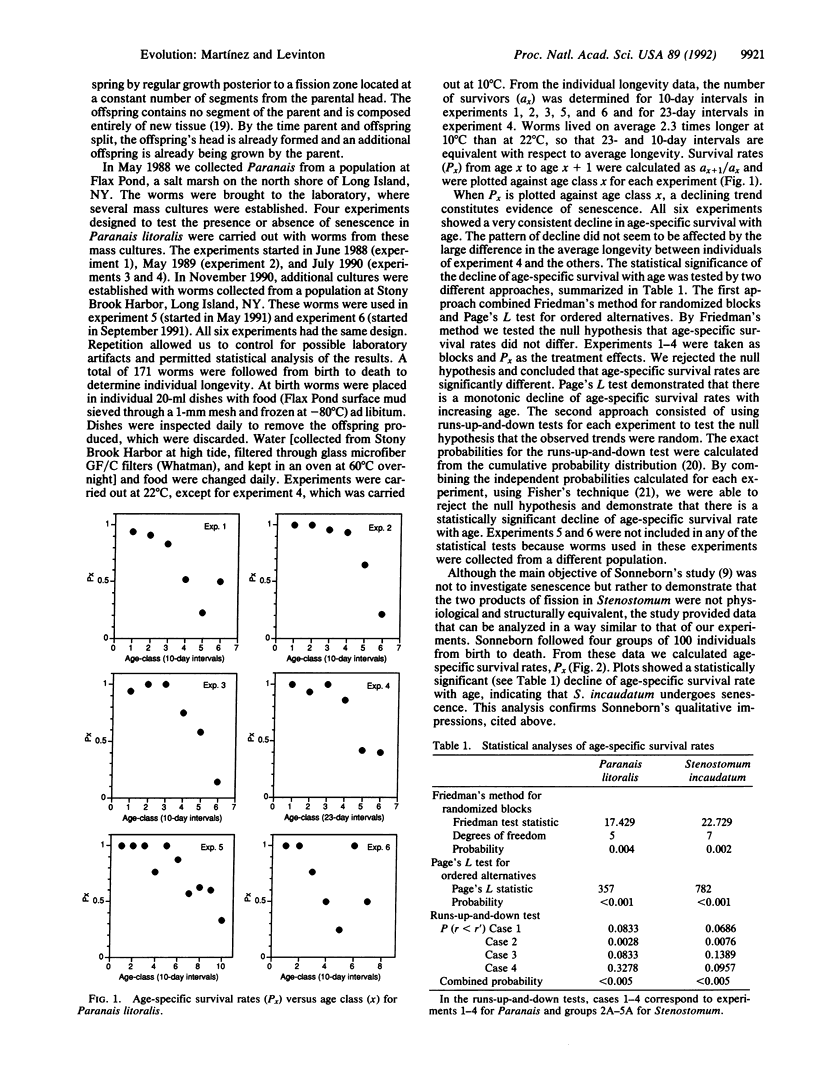
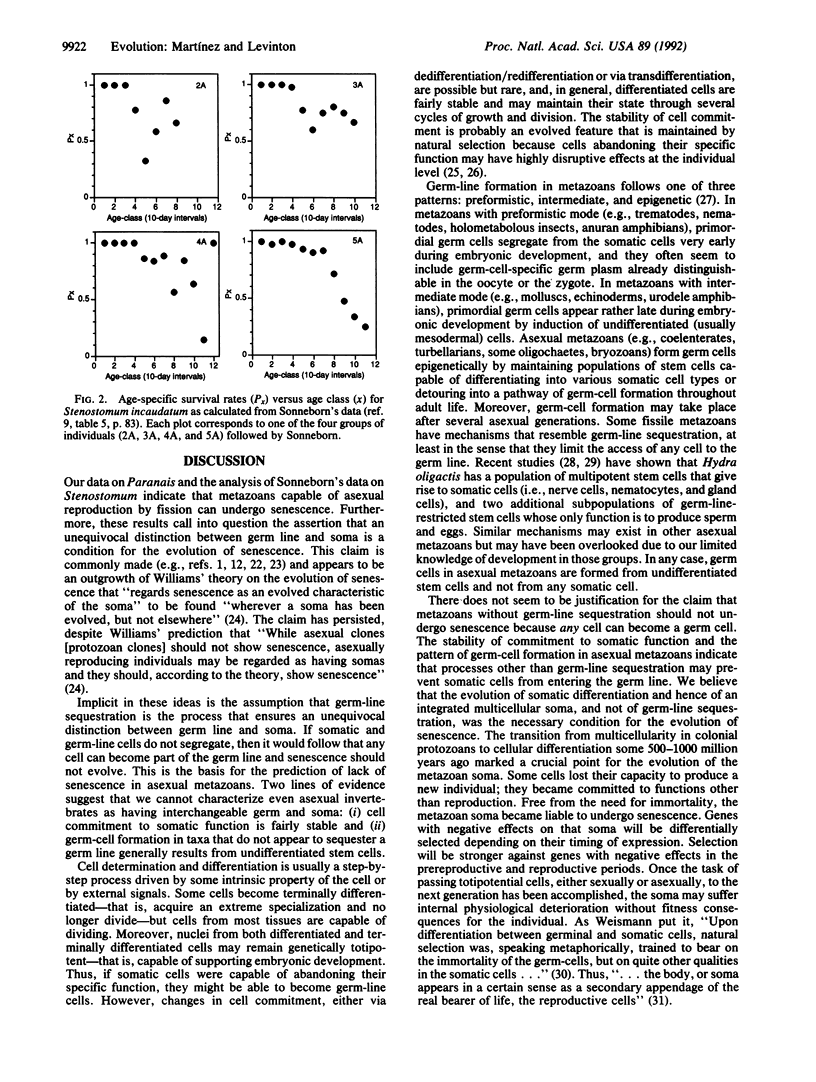
Abstract
August Weismann popularized the notion that metazoans have a potentially immortal germ line separated from a mortal soma, and evolutionary biologists regard senescence as an evolved characteristic of the soma. Many have claimed that metazoans that do not sequester their germ line have no clear distinction between germ line and soma, and consequently they should lack senescence. Here we present experimental evidence that senescence occurs in the asexually reproducing marine oligochaete Paranais litoralis. We also analyze data reported in Sonneborn's classical study and show that the rhabdocoel Stenostomum incaudatum undergoes senescence. We argue that the stability of commitment to somatic function and the fact that asexual metazoans form their germ cells from undifferentiated stem cells are sufficient to allow for senescence of the asexual metazoan's soma. Thus the evolution of somatic differentiation, and not germ-line sequestration, would be the necessary condition for the evolution of senescence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buss L. W. Evolution, development, and the units of selection. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1387–1391. doi: 10.1073/pnas.80.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. P., Jackson J. B. Do corals lie about their age? Some demographic consequences of partial mortality, fission, and fusion. Science. 1980 Aug 8;209(4457):713–715. doi: 10.1126/science.209.4457.713. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. B., Cremer T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum Genet. 1982;60(2):101–121. doi: 10.1007/BF00569695. [DOI] [PubMed] [Google Scholar]
- Littefield C. L. Cell lineages in Hydra: isolation and characterization of an interstitial stem cell restricted to egg production in Hydra oligactis. Dev Biol. 1991 Feb;143(2):378–388. doi: 10.1016/0012-1606(91)90088-k. [DOI] [PubMed] [Google Scholar]
- Littlefield C. L., Bode H. R. Germ cells in Hydra oligactis males. II. Evidence for a subpopulation of interstitial stem cells whose differentiation is limited to sperm production. Dev Biol. 1986 Aug;116(2):381–386. doi: 10.1016/0012-1606(86)90140-5. [DOI] [PubMed] [Google Scholar]
- TURNER C. L. The reproductive potential of a single clone of Pelmatohydra oligactis. Biol Bull. 1950 Oct;99(2):285–299. doi: 10.2307/1538744. [DOI] [PubMed] [Google Scholar]



