Abstract
Chronic obstructive pulmonary disease (COPD) with hypercapnia is associated with increased mortality. Non-invasive ventilation (NIV) can lower hypercapnia and ventilator loads but is hampered by a low adherence rate leaving a majority of patients insufficiently treated.
Recently, nasal high flow (NHF) has been introduced in the acute setting in adults, too. It is an open nasal cannula system for delivering warm and humidified air or oxygen at high flow rates (2–50 L/min) assisting ventilation. It was shown that this treatment can improve hypercapnia. The mechanism of reducing arterial carbon dioxide (CO2) is proposed through a reduction in nasal dead space ventilation, but there are no studies in which dead space volume was measured in spontaneously breathing subjects. In our case report we measured in a tracheostomized COPD patient CO2 and pressure via sealed ports in the tracheostomy cap and monitored transcutaneous CO2 and tidal volumes. NHF (30 L/min mixed with 3 L/min oxygen) was administered repeatedly at 15-minutes intervals. Inspired CO2 decreased instantly with onset of NHF, followed by a reduction in transcutaneous/arterial CO2. Minute ventilation on nasal high flow was also reduced by 700 ml, indicating that nasal high flow led to a reduction of dead space ventilation thereby improving alveolar ventilation.
In conclusion, NHF assist ventilation through clearance of anatomical dead space, which improves alveolar ventilation. Since the reduction in hypercapnia was similar to that reported with effective NIV treatment NHF may become an alternative to NIV in hypercapnic respiratory failure.
Keywords: Chronic obstructive pulmonary disease, Nasal high flow, Hypercapnic respiratory failure, Dead space ventilation
1. Introduction
An open nasal cannula system for delivering warm and humidified air or oxygen at higher flow rates (nasal high flow (NHF), 2–50 L/min) has been shown to assist ventilation and outcomes in the acute setting in adults and in children. Recently, NHF has been introduced for respiratory support in ICU settings, mainly in patients with acute hypoxemic respiratory failure [3], [6], [12], but some demonstrating an improvement in hypercapnia and clinical outcomes, too [6], [10], [11]. The mechanisms of reducing arterial CO2 is proposed through a reduction in nasal dead space ventilation [8], [9], but there are no studies in spontaneously breathing subjects. We therefore, measured tracheal CO2 on and off NHF in a spontaneously breathing patient with tracheostomy.
2. Case presentation
A 62-year-old man (smoker, 60 pack years) with severe chronic obstructive pulmonary disease (COPD GOLD IV) (FEV1: 0,8 L/min), cachexia (BMI 19,8 kg/m2), oxygen dependency (2L/min) and hypercapnic respiratory failure with arterial CO2 ranging from 65 to 75 mmHg was studied during wake. His current illness included critical illness polyneuro- and myopathie after he developed an acute exacerbation of COPD and was ventilated through a tracheostomy for 1 month. He regained muscle strength after neurological rehabilitation and was admitted to our pulmonary rehabilitation unit for considering decannulation of his tracheostomy tube. At the time of this study, his tracheostomy was capped with a tracheostomy button for several days and he was able to breathe spontaneously at rest during the day and continued non-invasive ventilation (NIV) with following settings: Spontaneous/Timed (S/T)-mode, inspiratory pressure 25 cmH2O, expiratory pressure 5 cm H2O, backup rate 15/min.
3. Methods
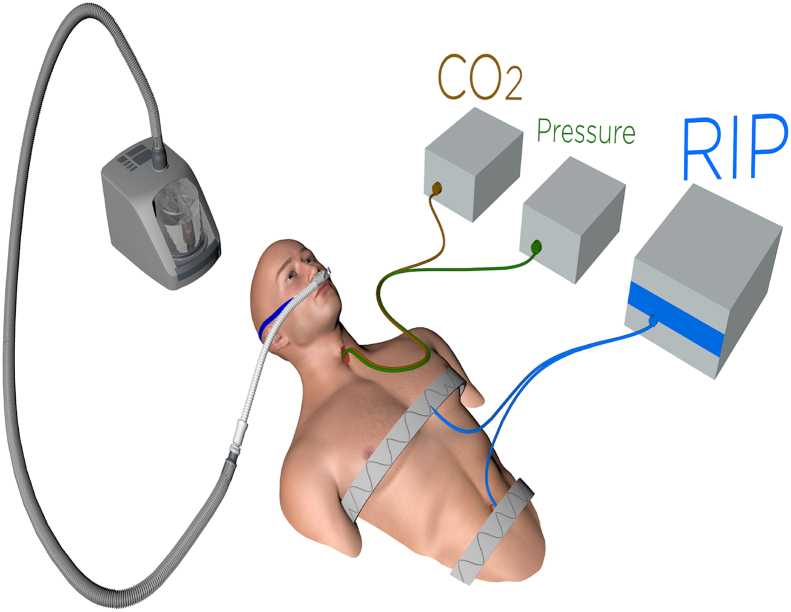
The patient was instrumented with intra-tracheal catheters for CO2 and pressure (PTRACH) measurements via sealed ports in the tracheostomy cap (Fig. 1). Tidal volume (VT) was measured by respiratory inductive plethysmography (RIP) and arterial CO2 was monitored transcutaneously (tc CO2). Inspired CO2 was calculated from the tidal CO2 tracing and tidal volume using the modified Fowler technique as previously described [4], [2]. The study was performed while the patient was resting in a sitting semi-recumbent position breathing effortless with 2 L/min oxygen (baseline). Nasal high flow (NHF 30 L/min mixed with 3 L/min O2 to match his FIO2 requirements) was administered repeatedly at 15-minutes intervals. Due to the higher inspiratory flow with NHF a higher oxygen concentration (3L/min) is required to achieve the same FIO2.
Fig. 1.
View of study setting: intra-tracheal catheters for CO2 and pressure (PTRACH) measurements, tidal volume (VT) was measured by respiratory inductive plethysmography (RIP) and CO2 was monitored transcutaneously (tcCO2). High flow was delivered via nasal cannula.
4. Results
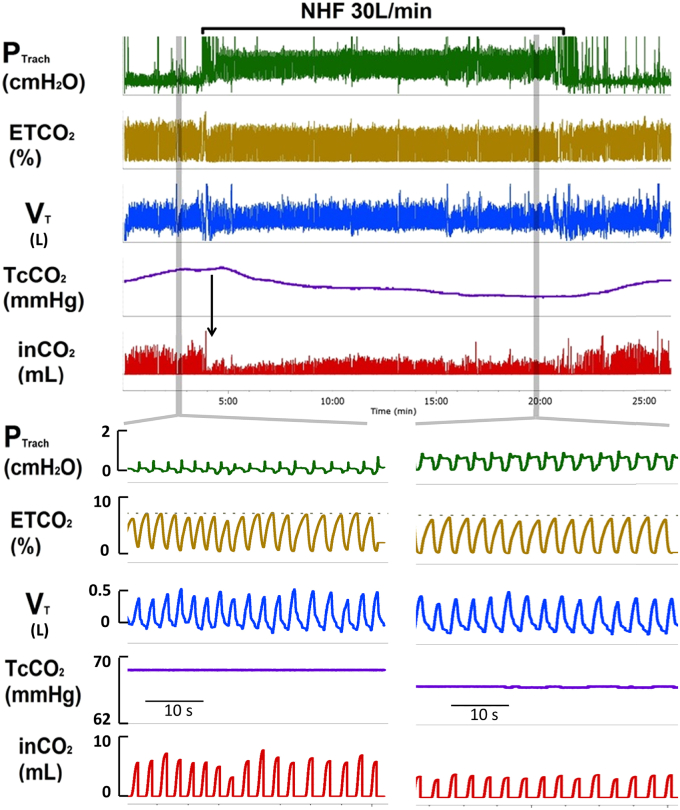
The effects of NHF on ventilation and arterial blood gases compared to oxygen are shown in Fig. 2. The top panel shows a 25 minute overview of experimental setup: tracheal pressure (PTRACH), end tidal tracheal CO2 (ET CO2), tidal volume (VT), transcutaneous CO2 (Tc CO2), inspired CO2, (in CO2). Grey bars highlight snapshots of 40 seconds at baseline (left lower panel) and NHF (right lower panel). As can be seen, NHF increased end-expiratory pressure (PEEP) by 1 cm H2O, reduced inspiratory CO2 from 6 ± 1 to 3 ± 0,5 ml, and tcCO2 from 68 to 63 mmHg. The reduction in inCO2 occurred instantly with onset of NHF (see vertical arrow in Fig. 2 upper panel) when tcCO2 remained unchanged. While tidal volume remained similar at 290 ± 15 ml, there was a reduction in respiratory rate from 26/min to 22/min leading to a reduction in minute ventilation from 7,2–6.5 L/min (by 700 ml). Based on the mass balance equation shown below, a reduction in minute ventilation (VE) should have increased arterial CO2. Rather, we show a decrease in tcCO2, which can only be explained by a reduction in dead space ventilation (VD) exceeding 700 ml/min. Given a respiratory rate of 22/min the NHF must have led to a reduction in dead space ventilation (VD) of more than 30 mls per breath.
Fig. 2.
Increase in tracheal pressure (PTRACH) due to NHF was associated with a decrease in transcutaneous (Tc) CO2 and inspired (in) CO2 while respiratory rate was reduced. Tracheal pressure (PTRACH), end-tidal CO2 (ET CO2), tidal volume (VT), transcutaneous CO2 (TcCO2), inspired CO2, (inCO2).
5. Summary and interpretation
We assume that the reduction in inspired CO2 by 50% is likely due to a washout in CO2 of the upper airway for the following reasons: A) it occurred instantly with onset of NHF when tcCO2 remained unchanged. B) the patient reduced minute ventilation on NHF by 700 ml/min and tcCO2 decreased which can only be explained by a reduction in dead space ventilation (VD) exceeding 700 ml/min based on the mass balance equation. This reduction was sufficient to reduce ventilatory loads as best documented by a concomitant ∼20% reduction in respiratory rate.
We also show, that in our patient, transcutaneous (arterial) CO2 was lowered by 7.4% with NHF. If this reduction would remain over an extended period, e.g., sleep, this magnitude of CO2 reduction would be similar to that using non-invasive ventilation via nasal or full face mask [5], [7].
6. Clinical implications
COPD with hypercapnia is associated with increased mortality [1] and current treatment options to lower hypercapnia and ventilatory loads require extensive treatment with invasive or non-invasive ventilator support [5]. Our data suggest that NHF could be used as an alternative option to lower arterial CO2 in a subset of patients. Moreover, the clearance of anatomical dead space by NHF may be especially beneficial for subjects who have high dead space ventilation due to tachypnea or due to rapid shallow breathing pattern as in our patient. Even a small reduction of dead space would lead to a relatively high increase in alveolar volume, since the ratio of dead space to tidal volume increases during shallow breathing.
In conclusion, NHF can treat hypercapnic respiratory failure in some patients through clearance of anatomical dead space, which improves alveolar ventilation, thus leading to reduction of arterial CO2.
Mass Balance Equation: arterial carbon dioxide tension (PaCO2), carbon dioxide production (VCO2), alveolar ventilation (VA), minute ventilation (VE), dead space ventilation (VD).
Funding of this work
This work was supported by the National Institutes of Health (NIHR01HL105546) and the medical technology company Heinen und Löwenstein GmbH&Co.
Conflicting interest
Dr. Nilius, Ms. Domanski and Dr. Schneider receive grants by Fisher & Paykel. Dr. Tatkov is full time employed by Fisher & Paykel.
Acknowledgements
Authors' funding
Dr. Nilius and Ms. Domanski receive grants by Heinen und Löwenstein GmbH&Co, Resmed, Fisher & Paykel.
Dr. Franke reports personal fees from PneumRx and personal fees from Olympus.
Dr. Schneider receives grants by Heinen und LöwensteinGmbH&CO, Fisher & Paykel and National Institutes of Health (NIHR01HL105546).
Dr. Fricke reports personal fees from Heinen und Löwenstein GmbH&Co.
Dr. Tatkov is full time employed by Fisher & Paykel Healthcare and reports personal fees from Fisher & Paykel Healthcare.
References
- 1.Ahamdi Z., Bornefalk-Hermansson A., Franklin K.A., Midgren B., Ekstroem M.P. Hypo- and hypercapnia predict mortalitiy in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir. Res. 2014 Mar 13:15–30. doi: 10.1186/1465-9921-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler W.S., Comroe J.H. Lung function studies. I. The rate of increase of arterial oxygen saturation during the inhalation of 100% O2. J. Clin. Invest. 1948 May;27:327–334. doi: 10.1172/JCI101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frat J.P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., Prat G., Boulain T., Morawiec E., Cottereau A., Devaquet J., Nseir S., Razazi K., Mira J.P., Argaud L., Chakarian J.C., Ricard J.D., Wittebole X., Chevalier S., Herbland A., Fartoukh M., Constantin J.M., Tonnelier J.M., Pierrot M., Mathonnet A., Béduneau G., Delétage-Métreau C., Richard J.C., Brochard L., Robert R. FLORALI Study Group and the REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015 Jun 4;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 4.Kars A.H., Bogaard J.M., Stijnen T., de Vries J., Verbraak AF,Hilvering C. Dead space and slope indices from the expiratory carbon dioxide tension-volume curve. Eur. Respir. J. 1997 Aug;10:1829–1836. doi: 10.1183/09031936.97.10081829. [DOI] [PubMed] [Google Scholar]
- 5.Koehlein T., Windisch W., Koehler D., Drabik A., Geiseler J., Hartl Szlvia, Karg O., Laier-Groeneveld G., Stefano N., Schoenhofer B., Schucher B., Wegschweider K., Cree A.P., Welte T. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomized, controlled clinical trial. Lancet Respir. Med. 2014 Sep;2(9):698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 6.Maggiore S.M., Idone F.A., Vaschetto R., Festa R., Cataldo A., Antonicelli F., Montini L., De Gaetano A., Navalesi P., Antonell M. Nasal high-flow versus Venturi mask oxygen therapy after extubation Effects on oxygenation, comfort, and clinical outcome. Am. J. Respir. Crit. Care Med. 2014 Aug;190(3):282–288. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 7.McEvoy R.D., Pierce R.J., Hillman D., Esterman A., Ellis E.E., Catcheside P.G., O'Donoghue F.J., Barnes D.J., Grunstein R.R. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009 Jul;64(7):561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 8.Möller W., Celik G., Feng S., Bartenstein P., Meyer G., Eickelberg O., Schmid O., Tatkov S. Nasal high flow clears anatomical dead space in upper airway models. J. Appl. Physiol. 2015 Jun 15;118(12):1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mündel T., Feng S., Tatkov S., Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J. Appl. Physiol. 1985;114(8):1058–1065. doi: 10.1152/japplphysiol.01308.2012. 2013 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilius G., Franke K.J., Domanski U., Rühle K.H., Kirkness J.P., Schneider H. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Adv. Exp. Med. Biol. 2013;755:27–34. doi: 10.1007/978-94-007-4546-9_4. [DOI] [PubMed] [Google Scholar]
- 11.Storgaard L., Frystyk M., Hockey H., Weinrich U.M. Poster of Aalborg University Hospital, presented at International Congress; Munich, Germany: 2014. Number of Exacerbations in COPD Patients Treated with a Nasal High Flow Heated and Humidified Oxygen. [Google Scholar]
- 12.Sztrymf B., Messika J., Mayot T., Lenglet H., Dreyfuss D., Ricard J.-D. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J. Crit. Care. 2012 Jun;27(3) doi: 10.1016/j.jcrc.2011.07.075. 324.e9-13. [DOI] [PubMed] [Google Scholar]