Abstract
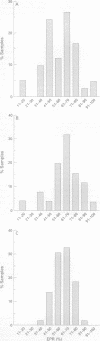
AIM--To investigate the avidity of specific IgG polyribosyl ribitol phosphate (PRP) antibodies induced by three Haemophilus influenzae type b (Hib) conjugate vaccines: PRP-meningococcal outer membrane protein complex (PRP-OMP), PRP-non-toxic mutant diphtheria toxin, CRM197 (HbOC) and PRP-tetanus toxoid (PRP-T). METHODS--One hundred and ten infants were immunised with one of the vaccines at two, three and four months of age. Blood samples were taken after each vaccination and serum stored at -20 degrees C. Serum samples collected after the third course (that is, at five months of age) were submitted to antibody avidity assessment, using a urea enzyme linked immunosorbent assay (ELISA). RESULTS--All three conjugate vaccines elicited IgG PRP antibodies of high median avidity. The resultant antibody populations were heterogeneous with regard to avidity, which in turn was independent of PRP antibody concentration. CONCLUSIONS--With the recent findings of a correlation between bactericidal killing and affinity, our data highlight the need for a protective level to be expressed qualitatively as well as quantitatively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984 Jun;149(6):1034–1035. doi: 10.1093/infdis/149.6.1034. [DOI] [PubMed] [Google Scholar]
- Black S. B., Shinefield H. R., Fireman B., Hiatt R. Safety, immunogenicity, and efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b vaccine in a United States population: possible implications for optimal use. J Infect Dis. 1992 Jun;165 (Suppl 1):S139–S143. doi: 10.1093/infdis/165-supplement_1-s139. [DOI] [PubMed] [Google Scholar]
- Black S. B., Shinefield H. R. Immunization with oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine on a large health maintenance organization population: extended follow-up and impact on Haemophilus influenzae disease epidemiology. The Kaiser Permanente Pediatric Vaccine Study Group. Pediatr Infect Dis J. 1992 Aug;11(8):610–613. [PubMed] [Google Scholar]
- Booy R., Aitken S. J., Taylor S., Tudor-Williams G., Macfarlane J. A., Moxon E. R., Ashworth L. A., Mayon-White R. T., Griffiths H., Chapel H. M. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet. 1992 Feb 29;339(8792):507–510. doi: 10.1016/0140-6736(92)90336-2. [DOI] [PubMed] [Google Scholar]
- Claesson B. A., Schneerson R., Robbins J. B., Johansson J., Lagergard T., Taranger J., Bryla D., Levi L., Cramton T., Trollfors B. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugate. J Pediatr. 1989 Jan;114(1):97–100. doi: 10.1016/s0022-3476(89)80611-0. [DOI] [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Asmar B. I., Thirumoorthi M. C. Systemic Haemophilus influenzae disease: an overview. J Pediatr. 1979 Mar;94(3):355–364. doi: 10.1016/s0022-3476(79)80571-5. [DOI] [PubMed] [Google Scholar]
- Eilat D. Anti-DNA antibodies: problems in their study and interpretation. Clin Exp Immunol. 1986 Aug;65(2):215–222. [PMC free article] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Fritzell B., Plotkin S. Efficacy and safety of a Haemophilus influenzae type b capsular polysaccharide-tetanus protein conjugate vaccine. J Pediatr. 1992 Sep;121(3):355–362. doi: 10.1016/s0022-3476(05)81786-x. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Anderson E. L., Osterholm M. T., Holmes S. J., McHugh J. E., Belshe R. B., Medley F., Murphy T. V. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992 Aug;121(2):187–194. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Chacko A., Lottenbach K. R., Sheetz K. E. Immunogenicity of Haemophilus influenzae type b polysaccharide-outer membrane protein conjugate vaccine in patients who acquired Haemophilus disease despite previous vaccination with type b polysaccharide vaccine. J Pediatr. 1989 Jun;114(6):925–933. doi: 10.1016/s0022-3476(89)80432-9. [DOI] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Hedman K., Lappalainen M., Seppäiä I., Mäkelä O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989 Apr;159(4):736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- Hetherington S. V., Lepow M. L. Correlation between antibody affinity and serum bactericidal activity in infants. J Infect Dis. 1992 Apr;165(4):753–756. doi: 10.1093/infdis/165.4.753. [DOI] [PubMed] [Google Scholar]
- Hetherington S. V., Rutkowski A. F. Antibody affinity in infants after immunization with conjugated capsular polysaccharide from Haemophilus influenzae type b. J Infect Dis. 1990 Nov;162(5):1185–1188. doi: 10.1093/infdis/162.5.1185. [DOI] [PubMed] [Google Scholar]
- Joynson D. H., Payne R. A., Rawal B. K. Potential role of IgG avidity for diagnosing toxoplasmosis. J Clin Pathol. 1990 Dec;43(12):1032–1033. doi: 10.1136/jcp.43.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Peltola H., Karanko V., Mäkelä P. H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983 Jun;147(6):1100–1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Schneerson R., Sutton A. Class-specific antibody response to Haemophilus influenzae type b capsular polysaccharide vaccine. J Infect Dis. 1983 Oct;148(4):767–767. doi: 10.1093/infdis/148.4.767. [DOI] [PubMed] [Google Scholar]
- Lucas A. H., Granoff D. M. A major crossreactive idiotype associated with human antibodies to the Haemophilus influenzae b polysaccharide. Expression in relation to age and immunoglobulin G subclass. J Clin Invest. 1990 Apr;85(4):1158–1166. doi: 10.1172/JCI114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger Y., Granoff D. M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992 Mar 18;267(11):1489–1494. [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Tudor-Williams G., Frankland J., Isaacs D., Mayon-White R. T., MacFarlane J. A., Slack M. P., Anderson E., Rees D. G., Moxon E. R. Haemophilus influenzae type b disease in the Oxford region. Arch Dis Child. 1989 Apr;64(4):517–519. doi: 10.1136/adc.64.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]