) and stomatal conductance (Gs, molH2O·m−2·s−1). Instantaneous water use efficiency (IWUE, μmolCO2/μmolH2O) is calculated as by dividing net photosynthetic rate by transpiration rate. 18 individuals across 6 treatments were randomly measured in sequence to avoid systematic error. All sampled individuals were tagged for the follow-up measurements. At BBOS and BOPS, we randomly selected one flag leave on each tagged individual to perform measurements. That is to say, for each tagged individual, different leaves were measured at different stages since it is hardly possible to keep all leaves alive during the whole growing season.
After the final measurement of the whole growing season, the measured leaves were sampled to measure foliar nitrogen content with H2SO4-H2O2-indophenol blue method. Photosynthetic nitrogen use efficiency (PNUE) was calculated as the ratio of Pnmax to foliar N content.
At each stage, soil of depth from 0–20 cm was sampled to measure soil water content and we found soil water content maintained stable across all treatments.
Stable carbon isotope
For each treatment, 2 canopy leaves from each of three measured individuals (N = 6 for each treatment) were collected immediately after harvesting in mid-October, then dried to constant mass at 70 °C and ground in the mill to pass 0.147-mm mesh. The carbon isotope composition of leaves was analyzed at the stable isotope lab of Chinese Academy of Sciences (Beijing, China) using an isotope ratio mass spectrometer (DELTA plus XP, analytical precision was about 0.1‰). The results were then expressed as δ13C values to characterize leaf long-term water use efficiency (LWUE), using a formula as34:
δ13C = (Rsam − Rstd)/Rstd × 1000‰, where sam and std refer to sample and standard material, respectively.
Statistical analysis
To evaluate the effects of cultivar, fertilizer level, and development stage on IWUE, PNUE, and δ13C values, as well as some other traits, three or two-way ANOVAs (for some traits without the effect of the stage) were conducted, and post-hoc Fisher-LSD tests were used to determine the significance level of the difference between different treatments. Homogeneity of variance was checked at the first step to ensure the validity of ANOVA tests. All the statistical analyses were run by JMP10.0 software packages.
Results
Instantaneous water use efficiency
Three-way ANOVAs revealed significant effects of cultivar, development stage and fertilizer level on Pnmax, E, IWUE and Gs (For Pnmax: cultivar: F1,36 = 112.50, p < 0.001; stage: F2,36 = 56.07, p < 0.001; fertilizer level: F2,36 = 214.10, p < 0.001; For E: cultivar: F1,36 = 331.69, p < 0.001; stage: F2,36 = 115.22, p < 0.001; fertilizer level: F2,36 = 12.72, p < 0.001; For IWUE: cultivar: F1,36 = 169.29, p < 0.001; stage: F2,36 = 88.49, p < 0.001; fertilizer level: F2,36 = 85.07, p < 0.001; For Gs: cultivar: F1,36 = 248.78, p < 0.001; stage: F2,36 = 35.67, p < 0.001; fertilizer level: F2,36 = 33.25, p < 0.001). Z16 generally had higher Pnmax than Z30, especially at the flowering peak stage. For both cultivars, Pnmax were higher at the beginning of boll opening stage than those at the flowering stage and boll opening peak stage. Moreover, higher fertilizer level enhanced Pnmax for both Z16 and Z30 (Table 1).
Table 1. Leaf instantaneous water use efficiency (IWUE μmolCO2/μmolH2O), net light-saturated photosynthetic rate (Pnmax, μmolCO2·m−2·s−1), transpiration rate (E, μmolH2O·m−2·s−1) and stomatal conductance (Gs, molH2O·m−2·s−1) of Z30 and Z16 under three fertilizer levels at different stages.
| Z30-Low | Z30-Medium | Z30-High | Z16-Low | Z16-Medium | Z16-High | ||
|---|---|---|---|---|---|---|---|
| FPS | IWUE | 4.02 ± 0.20 | 4.45 ± 0.26 | 5.75 ± 0.17 | 3.04 ± 0.10 | 3.81 ± 0.12 | 4.83 ± 0.08 |
| Pnmax | 7.21 ± 0.29 | 15.42 ± 0.43 | 19.29 ± 1.08 | 18.34 ± 0.21 | 20.24 ± 0.41 | 27.36 ± 0.98 | |
| E | 1.80 ± 0.09 | 3.49 ± 0.23 | 3.36 ± 0.22 | 6.05 ± 0.22 | 5.31 ± 0.06 | 5.66 ± 0.17 | |
| Gs | 0.08 ± 0.00 | 0.19 ± 0.01 | 0.22 ± 0.01 | 0.45 ± 0.02 | 0.41 ± 0.02 | 0.38 ± 0.02 | |
| BBOS | IWUE | 3.71 ± 0.07 | 3.87 ± 0.05 | 5.43 ± 0.16 | 2.87 ± 0.04 | 3.78 ± 0.06 | 4.70 ± 0.10 |
| Pnmax | 18.93 ± 0.31 | 21.88 ± 1.08 | 24.41 ± 0.84 | 18.35 ± 0.37 | 21.11 ± 0.57 | 28.55 ± 0.84 | |
| E | 5.11 ± 0.18 | 5.65 ± 0.24 | 4.51 ± 0.25 | 6.40 ± 0.16 | 5.59 ± 0.22 | 6.08 ± 0.31 | |
| Gs | 0.27 ± 0.01 | 0.35 ± 0.00 | 0.37 ± 0.01 | 0.36 ± 0.02 | 0.38 ± 0.01 | 0.53 ± 0.05 | |
| BOPS | IWUE | 7.55 ± 0.39 | 4.65 ± 0.12 | 5.36 ± 0.10 | 3.97 ± 0.11 | 4.53 ± 0.07 | 4.94 ± 0.02 |
| Pnmax | 14.88 ± 0.81 | 19.63 ± 1.02 | 22.57 ± 0.43 | 17.81 ± 0.20 | 20.62 ± 0.58 | 23.10 ± 0.81 | |
| E | 1.97 ± 0.08 | 4.22 ± 0.18 | 4.21 ± 0.13 | 4.49 ± 0.16 | 4.55 ± 0.12 | 4.68 ± 0.17 | |
| Gs | 0.15 ± 0.01 | 0.32 ± 0.02 | 0.33 ± 0.00 | 0.37 ± 0.02 | 0.31 ± 0.04 | 0.39 ± 0.00 |
Z16 had higher E than Z30 at all three stages. Generally, E remained relatively stable at flowering peak stage and boll opening peak stage but was higher at the beginning of boll opening stage, especially for Z30. For Z16, fertilizer level did not have substantial effect on E, but at the level of low fertilizer supply, E was also lower at flowering peak stage and boll opening peak stage for Z30 (Table 1).
Similar as E, Z16 had higher Gs and Z30 showed apparent low Gs at flowering peak stage and boll opening peak stage when fertilizer level was low. Pearson correlation was performed and detected strong correlation between Pn and Gs (r = 0.778). Correlation between E and Gs was even stronger (r = 0.847).
For IWUE, cultivar, fertilizer level, stage and all interactions had significant effects. Generally, Z30 had significantly higher IWUE than Z16. IWUE was significantly enhanced by higher fertilizer level. Interestingly, Z30 at the level of low fertilizer supply showed extremely high IWUE at boll opening peak stage (Table 1).
Carbon isotope composition (δ13C)
Z30 had significantly higher δ13C than Z16 but neither fertilizer level nor interaction between cultivar and fertilizer level had significant effect on δ13C (cultivar: F1,30 = 18.8, p = 0.001; fertilizer level: F2,30 = 2.6, p = 0.087; cultivar*fertilizer level: F2,30 = 0.04, p = 0.969; Fig. 1). Fisher-LSD test showed Z30 had obviously higher δ13C than Z16 at all three stages. Higher fertilizer levels seemed could enhance δ13C slightly but the effect was insignificant.
Figure 1. Foliar δ13C of Z30 and Z16 under three fertilizer levels.
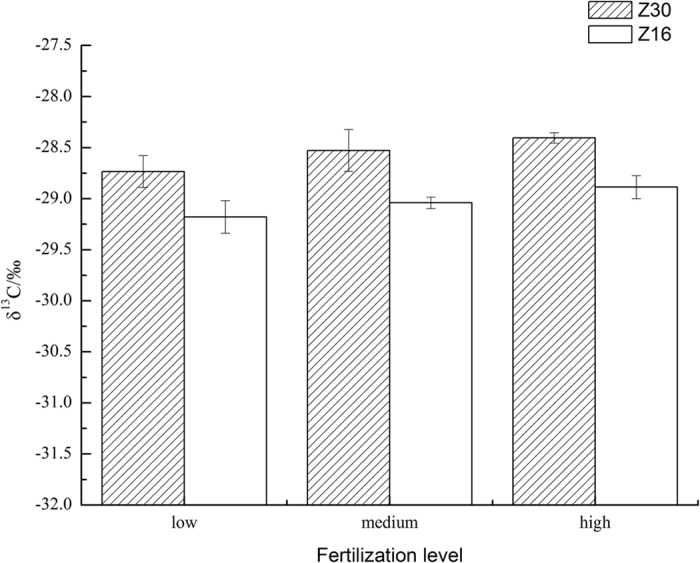
Z30: shaded columns; Z16: blank columns. The errors bars denote 1 standard error. N = 6 for each treatment.
Photosynthetic nitrogen use efficiency
At the boll opening peak stage, the only stage at which PNUE was examined, the introduction of Bt gene decreased Pnmax but enhanced foliar nitrogen content (Fig. 2a,b). Therefore, Bt gene decreased cotton’s PNUE as the ratio of Pnmax to foliar nitrogen concentration. For both cultivars, PNUE was significantly lower at low fertilizer level than at medium and high level, whereas no difference was detected between the latter two stages. Meanwhile, at all three fertilizer levels, Z30 had lower PNUE compared to Z16 (F1,12 = 79.506, p < 0.001), suggesting the interaction between cultivar and fertilizer level was insignificant (Fig. 2c).
Figure 2. Net light-saturated photosynthetic rates.
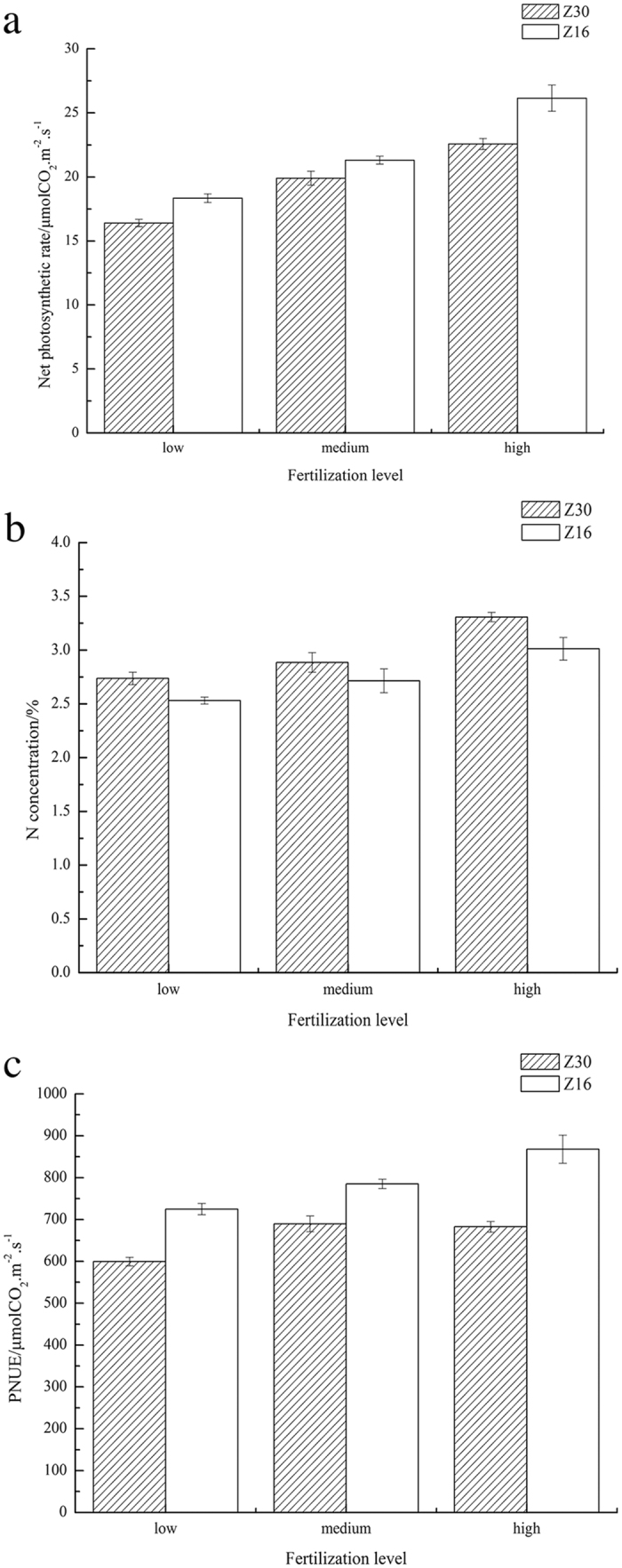
(a) Foliar total N concentrations (b) and photosynthetic nitrogen use efficiencies (c) of Z30 and Z16 under three fertilizer levels at boll opening peak stage. Z30: shaded columns; Z16: blank columns. The errors bars denote 1 standard error. N = 3 for each treatment.
Discussions
Leaf water use efficiency
IWUE, as defined above, is determined by both Pnmax and E. We may find the source of difference in IWUE by inspecting the difference in Pnmax and E between Z30 and Z16. Generally, the introduction of Bt gene decreased both Pnmax and E. At the flowering peak stage, Z30’s Pnmax was 39% of Z16’s at the level of low fertilization. At the level of medium and high fertilization, this number was 76% and 71%. Meanwhile, Z30’s E was 30% of Z16’s at the level of low fertilization. At the level of medium and high fertilization, this number was 66% and 59%. In another word, compared to Pn, Z30’s E reduced with more extent. The situation was similar for both beginning of boll opening stage and boll opening peak stage. Both Pnmax and E showed a strong positive correlation with Gs, which was consistent with previous studies36,37. Compared to Z16, Z30’s lower Gs was consistent with the inter-cultivar difference of Pnmax and E. Furthermore, relative to H2O, CO2 in the leaves has an additional diffusive resistance38, suggesting that the decrease of Gs would lead to less decrease in Pnmax than in E36,37. Rosenthal et al. also made the same conclusion in their study about cotton and sorghum39. They found the reduction of Gs would decrease both E and Pn but the reduction of Pn was not as obvious as E’s reduction. Although Rosenthal’s work mainly focused on the water deficit’s effect while our work focused on the effect of Bt gene’s introduction, we found similar changes of photosynthetic parameters. Therefore, we suggest Z30’s advantage on IWUE was after all resulted from the decrease Gs which led to the asynchronous reductions of Pn and E. The same pattern was found in another study of ours in Bt rice40. However, we do not know whether the decrease of Gs was related to an obvious change of foliar structure and a comparative dissection of both Z30 and Z16 leaves is helpful to uncover the mechanism of decreased Gs.
Foliar δ13C is a common and useful index of seasonally integrated, namely long-term WUE. Generally, δ13C is positively correlated to LWUE34,35. In our study, significantly higher δ13C value, namely higher LWUE in Z30 was consistent with the result of IWUE. This suggested the introduction of Bt gene truly enhanced water use efficiency of cotton. A previous study found differences in soil water content between Bt- and non-Bt-maize41, but we did not detect this difference after running a 3-way anova to explore if any factor might affect soil water content (cultivar: F1,48 = 0.9337, p = 0.3387; fertilizer level: F2,48 = 0.2421, p = 0.7859; stage: F2,48 = 1.6945, p = 0.1945). Therefore, we suggest the measured differences of photosynthetic parameters were not due to the deviation of soil water content but a direct consequence of the introduction of Bt gene.
With the advantage of both IWUE and LWUE, Z30 may be suitable for plantation in arid areas. This is important to use arid farmlands to enlarge planting areas and promote economic development. However, the advantage of higher IWUE is companying with the cost of decreased Pn and in theory would negatively affect growth and production. Indeed, we measured above-ground biomass and found Z30’s biomass was smaller than Z16’s although the difference was not significant (data not shown). Therefore, in terms of the overall situation, we suggest the introduction of Bt gene brings advantages that outweigh disadvantages.
Photosynthetic nitrogen use efficiency
As IWUE, PNUE is a ratio determined simultaneously by numerator (Pnmax) and denominator (foliar nitrogen concentration). Within cultivars, both Pnmax and N concentration increased with more fertilizer supply but the amplitude of Pnmax was bigger than foliar N. This result means PNUE positively correlated with N concentration within both Bt and conventional cultivars. The pattern is consistent with a previous study42 but is incompatible with another43.
Z16, which had higher Pnmax but lower N concentration, showed higher PNUE than Z30. This is contrast to the well-known tendency that Pnmax is generally positively related to mass-based foliar N concentration44,45,46,47. We suppose this unusual result is partly because of the relatively less photosynthetic nitrogen (here we mean the nitrogen participated in photosynthesis in the form of protein and enzymes) in Bt cotton, although it had higher foliar total nitrogen concentration relative to the conventional cultivar. Photosynthesis is an enzyme-mediated process that largely depends on Ribulose-1, 5-bisphosphate carboxylase-oxygenase (Rubisco enzyme). Rubisco enzyme, as the key enzyme that accounts for up to 30% of total leaf nitrogen, is often positively correlated to nitrogen availability and could directly influence photosynthetic capacity44,48,49. Bt cotton, which allocates part of its nitrogen resource to construct Bt protein that did not exist in conventional cotton, might have less available nitrogen to construct Rubisco enzyme and other nitrogen-demanded materials involved in photosynthesis. This explanation is similar to the result of Field & Mooney44 who claimed the expression of N-containing secondary compounds for defense against predation would lead to the decline of PNUE. However, nitrogen competition should not be the main factor affects PNUE in this study. According to a previous study involving a comparison of total protein content across 107 plants, protein in cotton leaves was about 1.26% of total weight50, which means 0.38% (3800 ppm) of total foliar weight was Rubisco enzyme. Assuming Bt protein content in cotton leaves is similar to that in maize51, namely 42 ppm, then the decrease of Rubisco enzyme due to the nitrogen competition by Bt cotton is just about 1%. Therefore, the obvious decrease of Pn in Z30 cannot be fully explained by the production of Bt protein. We infer the decrease of Gs might be the main factor lowered Pn since CO2 supply would be limited under this circumstance. In another study40, we found Gs of Bt rice decreased compared to conventional rice. Pu52 also found Bt cotton’s Gs decreased under both low and high selective pressures. Besides, Pu found chlorophyll content in Bt cotton was lower than that in conventional cotton. We did not measure chlorophyll content in our study, but considering Pu used the same cotton cultivar as we used, the decrease of chlorophyll content might also be a non-negligible factor led to the decrease of Pn in Bt cotton.
In our study, the enhancement of water use efficiency in Bt cotton was achieved by decreasing Gs. The reduction of Gs would limit CO2 supply in leaves and hence Pnmax also decreased, which further lead to the decrease of PNUE. That is to say, maintaining WUE may incur a cost of PNUE, as shown in several previous studies53,54,55. Because of the trade-off between WUE and PNUE, the advantage on WUE in Bt cotton could be neutralized by the disadvantage on PNUE, especially in those water-rich areas where water supply is not the major limiting factor for cotton plantation. But in arid areas where water is a limiting resource, Bt cotton would be advantageous and the decreased PNUE might be compensated by more fertilizer application.
To summarize, our results show that cotton displayed a significant lowering of Gs after introduction of the Bt gene. The decreased Gs enhanced WUE for the transgenic cultivar due to the asynchronous decrease of Pnmax and E. However, PNUE was decreased in the Bt cultivar due to the combinative effects of nitrogen trade-off and decrease of Gs and chlorophyll content. That is to say, with the advantage of saving water, it is possible that more fertilizer will be applied to Bt cotton to maintain normal yield and incur more severe agricultural non-point source pollution. Besides, considering the decreased Gs detected in this study and another study about Bt rice40 and observations from others of early aging and metabolic unbalance2, the risk of foliar structure damage cannot be neglected and need to arouse more attention.
Additional Information
How to cite this article: Guo, R. et al. Difference in leaf water use efficiency/photosynthetic nitrogen use efficiency of Bt-cotton and its conventional peer. Sci. Rep. 6, 33539; doi: 10.1038/srep33539 (2016).
Acknowledgments
We thank Yang W.J. and Ruan H for helping acquire photosynthetic data. Funding for this study was granted by the National Special Transgenic Project of China(2016ZX08012-005), the National Public Project of Environmental Protection (201509044) and the National Science and Technology Pillar Program (No. 2013BAD11B01).
Footnotes
Author Contributions B.L., S.S. and R.G. discussed and designed the study. Data acquisition was done by R.G. R.G. analyzed the data and wrote the manuscript, supported by B.L. and S.S. All authors read and approved the final manuscript.
References
- Zhou Y. Y. Cultivation techniques for cotton’s high yield and high quality (Golden Shield Press, 2005). [Google Scholar]
- Zheng S. F., Lu X. J. & Pan Z. Y. New cultivation techniques for cotton’s high quality and high efficiency growth (Anhui Science and Technology Press, 2007). [Google Scholar]
- Sims S. R. & Martin J. W. Effects of the Bacillus thuringiensis insecticidal proteins CryIA(b), CryIA(c), CryIIA, and CryIIIA on Folsomia candida and Xenylla grisea (Insecta: Collembola). Pedobiologia 41, 412–416 (1997). [Google Scholar]
- Watrud L. S. & Seidler R. J. Non-target ecological effects of plants, microbial, and chemical introductions to terrestrial system. Soil Chemistry and Ecosystem Health, Special Publication 521, 33–340 (1998). [Google Scholar]
- Saxena D. & Stotzky G. Insecticidal toxin from Bacillus thuringiensis is released from roots of transgenic Bt corn in vitro and in situ. FEMS Microbiology Ecology 33, 35–39 (2000). [DOI] [PubMed] [Google Scholar]
- Motavalli P. P., Kremer R. J., Fang M. & Means N. E. Impact of genetically modified crops and their management on soil microbially mediated plant nutrient transformations. Journal of Environmental Quality 33, 816–824 (2004). [DOI] [PubMed] [Google Scholar]
- LI X. G. et al. The effect of root exudates from two transgenic insect-resistant cotton lines on the growth of Fusarium oxysporum. Transgenic Research 18, 757–767 (2009). [DOI] [PubMed] [Google Scholar]
- Quist D. & Chapela I. H. Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature 414, 541–543 (2001). [DOI] [PubMed] [Google Scholar]
- Hanson B. D. et al. Interspecific hybridization: potential for movement of herbicide resistance from wheat to jointed goatgrass (Aegilops cylindrica). Weed Technology 19, 674–682 (2005). [Google Scholar]
- Ortiz-Garcia S. et al. Absence of detectable transgenes in local landraces of maize in Oaxaca, Mexico (2003–2004). Proceedings of the National Academy of Sciences 102, 12338–12343 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro-Nelson A. et al. transgenes in Mexican maize: molecular evidence and methodological considerations for GMO detection in landrace populations. Molecular Ecology 18, 750–761 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray C. E., Huang J., Hu R. F. & Rozelle S. Five years of Bt cotton in China - the benefits continue. The Plant Journal 31, 423–430 (2002). [DOI] [PubMed] [Google Scholar]
- Qaim. M. & Zilberman D. Yield effects of genetically modified crops in developing countries. Science 299, 900–902 (2003). [DOI] [PubMed] [Google Scholar]
- Felke M., Lorenz N. & Langenbruch G. A. Laboratory studies on the effects of pollen from Bt-maize on larvae of some butterfly species. Journal of Applied Entomology 126, 320–325 (2002). [Google Scholar]
- Carriere Y. et al. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proceedings of the National Academy of Sciences 100, 1519–1523 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvier M. et al. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 316, 1475–1477 (2007). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E. et al. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. Journal of Economic Entomology 96, 1031–1038 (2003). [DOI] [PubMed] [Google Scholar]
- Paris M. et al. persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bt toxins in mosquitoes. Pest Management Science 67, 122–128 (2011). [DOI] [PubMed] [Google Scholar]
- Lu Y. H. et al. Mirid bug outbreak in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154 (2010). [DOI] [PubMed] [Google Scholar]
- Zhao J. H., Ho P. & Azadi H. Benefits of Bt cotton counterbalanced by secondary pests? Perceptions of ecological change in China. Environmental monitoring and assessment 173, 985–994 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. The impact of introducing the Bacillus thuringiensis gene into cotton on boll nitrogen metabolism. Environmental and experimental botany 61, 175–180 (2007). [Google Scholar]
- Everman W. J., Thomas W. E., Burton J. D., York A. C. & Wilcut J. W. Absorption, translocation, and metabolism of glufosinate in transgenic and nontransgenic cotton, Palmer Amaranth (Amaranthus plameri), and pitted monringglory (lpomoea lacunosa). Weed science 57, 357–361 (2009). [Google Scholar]
- Sun C. X. et al. Determination of nutrient elements in transgenic insect-resistant cotton tissues by three different spectroscopical methods. Spectroscopy and spectral analysis 29, 3038–3041 (2009). [PubMed] [Google Scholar]
- Sterns S. C. The evolution of life histories (Oxford University Press, 1992). [Google Scholar]
- Giovannetti M., Sbrana C. & Turrini A. The impact of genetically modified crops on soil microbial communities. Rivista di biologia—biology forum 98, 393–417 (2005). [PubMed] [Google Scholar]
- Liu W. K. & Du L. F. Interactions between Bt transgenic crops and arbuscular mycorrhizal fungi: a new urgent issue of soil ecology in agroecosystems. Acta agriculture scandinavica section B—soil and plant science 58, 187–192 (2008). [Google Scholar]
- Liu W. K. Do genetically modified plants impact arbuscular mycorrhizal fungi? Ecotoxicology 19, 229–238 (2010). [DOI] [PubMed] [Google Scholar]
- Ren M. Z., Chen Q. J., Zhang R. & Guo S. D. Structural characteristic and genetic expression of nondulin-like gene and its promoter in cotton. Acta botanica sinica 46, 1424–1423 (2004). [Google Scholar]
- Yang X. J., Yang Z. M., Mao C. H. & Du J. Dependence of dielectric properties on Bt particle in EP/BT composites. Rare metals 25 (Sp. Iss.sl), 250–254 (2006). [Google Scholar]
- Wu J. H. et al. Transgenic cotton expressing synthesized scorpion insect toxin AaHIT gene confers enhanced resistance to cotton bollworm (Heliothis armigera) larvae. Biotechnology letters 30, 547–554 (2008). [DOI] [PubMed] [Google Scholar]
- Jiang W. L. Strategic research on how to solve the water problem in China. Impact of Science on Society 7, 24–29 (2010). [Google Scholar]
- State Statistical Bureau. China Statistical yearbook. Available at: http://www.stats.gov.cn/tjsj/ndsj/2009/indexch.htm (2009).
- Xie Y. X. et al. Source of nitrogen in wet deposition to a cotton agroecosystem at Tai lake region. Atmospheric environment 42, 5182–5192 (2008). [Google Scholar]
- Farquhar G. D., O’Leary M. H. & Berry J. A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9, 121–137 (1982). [Google Scholar]
- Farquhar G. D., Ehleringer J. R. & Hubick K. T. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537 (1989). [Google Scholar]
- Raschke K. Movements of stomata. Encyclopedia of Plant Physiology 7, 383–441 (1979). [Google Scholar]
- Cowan I. R. Regulation of water use in relation to carbon gain in higher plants. Encyclopedia of Plant Physiology 12B, 489–613 (1982). [Google Scholar]
- Nobel P. S. Biophysical plant physiology and ecology (W. H. Freeman Press, 1983). [Google Scholar]
- Rosenthal G. F. et al. Water deficit effects on transpiration and leaf growth. Agronomy Journal 79, 1019–1026 (1987). [Google Scholar]
- Guo R. Q. et al. Differential responses of leaf water-use efficiency and photosynthetic nitrogen-use efficiency to fertilization in Bt-introduced and conventional rice line. Photosynthetica 49, 507–514 (2011). [Google Scholar]
- Griffiths B. S. et al. A comparison of soil microbial community structure, protozoa and nematodes in field plots of conventional and genetically modified maize expressing the Bacillus thuringiensis Cry1Ab toxin. Plant and Soil 275, 135–146 (2005). [Google Scholar]
- Hidaka A. & Kitayama K. Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient availability gradients. Journal of Ecology 97, 984–991 (2009). [Google Scholar]
- Cordell S., Goldstein G., Mueller-Dombois D., Webb D. & Vitousek P. M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113, 188–196 (1998). [DOI] [PubMed] [Google Scholar]
- Field C. & Mooney H. A. The photosynthesis-nitrogen relationships in wild plants (Cambridge University Press, 1986). [Google Scholar]
- Evans J. R. Photosynthesis and nitrogen relationaships in leaves of C3 plants. Oecologia 78, 9–19 (1989). [DOI] [PubMed] [Google Scholar]
- Reich P. B. et al. Generality of leaf trait relationships: A test across six biomes. Ecology 80, 1955–1969 (1999). [Google Scholar]
- Wright I. J. et al. The world-wide leaf economics spectrum. Nature 428, 821–827 (2004). [DOI] [PubMed] [Google Scholar]
- Poorter H. & Evans J. R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116, 126–137 (1998). [DOI] [PubMed] [Google Scholar]
- Sims D. A., Seemann J. R. & Luo Y. The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen and CO2 for return on investment in leaves. Functional Ecology 12, 185–194 (1998). [Google Scholar]
- song B. H. et al. The comparison of leaf protein yield and their protein contents from 107 species of forage plants. Journal of Plant Resources and Environment 8(4), 52–54 (1999). [Google Scholar]
- De Vaufleury A. et al. Exposure and effects assessments of Bt-maize on non-target organisms (gastropods, microarthropods, mycorrhizal fungi) in microcosms. Pedobiologia 51, 185–194 (2007). [Google Scholar]
- Pu H. Q. The fitness effects of transgenic plants under the pressure of multi-factor selection in field tests. Dissertation for Master Degree of Nanjing University (2012). [Google Scholar]
- Field C., Merino J. & Mooney H. A. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreen. Oecologia (Berlin) 60, 384–389 (1983). [DOI] [PubMed] [Google Scholar]
- Evan H. D. & William H. S. Resource-use efficiency and drought tolerance in adjacent great basin and sierran plants. Ecology 72, 51–58 (1991). [Google Scholar]
- Gutierrez M. V. & Meinzer F. C. Carbon isotope discrimination and photosynthetic gas-exchange in coffee hedgerows during canopy development. Australia Journal of Plant Physiology 21, 207–219 (1994). [Google Scholar]


