Abstract
Background
The present study is unique in employing unusually difficult attention and working memory tasks to reveal subtle cognitive decrements among overweight/obese adolescents. It evaluated novel measures of background electroencephalographic (EEG) activity during one of the tasks and tested correlations of these and other measures with psychological and psychiatric predictors of obesity maintenance or progression.
Methods
Working memory and sustained attention tasks were presented to 158 female adolescents who were rated on dichotomous (body mass index percentile < vs. >=85) and continuous (triceps skinfold thickness) measures of adiposity.
Results
The results revealed a significant association between excess adiposity and performance errors during the working memory task. During the sustained attention task, overweight/obese adolescents exhibited more EEG frontal beta power as well as greater intraindividual variability in reaction time and beta power across task periods than their normal-weight peers. Secondary analyses showed that frontal beta power during the sustained attention task was positively correlated with anxiety, panic, borderline personality features, drug abuse, and loss of control over food intake.
Conclusions
The findings suggest that working memory and sustained attention decrements do exist among overweight/obese adolescent girls. The reliable detection of the decrements may depend on the difficulty of the tasks as well as the manner in which performance and brain activity are measured. Future studies should examine the relevance of these decrements to diet education efforts and treatment response.
Keywords: Overweight, obesity, working memory, attention, electroencephalography, psychopathology
Introduction
The typical consumer has been presented with a massive array of data describing the caloric and nutritional content [1,2] of food, “new” weight loss strategies [3], “functional” foods [4], and gimmicks. Apart from the burden that this abundance brings to bear on the motivational state of a consumer who wants to lose weight, there is also a significant cognitive burden. Indeed, there appears to be an assumption that the overweight or obese consumer has supra-normal skills in the cognitive domains of problem solving (e.g., calorie counting), attention, and working memory.
Unfortunately, this assumption about the skills of the typical overweight or obese consumer is inconsistent with the evidence. On intelligence tests, which are a global measure of cognitive function, the average score of overweight/obese adolescents is slightly lower than the average score of their normal weight peers [5]. Other studies find evidence of decrements in specific functional domains. One domain of particular interest [6] is response inhibition because it has an intuitively obvious connection to the cognitive control of behaviors, such as eating and overeating, and problem solving. Indeed, the literature contains many reports of below-average scores on response inhibition tests, such as the Stroop [7–12] and Go/NoGo [11,13,14]. These reports are buttressed by evidence in the neuroimaging literature showing that overweight or obese subjects exhibit decrements in brain structure or brain activation in frontal regions [8,9,14–18] influencing this cognitive domain.
Unlike the response inhibition decrements, decrements in other executive cognitive domains are rarely found [6] among overweight/obese adolescents. Modest decrements in working memory task performance have been demonstrated in some studies but not in others [16,19–21]. Also, impairments in sustained and divided attention have been shown inconsistently [16,22]. In part, the failure to detect group differences on these tasks may be an artifact of the difficulty level: the tasks (e.g., Digit Span, Trails A, and similar memory and attention tasks) typically used to index these domains are not sufficiently challenging to a neurologically-normal subject. To address this limitation, we administered versions of working memory and sustained attention tasks that were unusually difficult.
The present study was also unusual in examining electroencephalographic (EEG) activity during the sustained attention task and quantifying this activity in a unique manner. Unlike other investigations that have examined EEG spectral band power averaged over time [23–26], we focused on intraindividual variability and the range in spectral power across the task period. This focus on intraindividual variability derives from a theory which interprets, for example, the maximum in beta band power as an indication of a brief, supra-normal, compensatory effort triggered by the awareness of waning attention. In contrast, the theory views the minimum in beta power as an indication of waning attention unaccompanied by compensatory effort. It was accordingly hypothesized that the difference between the maximum and minimum would capture and better estimate individual differences in the electroencephalographic and behavioral correlates of impaired attention than the mean level.
The last notable contribution of the present study was its examination of psychological and psychiatric risk factors [27–30] implicated in a persistent course of obesity. Admittedly, questions about obesity persistence from adolescence to adulthood could be better addressed with a longitudinal design. But, in the absence of such a design, demonstrating an association of these psychological or psychiatric risk factors with task performance or EEG variables provides some insight into the relevance of the latter variables to a persistent course.
Methods
Participants and Procedures
Adolescent volunteers were recruited indirectly by contacting parents through posters, direct mail solicitations, and newspaper advertisements. These advertisements mentioned weight management problems, risk-taking tendencies, conduct problems, or a family history of risk-taking or drug/alcohol abuse as potential qualifiers. Similar advertisements were used to appeal directly to the adolescent population. Each interested volunteer (n=183) and one of her biological parents were asked to call a research assistant for additional information and eligibility screening. Volunteers (n=158) who reported no past or current pregnancy, psychosis, or major medical disorders (HIV, thyroid disease, seizure disorder, heart disease, hearing loss, uncorrected visual impairment) that would complicate body weight or electroencephalographic activity during the telephone and in-person interviews were deemed eligible and became participants in the protocol. They were paid for their time and effort.
Informed consent, HIPAA, and medical release documents approved by the university’s Institutional Review Board were reviewed and signed by the participant and parent on the day of data collection. On that day, the parent completed a questionnaire reviewing the child’s health history as well as a separate questionnaire that inquired about obesity, alcohol/drug dependence, and hypertension among first and second degree relatives. The participant completed several questionnaires assessing constructs with documented relevance to overeating or obesity. These measures were the Barratt Impulsiveness Scale (BIS-11) [31], Toronto Alexithymia Scale (TAS) [32,33], Borderline Symptom List (BSL) [34], and the Revised Questionnaire of Eating and Weight Patterns (QEWP-R) [35,36]. Other questionnaires and interviews were administered for the purpose of describing the general background characteristics of the sample. These included a modified Drug Abuse Screening Test (DAST) [37], the computerized Diagnostic Interview Scale for DSM-IV [38], and the 90-day Timeline Followback Interview for alcohol and drugs [39].
Height and weight were measured for each participant with a stadiometer (Health-o-meter™; McCook, IL). In addition, skinfold thickness was measured over the right and left triceps with calipers (Lange calipers, QuickMedical, Issaquah, WA) to provide a second measure of adiposity. The absence of recent cigarette use was verified with a breath carbon monoxide monitor (Vitalograph Inc., Lenexa, KS). Also, two saliva samples were collected. The first sample was used for same-day drug toxicology screening. The second sample was preserved for DNA extraction and genetic analyses to be performed at a later time.
Neurophysiological and Cognitive Task Procedures
Prior to the administration of the working memory and sustained attention tasks, Ag/AgCl EEG electrodes were applied to 64 scalp sites positioned by an electrode cap. A reference electrode was taped to each earlobe. A ground electrode was applied to the middle of the forehead. Interelectrode impedances were maintained below 10 Kilohms.
After the electrodes were applied, the participant was escorted into a sound-shielded chamber and seated in a comfortable chair facing a 14-inch computer monitor. The monitor was used for the presentation of visual stimuli. A panel with two response keys and a computer mouse were also placed in front of the participant.
The working memory task was self-paced and presented on the computer monitor over 23 trials. Each trial began with the simultaneous presentation of 3–7 visual objects scattered across 12 possible locations within a 3 by 4 grid. The participant was asked to commit the spatial locations of the objects to memory. When the memorized stimulus was removed after 5 s, it was replaced with an empty 3 by 4 grid, and the participant was asked to click the mouse in the grid locations where she previously saw an object. The software (Compumedics/Neuroscan Stim2, Charlotte, NC) allowed her to change her guesses before she selected the icon to advance to the next trial. No feedback about performance accuracy was provided.
The attention task was comprised of 400 trials presented over an uninterrupted 13.3 m period. On each trial, either a white noise burst or a 500 Hz pure tone, 75 dB SPL in intensity, was presented for 50 ms through foam-cushioned tubes inserted in the ear canals. The inter-trial interval was 2000 ms. The participant was instructed to ignore the frequently-occurring (p=0.8) noise burst and attend to, and press a response key to acknowledge, the rarely-occurring (p=0.2) pure tone.
During the attention task, the electroencephalogram was recorded from 64 electrodes placed across the scalp. Eyeblinks and eye movements were also recorded using a pair of electrodes placed diagonally above and below the left eye. The EEG and eye movement signals were appropriately amplified (gain=10K), routed to an A/D converter, and sampled at a rate of 500 Hz. EEG activity was not examined during the working memory task because the numerous hand and eye movements elicited by the task contaminated the recording and could not be effectively removed.
During off-line computations, the EEG record was filtered (bandpass=1–40 Hz, 24 db/octave roll-off, no phase shift), and segmented into 101, 4096-point non-overlapping epochs of 8.19 s in duration. A linear regression algorithm implemented in Scan version 4.4 software (Compumedics/Neuroscan, Inc., Charlotte, NC) was used to remove eye movement and eye blink artifacts from each epoch. The corrected epochs were separately processed through a Hanning window and a Fourier transform. The epochs were then averaged within each of 9, 81.9 s periods spanning the task to create a summary power spectrum for each period.
For each of the 9 periods during the attention task, relative power was calculated within delta (0–3.7 Hz), theta (3.9–7.8 Hz), alpha (7.9–12.8 Hz), and beta (12.9–29.7 Hz) frequency bands. The analysis focused on electrode sites at which relative power was maximal within the bands of interest: Fz beta power, Cz theta power, Pz alpha power. Activity in the delta band was not analyzed because activity within this band is not a significant or reliable feature of the electroencephalogram of an awake, healthy subject. When it is present, it is more likely an artifact of movement or lead sway than a genuine reflection of brain activity.
Analysis Plan
The analysis began with a MANOVA which screened for the presence of conjoint differences across all of the continuous measures listed in Tables 1 and 2 and thereby offered protection against the multiple comparison problem. Univariate ANOVAs were performed for the listed measures if and only if the MANOVA F statistic was significant at an alpha level less than 0.05. The grouping factors in the MANOVA were body mass index percentile (BMIP) status relative to the 85th percentile and race/ethnicity. A BMIP>=85, adjusted for age and sex, is the U.S. Center for Diseases Control's definition of an at-risk-for-overweight body mass.
Table 1.
Background and personality characteristics.
| BMIP<85, n=84 Mean (SD) | BMIP>=85, n=74 Mean (SD) | |
|---|---|---|
| Triceps Skinfold Thickness in mm* | 23.88 (7.56) | 40.29 (8.26) |
| Age in yrs | 15.57 (1.24) | 15.59 (1.30) |
| % Black or Hispanic* | 26.2 | 48.6 |
| DAST total score | .39 (.89) | .62 (1.24) |
| BIS-11 Attention | 15.70 (4.32) | 15.97 (4.10) |
| BIS-11 Motor | 20.65 (3.67) | 20.21 (2.98) |
| BIS-11 Nonplanning | 24.26 (5.16) | 25.60 (5.75) |
| BSL Total Score | .39 (.55) | .50 (.51) |
| TAS Difficulty Describing Feelings | 13.46 (2.79) | 13.37 (2.10) |
| TAS Externally Oriented Thinking | 23.55 (2.79) | 23.85 (2.95) |
| TAS Difficulty Identifying Emotions | 17.79 (4.17) | 17.13 (3.57) |
p<0.05.
Abbreviations: DAST, Drug Abuse Screening Test; BIS, Barratt Impulsiveness Scale; BSL, Borderline Symptom List; TAS, Toronto Alexithymia Scale.
Table 2.
EEG and task performance measures.
| BMIP<85 Mean (SD) | BMIP>=85 Mean (SD) | Partial η2 | |
|---|---|---|---|
| Visual Working Memory Task | |||
| % Correct* | 98.09 (2.89) | 96.88 (3.42) | .035 |
| % False Alarms* | 2.54 (4.11) | 4.19 (5.08) | .032 |
| Seconds per Trial | 4526.93 (949.11) | 4774.70 (1250.83) | .013 |
| Sustained Attention Task | |||
| Relative Beta Power @ Fz (Max-Min)* | .033 (.028) | .042 (.021) | .036 |
| Relative Beta Power @ Fz (avg over periods)* | .062 (.023) | .073 (.033) | .025 |
| Relative Alpha Power @ Pz (Max-Min) | .189 (.129) | .207 (.111) | .006 |
| Relative Alpha Power (avg over periods) | .458 (.149) | .463 (.116) | .000 |
| Relative Theta Power @ Cz (Max-Min) | .142 (.085) | .143 (.066) | .000 |
| Relative Theta Power (avg over periods) | .570 (.108) | .546 (.107) | .012 |
| Hit Rate (Max-Min) | .170 (.142) | .196 (.166) | .007 |
| Hit Rate (avg over periods) | .956 (.062) | .948 (.061) | .004 |
| False Alarm Rate (Max-Min) | .027 (.027) | .028 (.024) | .000 |
| False Alarm Rate (avg over periods) | .006 (.007) | .006 (.006) | .001 |
| Reaction Time in ms* (Max-Min) | 220.680 (127.330) | 271.850 (152.564) | .033 |
| Reaction Time in ms (avg over periods) | 474.222 (117.71) | 506.76 (113.94) | .019 |
p<0.05.
Another set of analyses included performance and EEG measures found to be significant in the prior analyses. Partial correlations, adjusting for age and race, were computed between these performance and EEG measures and a dimensional measure of adiposity, triceps skinfold thickness, that is not distorted by muscle weight as is the body mass index. Partial correlations were also computed to test the association of selected performance and EEG measures with psychological or psychiatric factors previously implicated in obesity maintenance or obesity progression.
Results
The multivariate test for the statistical effect of BMIP group was significant [Wilks’ Lambda=0.44, F(24,133)=7.04, p<0.001]. Including race/ethnicity and age in the MANOVA did not change the effect of BMIP. Accordingly, these factors were not examined in the univariate ANOVAs.
Background Characteristics
Analyses of the measures listed in Table 1 demonstrated group differences on two variables. Triceps skinfold thickness, an alternate measure of adiposity, was significantly greater [F(1,156)=169.6, p<0.001] in the BMIP>=85 group than in the BMIP<85 group. The groups also differed in the percent of group members who were Black or Hispanic versus white [χ2(df=1)=8.1, p<0.01].
Task Performance and EEG spectral power
Univariate ANOVAs of the dependent measures listed in Table 2 revealed reliable group differences across several measures. Statistically significant effects of BMIP were detected for the proportion of trials with correct responses [F(1,156)=5.73, p=0.018] and false alarms [F(1,156)=5.10, p=0.025] during the visual working memory task. Adolescent girls with a BMIP>=85 recalled fewer object locations correctly and executed more erroneous guesses about object locations than their peers with a BMIP<85.
During the sustained attention task, girls in the two groups exhibited a similar number of correct and incorrect responses, and similar reaction times. It was only through an examination of performance variability that group differences were discovered. Girls with an elevated BMIP were more variable in reaction time [F(1,156)=5.27, p=0.02] over the course of the task than girls in the comparison group. The groups did not differ significantly in mean reaction time [F(1,156)=3.09, p=0.08]. The increment in the group difference, i.e., the effect size, associated with the test of variability versus the test of the mean was modest: partial η2= 0.033 − 0.019 = 0.014 or 1.4% of the variance.
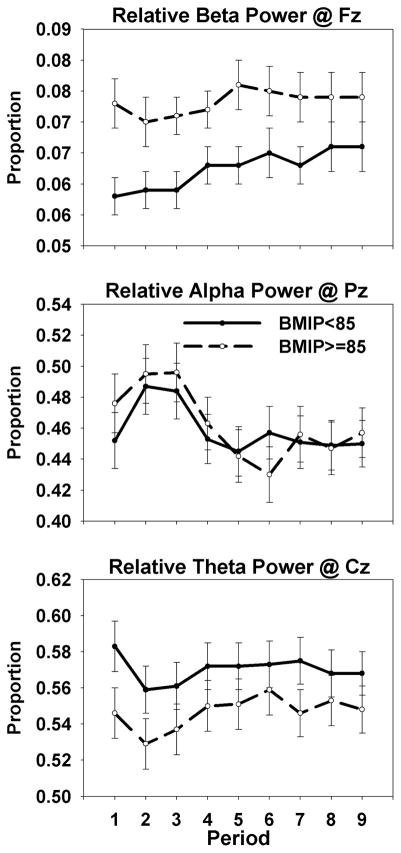
In the analysis of relative beta power, a similar though less obvious pattern was seen (compare the top panels of Figures 1 and 2). The group difference in intraindividual variability [F(1,156)=5.82, p=0.017] was modestly greater than the group difference in the mean [F(1,156)=4.06, p=0.045]. The added value, or increase in the effect size (partial η2 = .036 vs .025), associated with the test of intraindividual variability in frontal beta power was 0.011 or 1.1% of the variance.
Figure 1.

Group-averaged range in relative beta, alpha, and theta band power. Note the significant group effect for beta power only.
Figure 2.
Group-averaged mean of relative beta, alpha, and theta band power by task period.
No significant group differences on other EEG or performance measures were detected.
Regression Analyses
To provide converging evidence of an association between excess adiposity and the performance and EEG differences shown by the ANOVAs, a selected set of correlations were computed with triceps skinfold thickness as a replacement for BMI percentile. Age- and race-adjusted correlations are presented in Table 3.
Table 3.
Partial correlations, adjusting for age and race, of selected performance and EEG features during the sustained attention and working memory tasks with an alternative indicator of adiposity.
| Sustained Attention Beta Power (max-min) | Sustained Attention Beta Power (avg) | Sustained Attention Reaction Time (max-min) | Sustained Attention Reaction Time (avg) | Working Memory Hit Rate (proportion correct) | Working Memory False Alarm Rate (proportion incorrect) | |
|---|---|---|---|---|---|---|
| Triceps Skinfold Thickness |
r=.205 p=.01* |
r=.147 p=.06 |
r=.165 p=.03* |
r=.111 p=.16 |
r=−.182 p=.02* |
r=.183 p=.02* |
p<0.05
Tests of the correlations of triceps skinfold thickness with performance accuracy measures from the working memory task were statistically significant (Hit Rate: r= −.182, p=.02; False Alarm Rate: r=.183, p=.02). The table also shows that the correlations of intraindividual variability in both Fz relative beta power (r=.205, p=.01) and reaction time (r=.165, p=.03) with skinfold thickness were statistically significant. As was evident in the ANOVAs, variability showed a marginally better relationship with adiposity than the mean (beta power: r=.205 vs. r=.147; reaction time: r=.165 vs. r=.111).
Table 4 shows correlations of selected performance and EEG variables with psychological and psychiatric risk factors for adult obesity. The only variable consistently related to psychological or psychiatric problems, and therefore likely related to maintenance or progression of weight control problems, was beta power averaged over the vigil. It was significantly correlated with Binge Eating, Bulimia, Panic and Generalized Anxiety Disorders, as well as symptoms of Borderline Personality Disorder and drug abuse.
Table 4.
Point-biserial and product-moment correlations of selected task performance and EEG variables with psychiatric disorders and psychological symptoms previously associated with obesity in adults. The correlations are adjusted for age and race. Note the relatively consistent association of average beta power during the sustained attention task with these predictors.
| Sustained Attention Beta Power (max-min) | Sustained Attention Beta Power (avg) | Sustained Attention Reaction Time (max-min) | Sustained Attention Reaction Time (avg) | Working Memory Hit Rate (proportion correct) | Working Memory False Alarm Rate (proportion incorrect) | |
|---|---|---|---|---|---|---|
|
| ||||||
| ADD Dx N=4/158 | r=−.037 | r=−.071 | r=.044 | r=.049 | r=.113 | r=−.110 |
| p=.64 | p=.37 | p=.57 | p=.53 | p=.15 | p=.16 | |
|
| ||||||
| ADHD Dx N=3/158 | r=.124 | r=.133 | r=.016 | r=−.014 | r=.083 | r=−.070 |
| p=.12 | p=.09 | p=.83 | p=.86 | p=.29 | p=.37 | |
|
| ||||||
| Binge Eating Disorder Dx N=21/158 | r=.062 | r=.186 | r=.101 | r=.044 | r=−.068 | r=.041 |
| p=.44 | p=.020 | p=.20 | p=.58 | p=.39 | p=.60 | |
|
| ||||||
| Bulimia Nervosa Dx N=6/158 | r=.251 | r=.190 | r=.100 | r=.116 | r=−.010 | r=.000 |
| p=.002 | p=.017 | p=.20 | p=.14 | p=.89 | p=.99 | |
|
| ||||||
| Major Depressive Disorder Dx N=34/158 | r=.107 | r=.125 | r=.060 | r=.017 | r=−.035 | r=.021 |
| p=.18 | p=.12 | p=.45 | p=.83 | p=.65 | p=.78 | |
|
| ||||||
| Panic Attack Dx N=18/158 | r=.202 | r=.190 | r=.011 | r=.013 | r=.085 | r=−.082 |
| p=.01 | p=.017 | p=.89 | p=.87 | p=.28 | p=.30 | |
|
| ||||||
| Generalized Anxiety Disorder Dx N=7/158 | r=.117 | r=.274 | r=.032 | r=.104 | r=.036 | r=−.046 |
| p=.14 | p=.001 | p=.68 | p=.18 | p=.65 | p=.56 | |
|
| ||||||
| Borderline Symptom List Total Score | r=.192 | r=.164 | r=.062 | r=.087 | r=−.038 | r=.024 |
| p=.016 | p=.041 | p=.43 | p=.27 | p=.62 | p=.76 | |
|
| ||||||
| Days with loss of control over eating past 6 mos. | r=.179 | r=.170 | r=.138 | r=.031 | r=−.125 | r=.117 |
| p=.025 | p=.034 | p=.08 | p=.69 | p=.11 | p=.14 | |
|
| ||||||
| Drug Abuse Screening Test | r=.213 | r=.226 | r=.023 | r=−.056 | r=−.072 | r=.078 |
| p=.007 | p=.004 | p=.77 | p=.48 | p=.36 | p=.330 | |
A final set of correlations were computed between beta power averaged over the vigil and the disorders and problems just mentioned. These correlations partialed out the statistical effect of skinfold thickness and therefore attempted to resolve a question about adiposity as a likely cause of the elevation in beta power or a proxy for a neuropsychological disturbance that affects beta power. The findings of these analyses support the latter interpretation. Most of the correlations remained significant when this measure of adiposity was controlled: Binge Easting Disorder (r=.164, p=.04), Bulimia Nervosa (r=.171, p=.03), Panic Attack (r=.180, p=.02), Generalized Anxiety Disorder (r=.267, p=.001), Borderline Symptom List (r=.471, p=.01), loss of control over eating (r=.147, p=.06), DAST (r=.211, p=.008).
Discussion
The present study was designed to detect decrements in two cognitive domains among adolescents with excess adiposity. To reveal the decrements, we presented attention and working memory tasks that were unusually challenging. During the working memory task, for example, adolescents were asked to memorize and recall, on each trial, the spatial locations of 3–7 visual objects scattered across 12 possible locations. During the sustained attention task, they were asked to remain vigilant for 13.3 minutes and detect a rarely occurring auditory stimulus embedded within a series of background stimuli.
The result of the challenge was the detection of decrements that have not been consistently found in other studies employing simpler tasks [9,16,20]. For example, during the working memory task, adolescent girls with excess adiposity emitted more omission and comission errors than their normal weight peers. This performance decrement was also evident in analyses using a dimensional (triceps skinfold thickness; Table 3) indicator of overweight/obesity.
The findings from the auditory sustained attention task were more novel and interesting than those from the working memory task. It is particularly interesting that the range in reaction time across periods of the task was modestly better (Partial η2 = 0.033) than the mean reaction time (Partial η2 = 0.019) as an indicator of behavioral differences between the groups (Table 2). The results similarly showed that the range in EEG beta power was marginally better than mean beta power as a predictor of both triceps skinfold thickness (Table 3) and BMI percentile group membership (Table 2). We should note that we considered standard deviation as an alternative to the range. But, we were concerned that the standard deviation would assign excess weight to uninformative periods wherein task performance and EEG activity were stable.
Our demonstration of the modest superiority of a measure of variability versus a measure of central tendency is not new. Performance variability has recently become popular in studies of healthy aging [40,41] and Attention Deficit Hyperactivity Disorder (ADHD) [42]. The explanation for its popularity is most likely tied to the fact that the cognitive impairments seen in these groups are not severe. Participants with these issues often retain the ability to mobilize additional cognitive resources to maintain normal performance for the majority of the task period. Accordingly, their mean performance level does not reflect their subtle cognitive impairment as well as their variability in performance.
In the present study, participants in the BMIP>=85 group demonstrated an elevation in EEG beta power (Figure 2, top panel) that was sustained across task periods. One could interpret the elevation as an indication of compensatory effort or the mobilization of additional cognitive resources described above. Alternatively, one could view it not as a response to the challenges of the task but as a trait. The demonstration in this study of a significant association (Table 4) of enhanced frontal beta power with stable characteristics, such as borderline personality symptoms, and panic and anxiety disorders, and with GABRA2 genotype [43,44] in other studies, supports this view of elevated beta activity as a trait. Its association with stable characteristics suggests that it may predict a course of overweight/obesity that is likewise stable.
Conclusions
The present findings should not simply be viewed as a further description of the cognitive problems present in overweight/obese adolescents. Instead, the findings should be considered in the context of a larger discussion about obesity prevention and treatment. Given the disappointing success rate of lifestyle interventions for weight loss [45] and weight loss maintenance [46] in both adolescents and adults, one feels compelled to ask a question that was suggested in the Introduction to this article. That is, if we assume that the working memory and sustained attention decrements are present among many overweight/obese patients, then why would we expect a lengthy and complicated message to these patients about calorie counting, functional foods, and lifestyle changes to be successful? To the degree that these impairments are prevalent and severe, one might consider a re-design of weight loss interventions to compensate for them. In a re-designed intervention, it may be wise to reduce the amount and complexity of information to accomodate the working memory problem, and deliver it in an interesting manner to address the sustained attention problem.
Acknowledgments
This research was supported in part by U.S. Public Health Service grant P60AA03510. The technical assistance of Kristen Siedlarz, M.A., and Elisa Ferzacca, B.A. is greatly appreciated.
References
- 1.US Food and Drug Administration. Food labeling; nutrition labeling of standard menu items in restaurants and similar retail food establishments. Final rule Fed Regist. 2014;79:71155–71259. [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Food labeling; calorie labeling of articles of food in vending machines. Final rule Fed Regist. 2014;79:71259–71293. [PubMed] [Google Scholar]
- 3.Mata J, Todd PM, Lippke S. When weight management lasts. Lower perceived rule complexity increases adherence. Appetite. 2010;54:37–43. doi: 10.1016/j.appet.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Marinangeli CP, Jones PJ. Functional food ingredients as adjunctive therapies to pharmacotherapy for treating disorders of metabolic syndrome. Ann Med. 2010;42:317–333. doi: 10.3109/07853890.2010.484026. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg S, Werbeloff N, Fruchter E, Portuguese S, Davidson M, Weiser M. IQ and obesity in adolescence: a population-based, cross-sectional study. Pediatr Obes. 2014;9:419–426. doi: 10.1111/j.2047-6310.2013.00203.x. [DOI] [PubMed] [Google Scholar]
- 6.Reinert KR, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 2013;2013:820956. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado-Rico E, Rio-Valle JS, Gonzalez-Jimenez E, Campoy C, Verdejo-Garcia A. BMI predicts emotion-driven impulsivity and cognitive inflexibility in adolescents with excess weight. Obesity (Silver Spring) 2012;20:1604–1610. doi: 10.1038/oby.2012.47. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JI, Yates KF, Duong M, Convit A. Obesity, orbitofrontal structure and function are associated with food choice: a cross-sectional study. BMJ Open. 2011;1:e000175. doi: 10.1136/bmjopen-2011-000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 2011;19:1382–1387. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 11.Lillis J, Levin ME, Trafton JA. Elevated BMI and illicit drug use are associated with decreased ability to inhibit prepotent behaviors. Addict Behav. 2012;37:544–547. doi: 10.1016/j.addbeh.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Bauer LO, Kaplan RF, Hesselbrock VM. P300 and the Stroop effect in overweight minority adolescents. Neuropsychobiology. 2010;61:180–187. doi: 10.1159/000297735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Does inhibitory control capacity in overweight and obese children and adolescents predict success in a weight-reduction program? Eur Child Adolesc Psychiatry. 2009 doi: 10.1007/s00787-009-0049-0. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo K, Pontifex MB, Khan NA, Raine LB, Scudder MR, Drollette ES, Evans EM, Castelli DM, Hillman CH. The association of childhood obesity to neuroelectric indices of inhibition. Psychophysiology. 2012;49:1361–1371. doi: 10.1111/j.1469-8986.2012.01459.x. [DOI] [PubMed] [Google Scholar]
- 15.Bauer L, Dick D, Bierut L, Bucholz K, Edenberg H, Kuperman S, Kramer J, Nurnberger J, O'Connor S, Rice J, Rohrbaugh J, Schuckit M, Tischfield J, Porjesz B, Hesselbrock V. Obesity, smoking, and frontal brain dysfunction. Am J Addict. 2010;19:391–400. doi: 10.1111/j.1521-0391.2010.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22:1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, Pradhan K. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 2011;19:1095–1097. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunstad J, Spitznagel MB, Paul RH, Cohen RA, Kohn M, Luyster FS, Clark R, Williams LM, Gordon E. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 2008;50:246–251. doi: 10.1016/j.appet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49:675–678. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Zamzow J, Culnan E, Spiers M, Calkins M, Satterthwaite T, Ruparel K, Abrams D, Chiavacci R, Hakonarson H, Gur R. B-37The Relationship between Body Mass Index and Executive Function from Late Childhood through Adolescence. Arch Clin Neuropsychol. 2014;29:550. [Google Scholar]
- 22.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Kamzanova AT, Kustubayeva AM, Matthews G. Use of EEG workload indices for diagnostic monitoring of vigilance decrement. Hum Factors. 2014;56:1136–1149. doi: 10.1177/0018720814526617. [DOI] [PubMed] [Google Scholar]
- 24.Kathner I, Wriessnegger SC, Muller-Putz GR, Kubler A, Halder S. Effects of mental workload and fatigue on the P300, alpha and theta band power during operation of an ERP (P300) brain-computer interface. Biol Psychol. 2014;102:118–129. doi: 10.1016/j.biopsycho.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Roy RN, Bonnet S, Charbonnier S, Campagne A. Mental fatigue and working memory load estimation: interaction and implications for EEG-based passive BCI. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:6607–6610. doi: 10.1109/EMBC.2013.6611070. [DOI] [PubMed] [Google Scholar]
- 26.Kiroy VN, Warsawskaya LV, Voynov VB. EEG after prolonged mental activity. Int J Neurosci. 1996;85:31–43. doi: 10.3109/00207459608986349. [DOI] [PubMed] [Google Scholar]
- 27.Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D, Rossler W, Angst J. The associations between psychopathology and being overweight: a 20-year prospective study. Psychol Med. 2004;34:1047–1057. doi: 10.1017/s0033291703001697. [DOI] [PubMed] [Google Scholar]
- 28.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 29.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson SE, Cohen P, Naumova EN, Must A. Relationship of childhood behavior disorders to weight gain from childhood into adulthood. Ambul Pediatr. 2006;6:297–301. doi: 10.1016/j.ambp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47:385–395. [Google Scholar]
- 32.Elfhag K, Lundh LG. TAS-20 alexithymia in obesity, and its links to personality. Scand J Psychol. 2007;48:391–398. doi: 10.1111/j.1467-9450.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 33.Bagby RM, Taylor GJ, Ryan D. Toronto Alexithymia Scale: relationship with personality and psychopathology measures. Psychother Psychosom. 1986;45:207–215. doi: 10.1159/000287950. [DOI] [PubMed] [Google Scholar]
- 34.Bohus M, Kleindienst N, Limberger MF, Stieglitz RD, Domsalla M, Chapman AL, Steil R, Philipsen A, Wolf M. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42:32–39. doi: 10.1159/000173701. [DOI] [PubMed] [Google Scholar]
- 35.Nangle DW, Johnson WG, Carr-Nangle RE, Engler LB. Binge eating disorder and the proposed DSM-IV criteria: psychometric analysis of the Questionnaire of Eating and Weight Patterns. Int J Eat Disord. 1994;16:147–157. doi: 10.1002/1098-108x(199409)16:2<147::aid-eat2260160206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, Devlin M, Mitchell J, Hasin D, Horne RL. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord. 1993;13:137–153. [PubMed] [Google Scholar]
- 37.McCabe SE, Boyd CJ, Cranford JA, Morales M, Slayden J. A modified version of the Drug Abuse Screening Test among undergraduate students. J Subst Abuse Treat. 2006;31:297–303. doi: 10.1016/j.jsat.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University; 2002. [Google Scholar]
- 39.Dennis ML, Funk R, Godley SH, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (GAIN) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction. 2004;99(Suppl 2):120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- 40.Bielak AA, Cherbuin N, Bunce D, Anstey KJ. Intraindividual variability is a fundamental phenomenon of aging: evidence from an 8-year longitudinal study across young, middle, and older adulthood. Dev Psychol. 2014;50:143–151. doi: 10.1037/a0032650. [DOI] [PubMed] [Google Scholar]
- 41.Myerson J, Robertson S, Hale S. Aging and intraindividual variability in performance: analyses of response time distributions. J Exp Anal Behav. 2007;88:319–337. doi: 10.1901/jeab.2007.88-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuntsi J, Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Curr Top Behav Neurosci. 2012;9:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- 43.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer LO, Yang BZ, Houston RJ, Kranzler HR, Gelernter J. GABRA2 genotype, impulsivity, and body mass. Am J Addict. 2012;21:404–410. doi: 10.1111/j.1521-0391.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadden TA, Volger S, Tsai AG, Sarwer DB, Berkowitz RI, Diewald LK, Carvajal R, Moran CH, Vetter M, Group P-UR. Managing obesity in primary care practice: an overview with perspective from the POWER-UP study. Int J Obes (Lond) 2013;37(Suppl 1):S3–11. doi: 10.1038/ijo.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJ. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11:899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]