Abstract
Objectives. To determine the effectiveness of a statewide telephone service in identifying low-income women at risk for hereditary breast and ovarian cancer and referring them to free genetic counseling.
Methods. From June 2010 through August 2011, eligible callers to California’s toll-free breast and cervical cancer telephone service were screened for their family histories of breast and ovarian cancer. High-risk women were identified and called for a baseline survey and randomization to an immediate offer of genetic counseling or a mailed brochure on how to obtain counseling. Clinic records were used to assess receipt of genetic counseling after 2 months.
Results. Among 1212 eligible callers, 709 (58.5%) agreed to answer family history questions; 102 (14%) were at high risk (25% Hispanic, 46% White, 10% Black, 16% Asian, 3% of other racial/ethnic backgrounds). Of the high-risk women offered an immediate appointment, 39% received counseling during the intervention period, as compared with 4.5% of those receiving the brochure.
Conclusions. A public health approach to the rare but serious risk of hereditary breast and ovarian cancer can be successful when integrated into the efforts of existing safety net organizations.
Hereditary breast and ovarian cancer (HBOC) is rare but extremely serious. The lifetime risks of breast cancer and ovarian cancer among women with a BRCA1/2 gene mutation are as high as 66% and 46%, respectively,1 as compared with the overall average risks among women of 12.3% and 1.3%, respectively.2 Approximately 5% to 10% of all breast cancer cases are associated with BRCA1/2.1
For more than 2 decades, it has been possible to identify women with these deleterious gene mutations, and medical interventions can substantially reduce their cancer risk.3 However, genetic screening services are concentrated in academic medical centers,4 where low-income patients are less likely to receive care.5 This is true even among women diagnosed with breast cancer whose treatment options would be influenced by BRCA status.6 Among women both affected and as yet unaffected by breast cancer, other impediments to risk reduction include cost of testing, mistrust of medical care, lack of awareness of genetic services, and fear of discrimination.7–11 Consequently, fewer than 13% of women tested are of non-European descent.12
There is no known difference in risk of HBOC according to socioeconomic status,13 but consensus regarding the racial/ethnic distribution of the BRCA mutations has been slow to emerge. One population-based study involving the Northern California Breast Cancer Family Registry estimated the prevalence of deleterious BRCA1 mutations among newly diagnosed cancer patients as 3.5% for Hispanics, 1.3% for African Americans, and 0.5% for Asian Americans, compared with 8.3% for Ashkenazi Jews and 2.2% for patients of other non-Hispanic White ethnic backgrounds. The prevalence was particularly high among young (< 35 years) African American patients, at 16.7%, exceeded only by Ashkenazi Jews in that age group (66.7%).14 Thus, HBOC represents an early and ominous example of how limited access to advances in precision medicine can exacerbate health disparities by leaving further behind those who already experience an excess burden of disease.
Even in instances in which genetic counseling and testing are financially accessible, 2 critical challenges remain: identifying individuals at high risk and encouraging them to access life-saving counseling and testing as needed. The relatively low prevalence of any BRCA mutation means that many people must be screened to find those at high risk. This process can be daunting given that outreach to diverse low-income communities for far more common and less complex objectives often requires costly and elaborate new infrastructure. Furthermore, although many such interventions are found to be effective in randomized trials, they often have limited potential for subsequent real-world implementation.15
The likelihood that low-income women will eventually have ready access to genetic risk assessment and counseling referrals depends on the extent to which processes to identify those at high risk and link them with counseling can be integrated with existing health care structures and resources. On the basis of this principle, the practice-based study described here emphasized context, generalizability, relevance, feasibility, and particularly fit.16 Here “fit” refers to the development of new services that are desirable to and benefit all stakeholders—service providers, recipients, and researchers—through minimal adaptation of existing programs already in use (and, importantly, trusted) by diverse underserved communities, thus building capacity while holding down costs.
The expectation is that minimal adaptation will yield maximum potential for sustainability and that engagement of end users (stakeholders) in all phases of development and evaluation is essential to real-world applicability. Toward this end, we partnered with 2 end-user organizations whose respective missions and functions aligned with our primary study objectives of risk identification and provision of genetic counseling.
END USERS AND STAKEHOLDERS AS RESEARCH PARTNERS
With federal and state funding, the California Department of Health Services established the statewide toll-free telephone service known as Every Woman Counts (EWC) in 1995 to provide access to free breast and cervical cancer screening for women who are medically underserved.17 To date, EWC has served more than 1 million low-income callers speaking multiple languages. We recognized the EWC program as a novel channel for reaching low-income women for varied preventive services on the basis of a pilot study in which 49.5% of eligible EWC callers were willing to participate in research on a topic unrelated to the purpose of their call.18 Our findings suggested that the unexpectedly high rate of participation was attributable to the trust engendered by EWC’s provision of free screening, its multilingual capability, and its promotion of the service through credible local media. We designed the current study to assess the potential of this existing and effective communication channel to identify callers who are at high risk for HBOC and to refer them to genetic risk services.
The University of California, San Francisco (UCSF) Cancer Risk Program (CRP), the second stakeholder in our partnership, has sites on campus and at San Francisco General Hospital (SFGH). Through UCSF donor funds and foundation support at SFGH, free genetic counseling and testing have been offered at these sites to more than 1000 low-income women from around the region since 2003.
Our intervention was designed to identify low-income local EWC callers at high risk for HBOC and refer them to UCSF or SFGH for free genetic services, as well as to closely fit this new service into the policies and procedures of EWC and CRP. Our study involved 2 phases. The first phase, reported elsewhere,19 assessed the effects of 2 intervention models and their degree of fit for end users in a randomized controlled pilot test. Because it measured impact in the absence of a baseline survey, the pilot avoided testing effects, which are a threat to external validity.16 In addition to the pilot test, the first study phase included development and pretest of a new family history screening questionnaire.19 The second phase, reported here, was a randomized delayed control trial of the intervention, which is an amalgamation of the 2 models.
METHODS
Study participants were recruited from callers to EWC. At the start of every call, telephone information specialists collected demographic and contact information and entered it into a computer program, which we adapted to identify callers who met our initial eligibility criteria: residence within one of 6 San Francisco Bay Area counties (for ease of access to our sites offering free genetic counseling and testing), at least 25 years of age, and English or Spanish speaking (the languages in which genetic counseling was conducted at the program sites). EWC telephone specialists asked these callers whether they were willing to answer family history questions to determine their eligibility for a research study.
Referral for Genetic Counseling or Testing
Several assessment tools have been designed to predict the likelihood of a BRCA mutation and, thus, the appropriateness of genetic testing (e.g., BRCAPRO, Myriad II, BOADICEA, PENN II).20–23 However, there is no standard tool for determining the appropriateness of referrals to genetic counseling that can be administered via telephone. Eligibility for genetic testing requires complete enumeration of family members who have and have not had cancer, types of cancer, and age at diagnosis. By contrast, identification of those who should be referred to obtain information on such a history is based on a set of “red flags” such as breast cancer before the age of 50 years, breast and ovarian cancer, bilateral breast cancer, or male breast cancer.
We adapted the Pedigree Assessment Tool24 because of its ease of administration over the phone. Our “6-point scale,” with scores representing the sum of 10 separate item scores, was designed to assess the most highly weighted items first so that the screening process can conclude if a sum of 6 points is reached, triggering a referral to a genetic counselor. Details on the development of the scale have been presented elsewhere.19 We validated the tool by comparing it with genetic counselors’ assessments and the more recently developed Referral Screening Tool.25 We found that our 6-point scale was more conservative than counselors’ assessments (sensitivity = 27%, positive predictive value = 67%, specificity = 97%, negative predictive value = 86%) and that it was in generally good agreement with the Referral Screening Tool, although it was somewhat less conservative (sensitivity = 86%, positive predictive value = 45%, specificity = 96%, negative predictive value = 99%) in classifying personal and family histories as high risk (S. L. Stewart et al., unpublished data, 2016).
Randomized Delayed Intervention Control Trial
After participants had provided consent, information specialists administered our 6-point scale. Women with scores of at least 6 points were told that they would be called back by researchers to complete a survey and that they would be assigned to a study arm. This process was very similar to the usual EWC intake and referral, and it was designed to fit the capabilities of the information specialist team, whose members had a high school–level education.
Within 2 weeks, a genetic counseling assistant from CRP called participants and administered a 15- to 20-minute baseline survey. The genetic counseling assistant also created a computer-generated list of random assignments, placed each assignment in an envelope, and opened each envelope in sequence to randomize participants for a 1:1 allocation. Women randomized to the intervention were told that, because of their family history of breast or ovarian cancer (or both), we were able to offer them a free genetic counseling appointment. This followed CRP’s usual protocol of outcalls to women identified as high risk through collection of family history forms completed by patients in the SFGH mammography clinic.
Participants randomized to the delayed intervention control group were sent a brochure with information on HBOC and a phone number through which they could contact CRP for free genetic counseling. The intervention period was 2 months, during which we expected significantly more women in the immediate appointment group than the control group to obtain genetic counseling (the main study outcome). Two months after randomization (June 2010 through June 2012), a research associate phoned each woman to complete a follow-up survey asking whether she had obtained counseling. Although the survey identified participants who went elsewhere for genetic counseling, the intervention was evaluated on the basis of the counselors’ records alone. Because all study participants were defined as being at high risk, everyone who had not yet been counseled after 2 months, regardless of study arm, was offered a genetic counseling appointment. In appreciation for their time in completing the baseline and final surveys, respondents were offered $25 and $35, respectively.
In the course of the study, it became clear that travel distance to obtain genetic counseling was a deterrent for some women. Until very recently, evidence on remote counseling has been mixed, and there has persisted a tacit understanding that in-person genetic counseling is optimal. However, 2 recent trials (although not including diverse low-income women) demonstrated that telephone counseling is not inferior to in-person counseling with respect to psychosocial outcomes.26,27 On the assumption that counseling via telephone would be better than no counseling, we made phone counseling available to those who needed it beginning in the ninth month of our 15-month trial.
Statistical Analyses
The study was designed to have a sample size of 144 participants, providing 80% power to detect the difference between 30% of participants counseled within 2 months in the intervention group and 10% of participants counseled within 2 months in the control group at the .05 level (2 sided). When results were available for 72 participants (half the planned sample size), we conducted an interim analysis using an O’Brien–Fleming design28 with early stopping rules for futility (i.e., lack of an intervention effect) and efficacy (i.e., a large positive intervention effect); we used ADDPLAN 6 (Aptiv Solutions, Reston, VA) in performing the analysis. On the basis of the results of this interim analysis, recruitment was stopped, and all enrolled participants were followed to determine outcomes (enrollment continued pending the first 72 participants’ completion of the study).
We also used ADDPLAN 6 in conducting the final analysis of the primary outcome, receipt of counseling during the 2-month intervention period; we computed 95% confidence intervals (CIs) for differences in proportions, as well as overall one-sided P values. A t test (for age) and a χ2 test (for all other variables) were conducted to compare the study arms with respect to baseline demographic variables and counseling status. In addition, we used log-rank tests to compare the study arms in terms of time from randomization to counseling appointment (the secondary study outcome), and Kaplan–Meier curves were computed for each study arm. We used SAS version 9.3 (SAS Institute Inc, Cary, NC) in performing analyses of demographic variables and secondary outcomes; other than the exceptions just noted, statistical significance was assessed at the .05 level (2 sided).
RESULTS
Of the 23 619 EWC callers during the study enrollment period, 1212 (5%) met our initial eligibility criteria. There were no significant racial/ethnic differences among the 709 callers willing to be screened for family histories, 14.4% (n = 102) of whom had a score of 6 or above and were eligible for randomization (Table 1). After exclusion of those who were eligible but declined to participate for various reasons (n = 14), 88 women were randomized (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). This group included 13 women (15%) with a personal history of breast cancer.
TABLE 1—
Racial/Ethnic Distributions of Eligible Callers, Those Agreeing to Participate, and Those at High Risk: California’s Every Woman Counts Service, 2010–2011
| Race/Ethnicity | Women Eligible for Study,a No. (%) | Women Agreeing to Participate,b No. (%) | High-Risk Women,c No. (%) |
| Hispanic | 440 (36.3) | 282 (39.8) | 26 (25.5) |
| White | 386 (31.8) | 214 (30.2) | 47 (46.1) |
| Black | 111 (9.2) | 66 (9.3) | 10 (9.8) |
| Asian/Pacific Islander | 204 (16.8) | 114 (16.1) | 13 (12.7) |
| Multiracial | 24 (2.0) | 19 (2.7) | 3 (2.9) |
| Other/unknown | 47 (3.9) | 14 (2.0) | 3 (2.9) |
| Total | 1212 (100) | 709 (100) | 102 (100) |
Eligibility criteria: Spanish or English speaker, resident of one of 6 local San Francisco Bay Area counties, at least 25 years of age.
The overall participation rate was 58%, and this rate was comparable across the major racial/ethnic groups.
The percentage of women eligible for genetic counseling was 14.4%.
As can be seen in Table 2, the sample of women entered in the trial was diverse with regard to ethnicity and educational level, was predominantly English speaking, and was equally divided among those who were and were not employed; 25% of the women were foreign-born. Ages ranged from 28 to 69 years and were similar in the 2 groups (control mean = 49.7, SD = 9.9; intervention mean = 50.2, SD = 8.3; P = .79). There were no statistically significant differences between the study arms with respect to demographic variables.
TABLE 2—
Demographic Characteristics of the High-Risk Women Randomized to the Study: Callers to California’s Every Woman Counts Service, 2010–2011
| Characteristic | Control Group, No. (%) | Intervention Group, No. (%) | Total, No. (%) | χ2 P |
| Race/ethnicity | .29 | |||
| African American | 4 (9.1) | 4 (9.1) | 8 (9.1) | |
| Asian/Pacific Islander | 3 (6.8) | 9 (20.5) | 12 (13.6) | |
| Hispanic | 11 (25.0) | 10 (22.7) | 21 (23.9) | |
| Non-Hispanic White | 22 (50.0) | 20 (45.5) | 42 (47.7) | |
| Multiracial | 1 (2.3) | 1 (2.3) | 2 (2.3) | |
| Unknown | 3 (6.8) | 0 (0.0) | 3 (3.4) | |
| Language of Interview | .73 | |||
| English | 40 (90.9) | 39 (88.6) | 79 (89.8) | |
| Spanish | 4 (9.1) | 5 (11.4) | 9 (10.2) | |
| Birthplace | .24 | |||
| United States | 34 (77.3) | 29 (65.9) | 63 (71.6) | |
| Outside United States | 10 (22.7) | 15 (34.1) | 25 (28.4) | |
| Educational level | .31 | |||
| Grade 0–8 | 3 (6.8) | 1 (2.3) | 4 (4.5) | |
| Some high school or high school graduate | 8 (18.2) | 12 (27.3) | 20 (22.7) | |
| Some college | 15 (34.1) | 16 (36.4) | 31 (35.2) | |
| College graduate | 11 (25.0) | 13 (29.6) | 24 (27.3) | |
| Graduate school | 7 (15.9) | 2 (4.6) | 9 (10.2) | |
| Employment status | .40 | |||
| Employed full time | 11 (25.0) | 6 (13.6) | 17 (19.3) | |
| Employed part time | 10 (22.7) | 11 (25.0) | 21 (23.9) | |
| Not employed | 23 (52.3) | 27 (61.4) | 50 (56.8) | |
| Income level | .70 | |||
| > 200% of PL | 2 (4.5) | 2 (4.5) | 4 (4.5) | |
| ≤ 200% of PL | 39 (88.6) | 36 (81.8) | 75 (85.2) | |
| Don’t know | 1 (2.3) | 1 (2.3) | 2 (2.3) | |
| Question not asked | 2 (4.5) | 5 (11.4) | 7 (8.0) | |
| Health insurance coverage | .07 | |||
| No | 30 (68.2) | 34 (77.3) | 64 (72.7) | |
| Yes | 9 (20.5) | 2 (4.5) | 11 (12.5) | |
| Question not asked | 5 (11.4) | 8 (18.2) | 13 (14.8) |
Note. PL = poverty line. The sample size was n = 88.
The interim analysis, based on the first 72 participants enrolled, showed that 35.3% of the intervention group members and 5.3% of the control group members obtained counseling during the intervention period (P < .001, 1 sided). Because the P value was less than the prespecified interim analysis level of .003, enrollment was discontinued. A significantly greater proportion of women in the intervention group (for which there was an immediate offer of a genetic counseling appointment) than in the control (delayed intervention) group obtained counseling during the intervention period (38.6% vs 4.5%; P < .001; difference in percentages: 95% CI = 12.0%, 54.2%). Final trial outcomes are summarized in Table 3.
TABLE 3—
Receipt of Genetic Counseling During and After the Intervention Period: Callers to California’s Every Woman Counts Service, 2010–2011
| Group and Type of Counseling | Counseled During Intervention Period, No. (%) | Counseled After Intervention Period, No. (%) | Total Counseled, No. (%) | Not Counseled, No. (%) |
| Control group (n = 44) | 2 (4.5) | 19 (43.2) | 21 (47.7) | 23 (52.3) |
| In person | 1 (50.0) | 4 (21.1) | 5 (23.8) | |
| Telephone | 1 (50.0) | 15 (78.9) | 16 (76.2) | |
| Intervention group (n = 44) | 17 (38.6) | 13 (29.6) | 30 (68.2) | 14 (31.8) |
| In person | 12 (70.6) | 3 (25.0) | 15 (51.7) | |
| Telephone | 5 (29.4) | 9 (75.0) | 14 (48.3) | |
| Total | 19 (21.6) | 32 (36.4) | 51 (58.0) | 37 (42.0) |
Note. Type of counseling (telephone or in person) was missing for 1 intervention group participant. χ2 P = .001 for comparison of study arms with respect to final counseling status (counseled during the intervention period, counseled after the intervention period, not counseled).
An additional 36% of the participants obtained counseling when called after the 2-month intervention period. This was the first call for the control group, 43% of whom then obtained counseling, and the second for members of the intervention group who had not yet been counseled, 30% of whom were subsequently counseled. In all, 58% of the participants received genetic counseling. Again, there was no significant difference according to race/ethnicity in receipt of counseling.
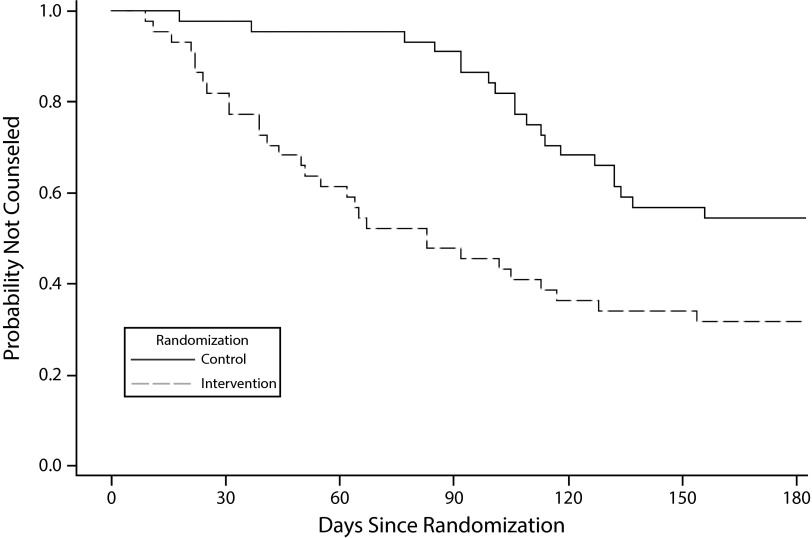
Figure 1 shows a Kaplan–Meier survival curve illustrating the probability of receiving counseling over time for each of the study arms. At the start of the intervention period, none of the women had been counseled. Moving forward over the 2-month intervention window (up to 60 days), the proportion of intervention group women not counseled dropped consistently and quite rapidly. By contrast, the proportion in the comparison group remained relatively stable until 85 days, when it began a similar drop. The intervention group continued to decline during that period, albeit more slowly than the control group, until both arms leveled off. The percentage of women counseled within 60 days of being offered counseling was similar among those in the intervention group (39%) and those in the control group (36%; data not shown).
FIGURE 1—
Probability of Not Being Counseled Over Time, by Study Arm: Callers to California’s Every Woman Counts Service, 2010–2011 (n = 88)
Other findings of note include the fact that 60% of women who were counseled received counseling via telephone. In addition, among the 51 women counseled, 5 were offered and accepted genetic testing. All of the results of these tests were negative.
DISCUSSION
In this study, we addressed disparities in services related to the rare but serious condition of HBOC. Low-income women throughout the country have had little or no access to risk assessment and risk reduction information and services, despite the fact that they are as likely as those who are more affluent and educated to carry gene mutations associated with an extremely high risk of breast and ovarian cancer. Most of the research exploring ascertainment of BRCA mutation carriers and awareness and use of genetic counseling in these populations has been conducted in clinical settings targeting women already affected by breast cancer.
In our randomized delayed intervention control trial, we were able to identify high-risk but predominantly unaffected women among callers seeking referrals to free cancer screening. We compared an intervention involving an immediate offer of a genetic counseling appointment with one consisting of information on HBOC sent by mail. Our findings demonstrated that, when a diverse population of low-income women call a trusted information and referral source, it is possible to both engage them in a topic that is different from the purpose of their call and recruit them for participation in research. The ethnic distribution of the women in our study who agreed to participate was similar to the distribution among EWC callers overall (Table 1). Numbers of high-risk women varied according to ethnic group at rates that generally corresponded with the incidence of breast cancer in these populations.
Our intervention was designed to fit as seamlessly as possible with the efforts of 2 end-user organizations. Existing staff (information specialists) of California’s EWC telephone service administered a simple family history screener similar to their usual procedure for assessing women’s eligibility for a free mammography. CRP genetic counseling assistants followed up with an outcall, just as they do normally in response to family history screeners filled out in the hospital’s mammography clinic. Not only did our results show a significantly larger effect on use of genetic counseling with the call and appointment offer than with a mailed brochure, but it was clear from calls made to women in both study arms after the intervention period that calling is both effective and essential in encouraging use of genetic counseling in this population. The number of women counseled in the intervention group was initially much higher than the number in the control group, and additional intervention group members obtained counseling when called a second time, after the 2-month intervention period.
The Kaplan–Meier analysis showed that the delayed intervention yielded essentially the same outcomes as the initial intervention. The proportion of intervention group women not counseled dropped over 60 days, after which the proportion for women in the control group began to drop when they were called for the follow-up survey and then offered counseling if they had not yet received it. Intervention women continued to receive counseling after 60 days. Some had scheduled an appointment during the intervention period but did not complete it within 2 months, and others may have simply put it off until the follow-up survey, when they were again offered the appointment. The likely reason that members of the control group did not catch up is that they were offered counseling only once.
The addition of genetic counseling by telephone enabled a substantial number of women to obtain counseling who would not have done so otherwise. The importance of telephone communication among low-income populations has been demonstrated in research on numerous health issues. In a review of interventions designed to increase repeat mammography, Vernon et al. noted that phone and in-person counseling strategies represent the most personalized forms of contact because of the opportunity for dialogue.15 Our study demonstrated that, in the case of the complex and highly personal issue of HBOC, more than one attempt is needed and justified.
Low-income women have been screened for family history of cancer and referred to genetic counseling in a small number of low-resource clinical settings,29 and a limited amount of research has been conducted.30,31 Mays et al. employed a graduate-level patient navigator to assist clinic patients in completing a detailed family history screener, and their results showed that 65 of 2436 patients were eligible for genetic counseling.30 Of these individuals, 47 (72%) were interested in counseling, 27 (57%) scheduled a counseling appointment, and 14 (11%) completed counseling. The intervention was deemed a success in that almost one fourth of interested patients were navigated to counseling.
Our study involved a simpler screener and thus identified a higher proportion of women as eligible for counseling. Importantly, our process of having information specialists with a high school–level education screen women as they called the EWC phone service may be a more efficient approach. In addition, our inclusion of a follow-up call by a genetic counseling assistant trained at the college level, also an existing process, yielded a much higher rate of genetic counseling (57%). Of the women in our study who received counseling, 10% were eligible for genetic testing, a rate slightly higher than that found in other predominantly unaffected populations.29,32 Although none of these women tested positive, this prevalence also falls within the range of what is expected given that the likelihood of a positive test in such a population is 10%.33
Limitations
Our study involved limitations. Members of our intended audience of callers seeking cancer screening were activated to protect themselves, at least with regard to early detection, and probably do not reflect the general population in terms of willingness to participate in research or to obtain genetic counseling. Eligibility was restricted to English and Spanish speakers, so we do not know what the impact would be among women who speak other languages. In addition, we targeted a small geographic area, and women in other regions may respond differently.
Conclusions
We recognize that a sustainable intervention must emphasize practicality and relevance in real-world contexts.34 Toward this end, we collaborated closely in the development of the intervention and the research with 2 organizations (EWC and CRP) wherein low-income women already obtain services in large numbers. Together, we sought to maximize the fit of the intervention with the existing structures and processes of these end-user organizations. These principles seem particularly relevant for the relatively rare but serious condition of HBOC, which clearly warrants surveillance and intervention in ways that may prove instructive for other challenges in the rapidly evolving realm of genetic risk. Rather than equitable access to precision medicine advances as an afterthought, innovations in discovery should be matched with efficient forms of delivery so that scientific progress can benefit all.
ACKNOWLEDGMENTS
This study was funded by the National Cancer Institute (grant 1R01CA129096). We also acknowledge the Avon Foundation and the National Breast Cancer Foundation for providing resources to offer genetic counseling and testing services to low-income women.
We thank the Every Woman Counts information specialists, without whom we would not have been able to identify the participants in our study, and Rachel Farrell, who served as the genetic counseling assistant and contributed to the success of the study.
HUMAN PARTICIPANT PROTECTION
This study was approved by the institutional review boards of the University of California, San Francisco, and the Cancer Prevention Institute of California. Participants provided verbal informed consent.
REFERENCES
- 1.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, editors. SEER cancer statistics review, 1975–2012. Available at: http://seer.cancer.gov/csr/1975_2012. Accessed July 6, 2016. [Google Scholar]
- 3.Cragun D, Pal T. Identification, evaluation, and treatment of patients with hereditary cancer risk within the United States. ISRN Oncol. 2013;2013:260847. doi: 10.1155/2013/260847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Society of Genetic Counselors. 2012 Professional Status Survey: executive summary. Available at: http://nsgc.org/p/cm/ld/fid=68. Accessed July 6, 2016.
- 5.Pal T, Radford C, Vadaparampil S, Prince A. Practical considerations in the delivery of genetic counseling and testing services for inherited cancer predisposition. Community Oncol. 2013;10(5):147–153. [Google Scholar]
- 6.Pal T, Bonner D, Kim J et al. Early onset breast cancer in a registry-based sample of African-American women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J. 2013;19(2):189–192. doi: 10.1111/tbj.12083. [DOI] [PubMed] [Google Scholar]
- 7.Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15(suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 8.Allford A, Qureshi N, Barwell J, Lewis C, Kai J. What hinders minority ethnic access to cancer genetics services and what may help? Eur J Hum Genet. 2014;22(7):866–874. doi: 10.1038/ejhg.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez AG, Aparicio-Ting FE, DeMajors SS, Miller AR. Interest, awareness, and perceptions of genetic testing among Hispanic family members of breast cancer survivors. Ethn Dis. 2006;16(2):398–403. [PubMed] [Google Scholar]
- 10.Vadaparampil ST, Quinn GP, Small BJ et al. A pilot study of hereditary breast and ovarian knowledge among a multiethnic group of Hispanic women with a personal or family history of cancer. Genet Test Mol Biomarkers. 2010;14(1):99–106. doi: 10.1089/gtmb.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbert CH, Kessler L, Collier A et al. Low rates of African American participation in genetic counseling and testing for BRCA1/2 mutations: racial disparities or just a difference? J Genet Couns. 2012;21(5):676–683. doi: 10.1007/s10897-012-9485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 13.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 14.John EM, Miron A, Gong G et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 15.Vernon SW, McQueen A, Tiro JA, Del Junco DJ. Interventions to promote repeat breast cancer screening with mammography: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102(14):1023–1039. doi: 10.1093/jnci/djq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow RE, Green LW, Taylor MV, Stange KC. An evidence integration triangle for aligning science with policy and practice. Am J Prev Med. 2012;42(6):646–654. doi: 10.1016/j.amepre.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.California Department of Health Care Services. Every Woman Counts. Available at: http://www.dhcs.ca.gov/services/Cancer/ewc/Pages/default.aspx. Accessed July 6, 2016.
- 18.Joseph G, Kaplan CP, Pasick RJ. Recruiting low-income healthy women to research: an exploratory study. Ethn Health. 2007;12(5):497–519. doi: 10.1080/13557850701616961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph G, Kaplan C, Luce J et al. Efficient identification and referral of low-income women at high risk for hereditary breast cancer: a practice-based approach. Public Health Genomics. 2012;15(3–4):172–180. doi: 10.1159/000336419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580–1590. doi: 10.1038/sj.bjc.6602175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DG, Eccles DM, Rahman N et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474–480. doi: 10.1136/jmg.2003.017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank TS, Deffenbaugh AM, Reid JE et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 23.Lindor NM, Johnson KJ, Harvey H et al. Predicting BRCA1 and BRCA2 gene mutation carriers: comparison of PENN II model to previous study. Fam Cancer. 2010;9(4):495–502. doi: 10.1007/s10689-010-9348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teller P, Hoskins KF, Zwaagstra A et al. Validation of the Pedigree Assessment Tool (PAT) in families with BRCA1 and BRCA2 mutations. Ann Surg Oncol. 2010;17(1):240–246. doi: 10.1245/s10434-009-0697-9. [DOI] [PubMed] [Google Scholar]
- 25.Bellcross CA, Lemke AA, Pape LS, Tess AL, Meisner LT. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11(11):783–789. doi: 10.1097/GIM.0b013e3181b9b04a. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MD, Valdimarsdottir HB, Peshkin BN et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32(7):618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinney AY, Butler KM, Schwartz MD et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst. 2014;106(12):dju328. doi: 10.1093/jnci/dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 29.Lee R, Beattie M, Crawford B et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9(4):306–312. doi: 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- 30.Mays D, Sharff ME, DeMarco TA et al. Outcomes of a systems-level intervention offering breast cancer risk assessments to low-income underserved women. Fam Cancer. 2012;11(3):493–502. doi: 10.1007/s10689-012-9541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph G, Beattie MS, Lee R et al. Pre-counseling education for low literacy women at risk of hereditary breast and ovarian cancer (HBOC): patient experiences using the Cancer Risk Education Intervention Tool (CREdIT) J Genet Couns. 2010;19(5):447–462. doi: 10.1007/s10897-010-9303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes KS, Roche C, Campbell CT et al. Prevalence of family history of breast and ovarian cancer in a single primary care practice using a self-administered questionnaire. Breast J. 2003;9(1):19–25. doi: 10.1046/j.1524-4741.2003.09105.x. [DOI] [PubMed] [Google Scholar]
- 33.Christinat A, Pagani O. Practical aspects of genetic counseling in breast cancer: lights and shadows. Breast. 2013;22(4):375–382. doi: 10.1016/j.breast.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Green LW, Nassar M. Furthering dissemination and implementation research: the need for more attention to external validity. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and Implementation Research in Health: Translating Science to Practice. Oxford, England: Oxford University Press; 2012. pp. 305–326. [Google Scholar]