Abstract
Background
Babies born to adolescent mothers have been shown to have poorer outcomes compared to those born to adults. Nutritional status may have an important role to play in improving the health of pregnant adolescents; however there is a lack of evidence regarding the adequacy of adolescent diets during pregnancy. This systematic review aims to examine what is known about the nutritional status of adolescent pregnant women.
Methods
A systematic search of the literature identified 21 studies which met the inclusion criteria for the review. Primary research papers using any methods were included where they were published in English between January 1995 and May 2015 and included measurements of nutrient intakes or biological markers of nutritional status in pregnant women aged 11–19 years. Individual study data was first summarised narratively before study means were pooled to give an estimate of nutritional status in the population.
Results
The results show that individual studies reported intakes of energy, fibre and a number of key micronutrients which were below recommended levels. Biological markers of iron and selenium status also showed cause for concern. Pooled analysis of individual means as a percentage of UK Dietary Reference Intakes showed intakes of vitamin D (34.8 % CI 0–83.1) to be significantly below recommendations (p = 0.05). Serum selenium levels were also found to be low (61.8 μg/L, CI 39–84).
Conclusions
This review has identified a number of areas where the nutritional status of pregnant adolescents is sub-optimal, which may have implications for the health of adolescent mothers and their babies. It was not however possible to examine the impact of supplement use or socio-demographic characteristics which limits the interpretation these results. Further work is needed to establish the characteristics of those most at risk within this population, how this differs from adult pregnant women and the role of supplementation in achieving adequate nutrition.
Electronic supplementary material
The online version of this article (doi:10.1186/s12884-016-1059-9) contains supplementary material, which is available to authorized users.
Keywords: Adolescent, Pregnancy, Nutrition, Systematic review
Background
Pregnancy during adolescence is often viewed as a social problem with women who have a child during the teenage years being more likely to suffer social isolation, poverty, lower levels of educational achievement and be unemployed or work in low paid jobs [1]. Rates of teenage conceptions both in the UK and internationally have reduced over recent years; however there are still a significant number of young women having pregnancies and giving birth at a young age. The rate of deliveries to young women aged 15–19 in the UK in 2012 was the highest in the European Union at 19.7 births per 1,000 females in the age group. This does however represent a reduction of more than a quarter (26.8 %) in the UK since 2004. The birth rates to young women in other developed countries have followed a similar pattern of decline, yet rates remain relatively high in the United States (29.4), New Zealand (24.9) and Australia (16.1) [2].
As well as the potential for adverse social outcomes associated with adolescent pregnancies there is evidence to suggest that health outcomes may be less favourable for younger mothers. A systematic review [3] aiming to assess the relationship between early first childbirth and increased risk of poor pregnancy outcomes found that very young maternal age (<15 years or less than 2 years after menarche) had a negative effect on both maternal and foetal growth and infant survival. It is suggested that young women who are still themselves growing may compete with the foetus for nutrients, which may in turn impair foetal growth and result in low birth weight babies or babies who are small for their gestational age. The review also found a moderate relationship between young maternal age and anaemia, premature birth and neonatal mortality.
It has long been established that good pregnancy nutrition has an important influence on birth outcomes, foetal growth and infant survival [4]. While specific nutritional issues may have changed since this early work, it is still maintained that mothers need to consume an adequate, yet not excessive diet in order to optimise pregnancy and birth outcomes [5]. Quantification of dietary adequacy in populations is difficult because individuals will have differing nutrient needs. This is especially true during phases of growth and physical change such as adolescence. However, the use of dietary reference intakes to estimate the adequacy of nutritional intakes has been established as acceptable [6] where the appropriate values for the age, sex and, in the case of pregnant women, stage of pregnancy are used. Evidence also suggests that nutritional needs change during the course of pregnancy with requirements for energy and several micronutrients increasing as pregnancy progresses [7].
Dietary habits of adolescent girls are often poorer than that of older women. The latest results of the UK National Diet and Nutrition Survey [8] showed that girls aged 11–18 years consumed 2.7 portions of fruit and vegetables per day compared to 4.1 portions in women aged 19–64, and adolescent girls also had some of the highest intakes of sugar-sweetened beverages within this dataset. A higher proportion of adolescent girls also had intakes of key vitamins and minerals below the lower reference nutrient intake level than adult women, including vitamin A, riboflavin, vitamin B12, folate, iron, calcium, magnesium, potassium, zinc and iodine. Dietary patterns across highly developed countries have been shown to have substantial similarities [9], while the same cannot be said for less developed regions.
While the evidence presented above suggests that adolescent girls often have a poorer diet than adult women in the general population this may not also necessarily be the case in those who are pregnant. Two systematic reviews have previously been conducted [10, 11] which explored nutritional intake and biochemical markers in pregnant adolescents living in developed countries. It was acknowledged in these reviews that there was a lack of good quality evidence in relation to these topics. However the author concluded that there was some consensus in the available literature that pregnant adolescents had intakes of energy, iron, folate, calcium, vitamin E and magnesium which were below the dietary recommended intakes. The review of biochemical markers reported that indicators of anaemia and iron status were compromised in this population; however no further conclusions could be drawn from the limited available evidence. It is therefore important that the most recent evidence relating to the nutritional intake and status of pregnant adolescents is examined in order to establish what the particular issues may be for this group. The aim of his systematic review was therefore to investigate the nutritional status of pregnant adolescents living in developed countries.
Methods
Search strategy
The search strategy was developed using search terms detailed in Table 1 and applied across nine key electronic databases (AMED, ASSIA, CINAHL, Child Development and Adolescent Studies, Cochrane Library, Health Source: Nursing, Maternity and Infant Care, MEDLINE and MEDLINE in Process, SCOPUS). Reference lists of identified papers were hand searched, and reference and citation functions were used where available.
Table 1.
Search terms
| Theme | Nutritional Intake | Pregnancy | Age | Nutritional Status |
|---|---|---|---|---|
| Search Terms | nutrient | pregnan* | adolescen* | biomarker* |
| nutrition* | gestatio* | teen | iron | |
| diet* | matern* | teenage* | folate | |
| eat* | mother* | youth | calcium | |
| food | gravid* | anaemi* | ||
| nutrition assessment (MH) | Pregnancy in adolescence (MH) | anemi* | ||
| Food habits (MH) | Biological markers (MH) | |||
| Dietary surveys (MH) |
* indicates truncation of search tearm
Table 1 search terms The main stages of the review including the number of references identified at each stage are illustrated in Fig. 1.
Fig. 1.
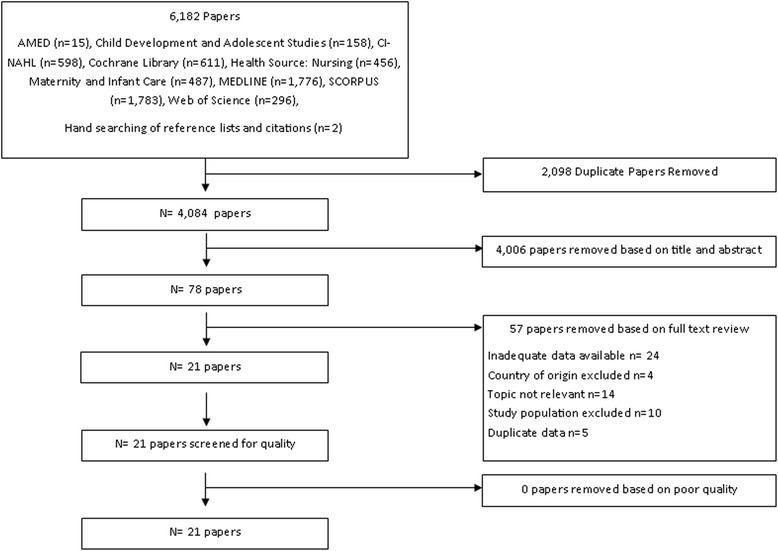
Protocol for systematic review
Inclusion criteria
Studies were limited to primary research papers using any methods published in English between 1995 and May 2015.
Studies were included where they provided data regarding either the nutritional intake or a biological marker of nutrient status of adolescents aged 11–19 years at any stage of pregnancy, from countries considered as having very high levels of human development by the United Nations Human Development Index [12]. This index has been selected as it provides a multidimensional model incorporating not only wealth but also health and education, and so provides a more reliable basis for assuming some commonality between countries of origin of the included studies. The definition of adolescence has been chosen to correspond with the World Health Organisation Growth Index [13].
Quality appraisal
The identified papers were assessed for risk of bias using the Critical Appraisal and Skills Program (CASP) checklist for systematic reviews [14] which was adapted to accommodate cross-sectional studies. Aspects of the studies giving an indication of methodological and interpretive rigor (e.g. research design, clear statement of research aims, recruitment of participants, consideration of confounding factors and reporting of results) were graded as either ‘good’ (+), ‘adequate or unclear’ (−/+) or poor (−), studies were then given an overall grade for quality.
Data extraction
Included studies were grouped depending on whether the study examined nutrient intake, biological markers of nutritional status or both. Information from the included studies was entered in to data extraction sheets using Microsoft Excel, one each for nutrient intakes and biological markers, and checked by a second reviewer.
Data synthesis
Reported data were first tabulated to explore patterns across the included studies and described narratively. Where 95 % confidence intervals were not reported they were calculated for the mean and used to assess the extent to which the study mean differed from the relevant reference value. Where the lower confidence limit was above the reference value the study mean was considered to be significantly higher, where the upper confidence limit was below the reference value the study mean was considered significantly lower, otherwise no significant difference was reported.
Analysis of micronutrient intakes was undertaken by comparing the reported data from included studies with the UK reference nutrient intake (RNI) [15] and US recommended daily allowance (RDA) [16] where available. Analysis has been undertaken using both UK and US thresholds as, while the majority of studies were undertaken in the USA, this review is also concerned with applying the results to the UK context. Energy intake was calculated by taking the mean of the single year of age estimated average requirements (EAR) for young women aged 11–19 with an increment applied in the third trimester; a population level EAR is only available in the UK [17]. For macronutrients an estimate of the percentage contribution to energy, if not provided, was calculated from the mean macronutrient intake and the mean energy intake.
In the case of biological markers, minimum thresholds for nutrient deficiency provided by WHO, UK and US authorities (where available) have been compared with reported study data. Nationally or internationally recognised cut offs for deficiency are not currently available for zinc, selenium, copper, magnesium and phosphorous, therefore these elements have been compared with suggested thresholds in academic literature [18–22].
Where data were available from two or more studies for a single nutrient or biological marker a pooled mean was calculated and weighted by the number of participants in each study. Mean measures of micronutrient and energy intake were expressed as a percentage of dietary reference values to allow for comparisons across different nutrients.
Sub-group analyses were performed by country of origin (USA only and UK only), stage of pregnancy (first, second and third trimester and reported average over the pregnancy) and age of adolescents (15 years and under and 16–19 years).
Results
A total of 4,084 unique papers were identified from the search of the literature with 78 studies remaining after title and abstract screening. Following examination of the full text of these papers a total of 21 papers were identified that met the review inclusion criteria. Details of the excluded studies are given in Additional file 1: Table S1. In brief, the main reasons for exclusion were not reporting appropriate data and the study population not meeting the inclusion criteria for age or pregnancy.
No studies were excluded for reasons of poor quality; after quality assessment 16 of the included studies were considered to be of good quality while the remaining five studies were of a satisfactory standard (Table 2).
Table 2.
Characteristics of included studies
| Study Information | Participants | Supplements | Measurement | Quality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Study design | Study groups | Number | Age | Inclusion criteria | Exclusion criteria | Supplement type and dose | Number with available data | Estimated compliance: N (%) | Data collection method (s) | Time period(s) | Quality rating |
| Baker et.al (2009) [23] | UK | Cross-sectional study | NA | 500 | 14–18 | Singleton pregnancy, age 14–18, gestational age < =20 weeks | Inability to provide informed consent, preeclampsia, clotting disorders, HIV/AIDS, haemoglobinopathies, diabetes, renal disease, hypertension, multiple gestations, history of 3= > previous miscarriages | Preconception folic acid | 498 | 34 (6.9) | 24 h recall (multiple) | Third trimester | + |
| Folic acid in early pregnancy | 498 | 220 (44.2) | |||||||||||
| Iron in early pregnancy | 498 | 39 (7.8) | |||||||||||
| Multivitamins in early pregnancy | 498 | 19 (3.8) | |||||||||||
| Folic acid only in 3rd trimester | 290 | 5 (1.7) | |||||||||||
| Iron and folic acid in 3rd trimester | 290 | 13 (4.5) | |||||||||||
| Iron only in 3rd trimester | 290 | 42 (14.5) | |||||||||||
| Multivitamins in 3rd trimester | 290 | 7 (2.4) | |||||||||||
| Castillo-Duran et.al (2001) [43] | Chile | RCT | Zinc supplemented | 249 | 16.4 (mean) | Beginning prenatal visits before 20 weeks gestation, aged <19 at estimated due date | Adolescents whose background included chronic diseases, drug abuse, mental retardation, illiteracy or pregnancy due to incest or rape | 20 mg Zinc sulphate daily | Intervention group - 249 | At least 50 % compliance | 24 h recall (multiple) | Second and third trimester | + |
| Placebo | 258 | 40 mg iron sulphate | All participants - 507 | ||||||||||
| Chan et.al (2006) [24] | USA | RCT | Control group, 2 intervention groups excluded from review* | 23 | 15–17 | Enrolled before 20 weeks gestation | Hypertension, diabetes, renal or liver disease, alcohol or tobacco use, using medicines effecting Ca metabolism | No additional supplements reported | Food diary (weighing not mentioned) | Second and third trimester | −/+ | ||
| Chang et.al (2003) [37] | USA | Retrospective chart review | NA | 918 | 12–17 | Self-reported racial group as African-American; singleton pregnancy | Birth results not available due to abortion, miscarriage or transfer of care | 90 mg carbonyl iron daily plus additional up to 120 mg for those classified as anaemic | 918 | Not reported | Not reported | Second and third trimester | + |
| Dawson et.al (2000) [35] | USA | RCT | One-A-Day without Iron | 20 | 16–20 | Aged 16–20, less than 16 weeks gestation, no iron supplementation for previous 30 days | Hypertension; diabetes; other medical problems; haemoglobin <11 g/dL; haematocrit <30 % | Vitamin A 5000 IU, vitamin D 400 IU, vitamin E 10 mg, vitamin C 60 mg, folic acid 0.4 mg, thiamine 1.5 mg, riboflavin 1.7 mg, niacin 20 mg, pyridoxine 2 mg, vitamin B- 6 mg, pantothenic acid 10 mg | 20 | Not reported | Venous blood sample | Second and third trimester and delivery | −/+ |
| One-A-Day with Iron | 20 | 18 mg Iron, Vitamin A 5000 IU, vitamin D 400 IU, vitamin E 10 mg, vitamin C 60 mg, folic acid 0.4 mg, thiamine 1.5 mg, riboflavin 1.7 mg, niacin 20 mg, pyridoxine 2 mg, vitamin B- 6 mg, pantothenic acid 10 mg | 20 | ||||||||||
| Derbyshire (2009) [39] | UK | Cross-sectional study | NA | 20 | 15–19 | Attending antenatal classes or community clinics | Incomplete diary | None reported | Food diary unweighed | Third trimester | + | ||
| Gadowsky et.al (1995) [42] | Canada | Cross-sectional study | NA | 58 | 14–19 | Not reported | Not reported | Folic Acid Mean 479 μg/day | 58 | 82 % | Venous blood sample | Third trimester | + |
| Elemental Iron Mean 31.5 mg/day | |||||||||||||
| Cyanocobalamin (B12) Mean 2.24 μg/d | |||||||||||||
| Giddens et.al (2000) [27] | USA | RCT (subset from a larger study) | NA | 59 | 13–18 | Singleton pregnancies, between 13 and 19 weeks gestation | Not reported | Reported that any contribution from supplements was not included in analysis | Food diary weighed | Mean over pregnancy | −/+ | ||
| Ginde et.al (2010) [32] | USA | Secondary analysis of cross sectional survey | NA | 84 | 13–19 | Not reported | Not reported | Some participants taking vitamin D supplements | Not reported | Not reported | Venous blood sample | Mean over pregnancy | + |
| Gutierrez et.al (1999) [28] | USA | Cross-sectional study | NA | 46 | 13–18 | Self-identified ethnicity as Mexican American, primigravida, 13–18 years of age | History of miscarriage or health problems, involved in competitive athletic performances or heavy exercise, planned to move away during study period | None reported | 24 h recall (single) | Second and third trimester | + | ||
| Iannotti et.al (2005) [36] | USA | Cross-sectional study | NA | 80 | 13–18 | Self-reported racial group as African-American; singleton pregnancy | Not reported | None reported | 10 ml Venous blood sample | Second and third trimesters | + | ||
| Job et.al (1995) [41] | Australia | Cross-sectional study | NA | 35 | 13–19 | Not reported | Not reported | None reported | 24 h recall | Mean over pregnancy | + | ||
| Lee et.al (2013) [25] | USA | Cross-sectional study | NA | 156 | 13–18 | Age 18 or under, carrying a single fetus, 12–30 weeks gestation at recruitment | Not reported | Reported that any contribution from supplements was not included in analysis | 24 h recall (multiple) | <23 weeks gestation, 23–30 weeks gestation and Mean over pregnancy | + | ||
| McGuire et.al. (2010) [31] | USA | Cross-sectional study | NA | 80 | Under 18 (mean 16.5) | Self-reported ethnic group as African American, singleton pregnancy | Not reported | Routinely prescribed prenatal supplements containing 400 IU vitamin D | Data not available | Venous blood sample | Second and third trimester | + | |
| Meier et.al (2002) [34] | USA | RCT | Iron supplemented | 20 | 15–18 | Not reported | Iron deficiency anaemia at recruitment | 60 mg elemental iron & 1 mg folic acid | 20 | Venous blood sample | Second and third trimesters | + | |
| Placebo | 17 | 1 mg folic acid | 17 | ||||||||||
| Mistry et.al (2014) [40] | UK | Cross-sectional study | Appropriate for gestational age babies | 107 | 14–18 | Not reported | Inability to provide informed consent, pre-eclampsia, clotting disorders, HIV/AIDS, Haemoglobinpathies, diabetes, renal disease, hypertension, multiple pregnancy and previous miscarriage | None reported | 30 ml venous blood sample | Third trimester | + | ||
| Small for gestational age babies | 9 | ||||||||||||
| O’Brien et.al (2003) [33] | USA | Cross-sectional study | NA | 23 | Mean 16.5 | First, singleton pregnancies; no medical problems; no medications known to influence calcium metabolism; non-smokers; no history of drug or alcohol abuse | Not reported | Prenatal supplement including 5 mmol Ca | 23 | 39 % | Not reported | Third trimester | + |
| Pobocik et.al (2003) [38] | Guam (USA Teritory) | Cross-sectional study | NA | 434 | 14–20 | Not reported | Not reported | Reported that any contribution from supplements was not included in analysis | 24 h recall (single) | Mean over pregnancy | −/+ | ||
| Rycel et.al (2009) [44] | Poland | Retro-spective cohort | NA | 102 | 15–18 | Not reported | Not reported | none reported | Venous blood sample | Before and after delivery | −/+ | ||
| Young et.al (2010) [29] | USA | Cross-sectional study | NA | 92 | 14–18 | Healthy, singleton pregnancy | HIV, diabetes, pre-eclampsia, eating disorders, malabsorption diseases, self-reported drug use | Prenatal supplement including 27 mg iron | 92 | Not reported | Venous blood sample | Second trimester and delivery | + |
| Young et.al (2012) [30] | USA | Cross-sectional study | NA | 171 | Under 18 (mean 17.1) | Healthy, singleton pregnancy | HIV, diabetes, pre-eclampsia, eating disorders, malabsorption diseases | 400 IU Vitamin D supplement given to participants found to be deficient | 46 (estimated from reported percentages) | 26.4 % - daily, 35.8 % at least twice per week | 10 ml venous blood sample | Delivery | + |
Of the included studies, six provided information on dietary intakes only, 12 on biological markers only and three reported both types of information. Nutrient intakes from food sources were reported (therefore excluding any contribution from supplements) in all but one paper [23]. However, the majority (10 out of 15) of papers reporting biological markers also reported that participants were taking nutritional supplements, details of which along with other characteristics of the included studies are shown in Table 2. Due to inconsistencies in the type, dose, duration and compliance with supplement use it was not possible to quantify the impact of supplements on the results.
Of the 21 included studies 14 were carried out in the USA [24–37], one in the US territory of Guam [38], three in the UK [23, 39, 40] and one in each of Australia [41], Canada [42], Chile [43] and Poland [44]. Nutritional status was a primary outcome measure in all but one of the included studies where the primary outcomes were birth weight and prematurity [43].
The study designs of the included studies are listed within Table 2. The majority of the studies were cross-sectional surveys. Five studies were randomised controlled trials, where baseline dietary assessments before randomisation, or data from the control group only, permitted the inclusion of nutritional intake or biomarker data cross-sectionally. One study was a retrospective cohort analysis and one was a retrospective chart review.
Participants were all aged between 12 and 20 years with the majority being aged 16 and over. The majority of studies selected participants using convenience samples; other sampling methods used were purposive [26], representative probability sample [32], stratified random sample [44] and a retrospective medical chart review including all eligible records [37].
The majority of studies reported a range of ethnicities in the sample with the exception of three studies where all participants were African American [31, 36, 37], one including only Mexican American participants [28] and one where all participants were White [26]. Where reported the majority of participants had a BMI in the healthy range. Six studies reported participant’s weight gain from recruitment to delivery which ranged from 14 to 17 kg.
All but two of the studies reporting biological markers collected venous blood samples which were analysed in laboratories using standard testing procedures. One study which was a medical records review [37] did not provide details of how samples were collected. One study also assessed biological markers of calcium absorption using a 24 h urine collection followed by daily spot urine collections [33]. Data relating to participants nutrient intakes used a variety of data collection methods. Three studies used food diaries [24, 27, 39], one of which was weighed [27]. The remaining studies used single [28, 38, 41] or multiple [25, 43, 23] 24 h recalls. Four out of the nine studies reporting nutrient intakes stated that dietary assessments were carried out by a trained nutritionist or similar professional [25, 26, 43, 27].
Nutrient intakes
Energy intake was reported by nine studies with seven of these reporting intakes below the recommendations at one or more time point (Table 3). Four studies also reported gestational weight gain which ranged from 14 to 17 kg. Pooled analysis of the percentage of the EAR for energy in these 10 studies revealed wide confidence limits around the estimated mean, with an average intake 9 % lower than the UK EAR (mean % EAR, 91.2 %, CI 29.6–152.8 %). Analysis of energy intake by trimester and study country of origin did not show any significant differences (UK studies 89.1 % CI 39.2–139.1 %, US studies 100.4 %, CI 24.2–176.6 %). Analysis of those studies reporting gestational weight gain only showed young women to be achieving a higher percentage of the EAR for energy (99.1 %, CI 41.0–157.2 %) than those studies which did not report weight gain (90.2 %, CI 27.9–152.5 %) but this difference was not statistically significant.
Table 3.
Mean energy intake in individual studies compared to UK estimated average requirement
| Study | Second Trimester | Third Trimester | Mean over pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | ||
| Energy kcal/day a | Baker (2009) | 290 | ↓2147 | 2075, 2219 | ||||||
| Castillo-Duran (2001) a | 249 | ↓1887 | 1825, 1949 | 249 | ↓2030 | 1968, 2092 | ||||
| Castillo-Duran (2001) b | 258 | ↓1863 | 1790, 1936 | 258 | ↓1982 | 1927, 2038 | ||||
| Chan (2006) | 23 | ↔2223 | 2057, 2389 | 23 | ↓2276 | 2059, 2493 | ||||
| Derbyshire (2009) | 20 | ↓2273 | 2052, 2494 | |||||||
| Giddens (2000) | 59 | ↓2342 | 2187, 2497 | |||||||
| Gutierrez (1999) | 46 | ↔2390 | 2150, 2630 | 46 | ↔2620 | 2389, 2851 | ||||
| Job (1995) | 70 | ↓2134 | 1949, 2320 | |||||||
| Lee (2013) | 133 | ↓2303 | 2161, 2446 | 156 | ↔2273 | 2167, 2379 | ||||
| Pobocik (2003) | 434 | ↑2487 | 2388, 2586 | |||||||
aComparison to UK EARs, First and Second Trimester requirement (Average UK EAR for Females aged 11–19) 2355 kcal/day, Third Trimester requirement 2546 kcal/day, Mean over pregnancy requirement (average of three trimester values) 2419 kcal/d, ↑ Study mean higher than reference value (p < 0.05), ↔ Study mean not different to reference value (p < 0.05), ↓ Study mean lower than reference value (p < 0.05)
Mean intakes of macronutrients are show in Table 4. Intakes of protein and total carbohydrate were roughly in line with recommendations. There were too few studies reporting intakes of total fat, fat types or sugars to permit conclusions to be drawn. Three studies reported any measurements of dietary fibre, all of which were below recommended levels.
Table 4.
Mean intakes of macronutrients (g/day or percent of energy) and dietary fibre (g/day) in individual studies compared to UK and US dietary reference values
| Comparison to UK Dietary Reference Value | Comparison to US Dietary Reference Value | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | Study | Second Trimester | Third Trimester | Mean over pregnancy | Second Trimester | Third Trimester | Mean over pregnancy | ||||||||||||
| N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | ||
| Protein grams/day a | Castillo-Duran (2001) a | 249 | ↑60 | 58, 62 | 249 | ↑62 | 59, 64 | 249 | ↓60 | 58, 62 | 249 | ↓62 | 59, 64 | ||||||
| Castillo-Duran (2001) b | 258 | ↑59 | 57, 61 | 258 | ↑60 | 58, 61 | 258 | ↓59 | 57, 61 | 258 | ↓60 | 58, 61 | |||||||
| Chan (2006) | 23 | ↑76 | 65, 87 | 23 | ↑76 | 65, 87 | 23 | ↔76 | 65, 87 | 23 | ↔76 | 65, 87 | |||||||
| Derbyshire (2009) | 20 | ↑72 | 65, 79 | 20 | ↔72 | 65, 79 | |||||||||||||
| Giddens (2000) | 59 | ↑82 | 77, 87 | 59 | ↑82 | 77, 87 | |||||||||||||
| Gutierrez (1999) | 46 | ↑111 | 98, 124 | 46 | ↑118 | 105, 130 | 46 | ↑111 | 98, 124 | 46 | ↑118 | 105, 130 | |||||||
| Job (1995) | 70 | ↑73 | 73, 73 | 70 | ↑73 | 73, 73 | |||||||||||||
| Lee (2013) | 133 | ↑81 | 76, 87 | 156 | ↑70 | 65, 75 | 133 | ↑81 | 76, 87 | 156 | ↔70 | 65, 75 | |||||||
| Pobocik (2003) | 434 | ↑99 | 95, 103 | 434 | ↑99 | 95, 103 | |||||||||||||
| Englyst Fibre grams/day b | Derbyshire (2009) | ↓12 | 11, 13 | ||||||||||||||||
| AOAC Fibre grams/day c | Giddens (2000) | 59 | ↓14 | 13, 15 | |||||||||||||||
| Lee (2013) | 133 | ↓13 | 12, 14 | 156 | ↓14 | 13,14 | |||||||||||||
| N= | % of Energy | N= | % of Energy | N= | % of Energy | ||||||||||||||
| Total Carbohydrate | Derbyshire (2009) | 20 | 54 % | ||||||||||||||||
| Giddens (2000) | 59 | 50 % | |||||||||||||||||
| Lee (2013) | 133 | 51 % | 156 | 52 % | |||||||||||||||
| Gutierrez (1999) | 46 | 56 % | 46 | 56 % | |||||||||||||||
| Total Fat | Chan (2006) | 23 | 29 % | 23 | 29 % | ||||||||||||||
| Gutierrez (1999) | 46 | 29 % | 46 | 27 % | |||||||||||||||
| Saturated Fat | Chan (2006) | 23 | 9 % | 23 | 10 % | ||||||||||||||
| Total Sugars | Derbyshire (2009) | 20 | 25 % | ||||||||||||||||
| Added Sugars | Lee (2013) | 133 | 17 % | 156 | 17 % | ||||||||||||||
a Comparison to UK RNI 51 g/day and US RDA 71 g/day, b Comparison to UK RNI 18 g/day, c Comparison to US RDA 28 g/day, ↑ Study mean higher than reference value (p < 0.05), ↔ Study mean not different to reference value (p < 0.05), ↓ Study mean lower than reference value (p < 0.05)
Tables 5 and 6 show the pattern of micronutrient intakes across the included studies compared to UK and US Dietary Reference Values (DRVs).
Table 5.
Intake of micronutrients in individual studies compared to UK and US dietary reference values - minerals
| Comparison to UK Dietary Reference Value | Comparison to US Dietary Reference Value | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | Study | Second Trimester | Third Trimester | Mean over pregnancy | Second Trimester | Third Trimester | Mean over pregnancy | ||||||||||||
| N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | ||
| Calcium mg/day a | Baker (2009) | 290 | ↔840 | 800, 880 | 290 | ↓840 | 800, 880 | ||||||||||||
| Chan (2006) | 23 | ↔835 | 711, 959 | 23 | ↔862 | 714, 1010 | 23 | ↓835 | 711, 959 | 24 | ↓862 | 714, 1010 | |||||||
| Derbyshire (2009) | 20 | ↑1007 | 867, 1147 | 20 | ↓1007 | 867, 1147 | |||||||||||||
| Giddens (2000) | 59 | ↑989 | 904, 1074 | 59 | ↓989 | 904, 1074 | |||||||||||||
| Gutierrez (1999) | 46 | ↑1561 | 1334, 1789 | 46 | ↑1655 | 1424, 1886 | 46 | ↑1561 | 1334, 1789 | 46 | ↑1655 | 1424, 1886 | |||||||
| Job (1995) | 70 | ↔923 | 756, 1090 | 70 | ↓923 | 756, 1090 | |||||||||||||
| Lee (2013) | 133 | ↑916 | 838, 995 | 156 | ↑886 | 824, 948 | 133 | ↓916 | 838, 995 | 156 | ↓886 | 824, 948 | |||||||
| Pobocik (2003) | 434 | ↓743 | 689, 797 | 434 | ↓743 | 689, 797 | |||||||||||||
| Phosphorous mg/day b | Chan (2006) | 23 | ↑934 | 811, 1057 | 23 | ↑961 | 812, 1110 | 23 | ↓934 | 811, 1057 | 24 | ↓961 | 812, 1110 | ||||||
| Giddens (2000) | 59 | ↑1340 | 1248, 1432 | 59 | ↔1340 | 1248, 1432 | |||||||||||||
| Lee (2013) | 133 | ↑1264 | 1182, 1347 | 156 | ↑1196 | 1131, 1261 | 133 | ↔1264 | 1182, 1347 | 156 | ↔1196 | 1131, 1261 | |||||||
| Pobocik (2003) | 434 | ↑1338 | 1279, 1397 | 434 | ↑1338 | 1279, 1397 | |||||||||||||
| Iron mg/day c | Baker (2009) | 290 | ↑17 | 15, 19 | 290 | ↓17 | 15, 19 | ||||||||||||
| Castillo-Duran (2001) a | 249 | ↑15.5 | 15, 16 | 249 | ↑16.8 | 16, 17 | 249 | ↓15.5 | 15, 16 | 249 | ↓16.8 | 16, 17 | |||||||
| Castillo-Duran (2001) b | 258 | ↑16.6 | 16, 17 | 258 | ↑16.8 | 16, 17 | 258 | ↓16.6 | 16, 17 | 258 | ↓16.8 | 16, 17 | |||||||
| Chan (2006) | 23 | ↑22 | 18, 26 | 23 | ↑25 | 20, 30 | 23 | ↓22 | 18, 26 | 23 | ↔25 | 20, 30 | |||||||
| Derbyshire (2009) | 20 | ↓12.6 | 11, 14 | 20 | ↔12.6 | 11, 14 | |||||||||||||
| Giddens (2000) | 59 | ↔16 | 15, 17 | 59 | ↓16 | 15, 17 | |||||||||||||
| Gutierrez (1999) | 46 | ↑17.7 | 15, 20 | 46 | ↑22.7 | 17, 28 | 46 | ↓17.7 | 15, 20 | 46 | ↔22.7 | 17, 28 | |||||||
| Job (1995) | 70 | ↓11.2 | 10, 12 | 70 | ↓11.2 | 10, 12 | |||||||||||||
| Lee (2013) | 133 | ↑18.8 | 17, 20 | 156 | ↑18.6 | 17, 20 | 133 | ↓18.8 | 17, 20 | 156 | ↓18.6 | 17, 20 | |||||||
| Pobocik (2003) | 434 | ↑20 | 19, 21 | 434 | ↓20 | 19, 21 | |||||||||||||
| Magnesium mg/day d | Baker (2009) | 290 | ↓236 | 227, 245 | ↓236 | 227, 245 | |||||||||||||
| Chan (2006) | 23 | ↓263 | 230, 296 | 23 | ↓264 | 231, 297 | ↓263 | 230, 296 | ↓264 | 231, 297 | |||||||||
| Derbyshire (2009) | 20 | ↓244 | 218, 270 | ↓244 | 218, 270 | ||||||||||||||
| Giddens (2000) | 59 | ↓252 | 234, 270 | ↓252 | 234, 270 | ||||||||||||||
| Lee (2013) | 133 | ↓237 | 222, 253 | 156 | ↓231 | 218, 244 | ↓237 | 222, 253 | ↓231 | 218, 244 | |||||||||
| Pobocik (2003) | 434 | ↓270 | 258, 282 | ↓270 | 258, 282 | ||||||||||||||
| Potassium mg/day e | Chan (2006) | 23 | ↓2802 | 2512, 3092 | 23 | ↓2954 | 2635, 3273 | 23 | ↓2802 | 2512, 3092 | 23 | ↓2954 | 2635, 3273 | ||||||
| Derbyshire (2009) | 20 | ↓2948 | 2659, 3237 | 20 | ↓2948 | 2659, 3237 | |||||||||||||
| Zinc mg/day f | Baker (2009) | 290 | ↑8.1 | 7.8, 8.4 | 290 | ↓8.1 | 7.8, 8.4 | ||||||||||||
| Castillo-Duran (2001) a | 249 | ↑7.4 | 7.1, 7.7 | 249 | ↑7.7 | 7.4, 8 | 249 | ↓7.4 | 7.1, 7.7 | 249 | ↓7.7 | 7.4, 8 | |||||||
| Castillo-Duran (2001) b | 258 | ↑7.4 | 7.1, 7.7 | 258 | ↑7.4 | 7.2, 7.6 | 258 | ↓7.4 | 7.1, 7.7 | 258 | ↓7.4 | 7.2, 7.6 | |||||||
| Chan (2006) | 23 | ↑16 | 11.9, 20.1 | 23 | ↑18 | 13.5, 22.5 | 23 | ↔16 | 11.9, 20.1 | 23 | ↑18 | 13.5, 22.5 | |||||||
| Derbyshire (2009) | 20 | ↑8.1 | 7.4, 8.9 | 20 | 7.4, 8.9 | ||||||||||||||
| Giddens (2000) | 59 | ↑11.6 | 10.5, 12.7 | 59 | ↑11.6 | 10.5, 12.7 | |||||||||||||
| Gutierrez (1999) | 46 | ↑14.5 | 12.6, 16.4 | 46 | ↑15.3 | 13.4, 17.2 | 46 | ↑14.5 | 12.6, 16.4 | 46 | ↑15.3 | 13.4, 17.2 | |||||||
| Job (1995) | 70 | ↑9.5 | 8.5, 10.5 | 70 | ↑9.5 | 8.5, 10.5 | |||||||||||||
| Lee (2013) | 133 | ↑12.8 | 156 | ↑12.6 | 11.4, 13.8 | 133 | ↑12.8 | 156 | ↑12.6 | 11.4, 13.8 | |||||||||
| Pobocik (2003) | 434 | ↑13 | 12.2, 13.8 | 434 | ↑13 | 12.2, 13.8 | |||||||||||||
| Sodium mg/day g | Chan (2006) | 23 | ↑3316 | 2809, 3823 | 23 | ↑3323 | 2812, 3834 | ↑3316 | 2809, 3823 | ↑3323 | 2812, 3834 | ||||||||
| Derbyshire (2009) | 20 | ↑3089 | 2722, 3456 | ↑3089 | 2722, 3456 | ||||||||||||||
| Copper μg/day h | Giddens (2000) | 59 | ↑1200 | 1098, 1302 | 59 | ↑1200 | 1098, 1302 | ||||||||||||
| Lee (2013) | 133 | ↑1100 | 1015, 1185 | 156 | ↑1085 | 1021, 1151 | 133 | ↑1100 | 1015, 1185 | 156 | ↑1085 | 1021, 1151 | |||||||
| Selenium μg/day i | Giddens (2000) | 59 | ↑116 | 109, 123 | 59 | ↑116 | 109, 123 | ||||||||||||
aComparison to UK RNI 800 mg/day and US RDA 1300 mg/day, bComparison to UK RNI 625 mg/day and US RDA 1250 mg/day, cComparison to UK RNI 14.8 mg/day and US RDA 27 mg/day, dComparison to UK RNI 300 mg/day and US RDA 400 mg/day, eComparison to UK RNI 3500 mg/day and US RDA 4700 mg/day, fComparison to UK RNI 7 mg/day and US RDA 12 mg/day, gComparison to UK RNI 1500 mg/day and US RDA 1600 mg/day, hComparison to UK RNI 1000 μg/day and US RDA 1000 μg/day, iComparison to UK RNI 60 μg/day and US RDA 60 μg/day, ↑ Study mean higher than reference value (p < 0.05), ↔ Study mean not different to reference value (p < 0.05), ↓ Study mean lower than reference value (p < 0.05)
Table 6.
Intake of micronutrients in individual studies compared to UK and US dietary reference values - vitamins
| Comparison to UK Dietary Reference Value | Comparison to US Dietary Reference Value | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | Study | Second Trimester | Third Trimester | Mean over pregnancy | Second Trimester | Third Trimester | Mean over pregnancy | ||||||||||||
| N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | ||
| Vitamin D μg/day a | Baker (2009) | 290 | ↓2.1 | 2, 2.3 | 290 | ↓2.1 | 2, 2.3 | ||||||||||||
| Chan (2006) | 23 | ↓2.8 | 1.9, 3.7 | 23 | ↓3.1 | 2.2, 4 | 23 | ↓2.8 | 1.9, 3.7 | 23 | ↓3.1 | 2.2, 4 | |||||||
| Derbyshire (2009) | 20 | ↓2.0 | 1.5, 2.6 | 20 | ↓2.0 | 1.5, 2.6 | |||||||||||||
| Giddens (2000) | 59 | ↓6.4 | 5.7, 7.1 | 59 | ↓6.4 | 5.7, 7.1 | |||||||||||||
| Lee (2013) | 133 | ↓5.4 | 4.7, 6.1 | 156 | ↓5.1 | 4.6, 5.6 | 133 | ↓5.4 | 4.7, 6.1 | 156 | ↓5.1 | 4.6, 5.6 | |||||||
| Vitamin E b | Baker (2009) | 290 | ↓8.9 | 8.3, 9.5 | |||||||||||||||
| Derbyshire (2009) | 20 | ↓7.7 | 6.5, 8.9 | ||||||||||||||||
| Gutierrez (1999) | 46 | ↓10.7 | 10.7 | 46 | ↓11.2 | 8.2, 14.1 | |||||||||||||
| Lee (2013) | 133 | ↓6.9 | 6.2, 7.6 | 156 | ↓6.9 | 6.4, 7.4 | |||||||||||||
| Pobocik (2003) | 434 | ↓8 | 7.2, 8.8 | ||||||||||||||||
| Vitamin C c | Baker (2009) | 290 | ↑160 | 146, 174 | 290 | ↑160 | 146, 174 | ||||||||||||
| Derbyshire (2009) | 20 | ↑138 | 111, 165 | 20 | ↑138 | 111, 165 | |||||||||||||
| Giddens (2000) | 59 | ↑128 | 112, 144 | 59 | ↑128 | 112, 144 | |||||||||||||
| Gutierrez (1999) | 46 | ↑252 | 208, 296 | 46 | ↑231 | 190, 271 | 46 | ↑252 | 208, 296 | 46 | ↑231 | 190, 271 | |||||||
| Job (1995) | 70 | ↑135 | 92, 178 | 70 | ↑135 | 92, 178 | |||||||||||||
| Lee (2013) | 133 | ↑97 | 81, 113 | 156 | ↑106 | 94, 118 | 133 | ↑97 | 81, 113 | 156 | ↑106 | 94, 118 | |||||||
| Pobocik (2003) | 434 | ↑167 | 150, 184 | 434 | ↑167 | 150, 184 | |||||||||||||
| Folate d | Baker (2009) | 290 | ↔285 | 269, 301 | 290 | ↓285 | 269, 301 | ||||||||||||
| Derbyshire (2009) | 20 | ↓227 | 205, 249 | 20 | ↓227 | 205, 249 | |||||||||||||
| Giddens (2000) | 59 | ↔312 | 277, 347 | 59 | ↓312 | 277, 347 | |||||||||||||
| Gutierrez (1999) | 46 | ↑447 | 355, 540 | 46 | ↑393 | 340, 445 | 46 | ↓447 | 355, 540 | 46 | ↓393 | 340, 445 | |||||||
| Lee (2013) | 133 | ↑829 | 723, 935 | 156 | ↑849 | 645, 1053 | 133 | ↑829 | 723, 935 | 156 | ↑849 | 645, 1053 | |||||||
| Pobocik (2003) | 434 | ↔292 | 269, 315 | 434 | ↓292 | 269, 315 | |||||||||||||
| Riboflavin e | Chan (2006) | 23 | ↑2.3 | 1.8, 2.8 | 23 | ↑2.4 | 2, 2.8 | 23 | ↑2.3 | 1.8, 2.8 | 23 | ↑2.4 | 2, 2.8 | ||||||
| Giddens (2000) | 59 | ↑2.3 | 59 | ↑2.3 | 2.1, 2.5 | ||||||||||||||
| Lee (2013) | 133 | ↑2.5 | 2.3, 2.7 | 156 | ↑2.4 | 133 | ↑2.5 | 2.3, 2.7 | 156 | ↑2.4 | 2.2, 2.6 | ||||||||
| Pobocik (2003) | 434 | ↑2.1 | 434 | ↑2.1 | 2, 2.2 | ||||||||||||||
| B12 f | Baker (2009) | 290 | ↑5.3 | 4.7, 5.9 | 290 | ↑5.3 | 4.7, 5.9 | ||||||||||||
| Chan (2006) | 23 | ↑5 | 3.8, 6.2 | 23 | ↑5.2 | 3.7, 6.7 | 23 | ↑5 | 3.8, 6.2 | 23 | ↑5.2 | 3.7, 6.7 | |||||||
| Giddens (2000) | 59 | ↑5.3 | 4.6, 6 | 59 | ↑5.3 | 4.6, 6 | |||||||||||||
| Lee (2013) | 133 | ↑5.6 | 5, 6.2 | 156 | ↑5.5 | 5, 6 | 133 | ↑5.6 | 5, 6.2 | 156 | ↑5.5 | 5, 6 | |||||||
| Pobocik (2003) | 434 | ↑5.5 | 4.8, 6.2 | 434 | ↑5.5 | 4.8, 6.2 | |||||||||||||
| Thiamin g | Baker (2009) | 290 | ↑1.6 | 1.5, 1.7 | 290 | ↑1.6 | 1.5, 1.7 | ||||||||||||
| Giddens (2000) | 59 | ↑2.1 | 1.9, 2.3 | 59 | ↑2.1 | 1.9, 2.3 | |||||||||||||
| Lee (2013) | 133 | ↑2.1 | 1.9, 2.3 | 156 | ↑2.1 | 1.9, 2.3 | 133 | ↑2.1 | 1.9, 2.3 | 156 | ↑2.1 | 1.9, 2.3 | |||||||
| Pobocik (2003) | 434 | 2.4 | 2.3, 2.5 | 434 | 2.4 | 2.3, 2.5 | |||||||||||||
| Niacin h | Baker (2009) | 290 | ↑33 | 32, 35 | 290 | ↑34 | 32, 35 | ||||||||||||
| Giddens (2000) | 59 | ↑24 | 22, 26 | 59 | ↑24 | 22, 26 | |||||||||||||
| Lee (2013) | 133 | ↑28 | 26, 30 | 156 | ↑26 | 24, 27 | 133 | ↑28 | 26, 30 | 156 | ↑26 | 24, 27 | |||||||
| Pobocik (2003) | 434 | ↑30 | 29, 31 | 434 | ↑30 | 29, 31 | |||||||||||||
| B6 i | Baker (2009) | 290 | ↑2.3 | 2.1, 2.4 | 290 | ↑2.3 | 2.1, 2.4 | ||||||||||||
| Giddens (2000) | 59 | ↑1.9 | 1.7, 2.1 | 59 | ↑1.9 | 1.7, 2.1 | |||||||||||||
| Lee (2013) | 133 | ↑2.2 | 2, 2.4 | 156 | ↑2.1 | 1.9, 2.3 | 133 | ↑2.2 | 2, 2.4 | 156 | ↑2.1 | 1.9, 2.3 | |||||||
| Pobocik (2003) | 434 | ↑2 | 1.9, 2.1 | 434 | ↑2 | 1.9, 2.1 | |||||||||||||
| Vitamin A j | Baker (2009) | 290 | ↑759 | 651, 867 | 290 | ↑759 | 651, 867 | ||||||||||||
| Derbyshire (2009) | 20 | ↑555 | 439, 671 | 20 | ↓555 | 439, 671 | |||||||||||||
| Giddens (2000) | ↑105 | 59 | ↑1053 | 907, 1199 | ↑1053 | 59 | ↑1053 | 907, 1199 | |||||||||||
| Gutierrez (1999) | 46 | ↑2492 | 1466, 3518 | 46 | ↑197 | 1432, 2523 | 46 | ↑2492 | 1466, 3518 | 46 | ↑1978 | 1432, 2523 | |||||||
| Job (1995) | 70 | ↑973 | 802, 1144 | 70 | ↑973 | 802, 1144 | |||||||||||||
| Lee (2013) | 133 | ↑698 | 614, 783 | 156 | ↑666 | 615, 717 | 133 | ↑698 | 614, 783 | 156 | ↓666 | 615, 717 | |||||||
| Pobocik (2003) | 434 | ↑109 | 944, 1242 | 434 | ↑1093 | 944, 1242 | |||||||||||||
| Vitamin K k | Lee (2013) | 133 | ↔70 | 57, 83 | 156 | ↔70 | 59, 81 | ||||||||||||
a Comparison to UK RNI 10 μg/day and US RDA 15 μg/day, b Comparison to US RDA 15 mg/day, c Comparison to UK RNI 40 mg/day and US RDA 80 mg/day, d Comparison to UK RNI 200 μg/day and US RDA 600 μg/day, e Comparison to UK RNI 1.1 mg/day and US RDA 1.4 mg/day, f Comparison to UK RNI 1.5 μg/day and US RDA 2.6 μg/day, g Comparison to UK RNI 0.8 mg/day and US RDA 1.4 mg/day, h Comparison to UK RNI 14 mg/day and US RDA 18 mg/day, i Comparison to UK RNI 1.2 mg/day and US RDA 1.9 mg/day, j Comparison to UK RNI 600 μg/day and US RDA 750 μg/day, k Comparison to US AI 75 μg/day, ↑ Study mean higher than reference value (p < 0.05), ↔ Study mean not different to reference value (p < 0.05), ↓ Study mean lower than reference value (p < 0.05)
The individual study results show that the majority of reported nutrient intakes were significantly below both the UK RNI and US RDA for vitamin D, potassium and magnesium and below the US RDA for calcium, vitamin E, folate, phosphorous and iron. Zinc intakes reported as the mean intake over pregnancy were low whereas this was not the case in the studies reporting intakes in the second or third trimesters specifically.
Results of the pooled analyses however showed that only intake of vitamin D remained significantly below both the UK RNI and US RDA, and intakes of potassium below the US RDA. Sub-group analysis showed that micronutrient intake was lower in UK based studies than those based in the USA for all micronutrients with the exception of vitamin C, however vitamin D was the only micronutrient where the percentage of the DRV in UK based studies was below the UK RNI (21.4 %, CI 0–63.5 %) and US RDA (14.3 %, CI 0–42.3 %). Results of the pooled analysis of micronutrients are shown in Fig. 2. Detailed results of the sub-group analysis of nutrient intakes are available in Additional file 1: Table S2 and Additional file 1: Table S3.
Fig. 2.
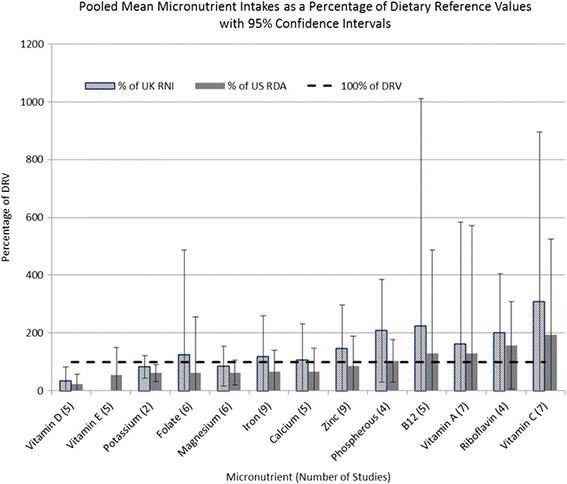
Pooled weighted mean micronutrient intake compared to UK and US dietary reference values
Micronutrient analysis by stage of pregnancy showed that intake of vitamin D in the second (28 % of the UK RNI, CI 26–30 %) and third (31.2 % of the UK RNI, CI 0–47 %) trimesters was below recommendations; this was not the case for measures reported as a mean over pregnancy (54 % of the UK RNI, CI 0–114.3 %). In the third trimester the percentage of the US RDA represented by intakes of magnesium (19.9 %, CI 20.4–98.6 %) and potassium (62.8 %, CI 32.5–93.1 %) were also below recommendations.
Biological markers
Table 7 shows the pattern of biological markers reported across the included studies compared to WHO, UK and US minimum thresholds for deficiency where available. As the recommended cut off points given by all three authorities are consistent, the results are presented in one combined table for clarity. Other nutrients were considered compared to deficiency thresholds suggested in the academic literature as previously discussed.
Table 7.
Biological markers of nutritional status in individual studies compared to reference values
| Study ID | First Trimester | Second Trimester | Third Trimester | Delivery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | N= | Mean | Confidence Interval | ||
| Haemoglobin (g/L) a | Baker (2009) | 404 | ↑122 | 121, 123 | 362 | ↓108 | 107, 109 | ||||||
| Chang (2003) | 445 | ↑121 | 121, 122 | 319 | ↑112 | 112, 114 | 836 | ↓107 | 107, 109 | ||||
| Dawson (2000) a | 21 | ↑124 | 123, 125 | 21 | ↑115 | 114, 116 | 20 | ↑114 | 111, 112 | ||||
| Dawson (2000) b | 20 | ↑125 | 124, 126 | 20 | ↑119 | 118, 120 | 21 | ↑116 | 112, 114 | ||||
| Dawson (2000) a | 21 | ↑114 | 113, 115 | ||||||||||
| Dawson (2000) b | 20 | ↑120 | 119, 121 | ||||||||||
| Gadowsky (1995) | 50 | ↑119 | 115, 123 | ||||||||||
| Iannotti (2005) | 35 | ↔111 | 107, 115 | 70 | ↔107 | 104, 110 | |||||||
| McGuire Davies (2010) | 78 | ↑118 | 118, 118 | 76 | ↓107 | 107, 108 | |||||||
| Meier (2002) a | 20 | ↑125 | 122, 129 | 15 | ↑123 | 119, 128 | |||||||
| Meier (2002) b | 17 | ↑128 | 124, 132 | 16 | ↑117 | 112, 123 | |||||||
| Meier (2002) a | 19 | ↑116 | 112, 120 | ||||||||||
| Meier (2002) b | 16 | ↑113 | 110, 116 | ||||||||||
| Rycel (2009) | 102 | ↑120 | 120, 229 | ||||||||||
| Rycel (2009) | 102 | ↓103 | 103, 103 | ||||||||||
| Young (2010) | 48 | ↑113 | 62 | ↑117 | 113, 121 | ||||||||
| Ferritin (μg/L or ng/ml) b | Gadowsky (1995) | 50 | ↓7.4 | 5.7, 9.1 | |||||||||
| Iannotti (2005) | 44 | ↑33 | 26.8, 40.6 | 59 | ↔15 | 12.6, 17.8 | |||||||
| Meier (2002) a | 19 | ↑42 | 31.3, 52.8 | 15 | ↔25 | 14.6, 35.4 | |||||||
| Meier (2002) b | 17 | ↑57 | 36.5, 77.5 | 15 | ↓6.8 | 5.2, 8.5 | |||||||
| Meier (2002) a | 19 | ↑46 | 24, 68.8 | ||||||||||
| Meier (2002) b | 16 | ↓10 | 7.9, 13 | ||||||||||
| Young (2010) | 81 | ↔16 | 12.9, 20.1 | 88 | ↔17 | 14.9, 20.3 | |||||||
| Haematocrit (g/L) c | Chang (2003) | 445 | ↑36 | 35.7, 36.3 | 319 | ↔33 | 32.7, 33.3 | 836 | ↓32 | 31.8, 32.2 | |||
| Gadowsky (1995) | 50 | ↑36 | 34.8, 37.2 | ||||||||||
| Iannotti (2005) | 35 | ↔33 | 32, 34 | 70 | ↓32 | 31.3, 32.7 | |||||||
| Rycel (2009) | 102 | 34 | 34.6, 34.8 | ||||||||||
| Rycel (2009) | 102 | ↓31 | 31.4, 31.6 | ||||||||||
| Zinc μmol/L-1 d | Castillo-Duran (2001) a | 249 | ↑11.9 | 11.7, 12.1 | 249 | ↑10.9 | 10.7, 11.1 | ||||||
| Castillo-Duran (2001) b | 258 | ↑11.7 | 11.5, 11.9 | 258 | ↑10.9 | 10.7, 11.1 | |||||||
| Castillo-Duran (2001) a | 249 | ↑10.5 | 10.3, 10.7 | ||||||||||
| Castillo-Duran (2001) b | 258 | ↑10.2 | 10, 10.4 | ||||||||||
| Chan (2006) | 23 | ↑26.3 | 23.2, 29.4 | ||||||||||
| Mistry (2014) a | 107 | ↑9.71 | 8.8, 10.5 | ||||||||||
| Mistry (2014) b | 19 | ↑10.8 | 7.8, 13.9 | ||||||||||
| Magnesium mmol/l e | Chan (2006) | 23 | ↑0.99 | 0.9, 1.1 | |||||||||
| Phosphorous mg/dl f | Chan (2006) | 23 | ↑5 | 4, 6 | |||||||||
| Copper μg/dl g | Mistry (2014) a | 107 | ↑206 | 1991, 2128 | |||||||||
| Mistry (2014) b | 19 | ↑196 | 1712, 2207 | ||||||||||
| Selenium μg/L h | Dawson (2000) a | 21 | ↓49 | 46, 52 | 21 | ↓55 | 52.9, 57.1 | 21 | ↑114 | 108.9, 119.1 | |||
| Dawson (2000) b | 20 | ↓44 | 39.6, 48.4 | 20 | ↓53 | 50.8, 55.2 | 20 | ↓55 | 50.2, 59.8 | ||||
| Dawson (2000) a | 21 | ↓50 | 46.2, 53.8 | 21 | ↔85 | 76.9, 93.1 | |||||||
| Dawson (2000) b | 20 | ↓44 | 39.6, 48.4 | 20 | ↓62 | 57.2, 66.8 | |||||||
| Mistry (2014) a | 107 | ↓65 | 62.7, 67.5 | ||||||||||
| Mistry (2014) b | 19 | ↓49 | 45.9, 52.9 | ||||||||||
| Red blood cell folate (nmol/l) i | Baker (2009) | 266 | ↑647 | 616, 680 | |||||||||
| Serum folate (nmol/l) j | Baker (2009) | 291 | ↑12 | 12, 14 | |||||||||
| Chan (2006) | 23 | ↔13 | 10, 17 | ||||||||||
| Vitamin A (μg/dL) | Chan (2006) | 23 | ↑38 | 31, 45 | |||||||||
| B12 (pmol/l) k | Baker (2009) | 293 | ↑177 | 169, 185 | |||||||||
| Chan (2006) | 23 | ↑265 | 216, 315 | ||||||||||
| Gadowsky (1995) | 50 | ↑170 | 146, 194 | ||||||||||
| Homocysteine (μmol/L) l | Baker (2009) | 293 | 7.9 | 7.6, 8.2 | |||||||||
| Gadowsky (1995) | 50 | 6.1 | 3, 9 | ||||||||||
| 25OHD (nmol/L) m | Baker (2009) | 263 | ↑33 | 30.4, 35.8 | |||||||||
| Chan (2006) | 23 | ↑57 | 48, 67 | ||||||||||
| Ginde (2010) | |||||||||||||
| McGuire Davies (2010) | 44 | ↑52 | 46, 58 | 36 | ↑56 | 50, 63 | |||||||
| O’Brien (2003) | 23 | ↑50 | 41, 60 | ||||||||||
| Young (2012) | 171 | ↑54 | 51, 59 | ||||||||||
a Target value 110 g/L, b Target value 15 μg/L, c Target value33 g/L, d Target value 6.12 μmol/L-1, e Target value 0.9 mmol/L, f Target value 2.5 mg/dl, g Target value 63.7 μg/dl, h Target value 90 μg/L, i Target value 340 nmol/L, j Target value 20 μg/dl, k Target value 150pmol/L, l Target value less than 13 μmol/L, m Target value 25 nmol/L, ↑ Study mean higher than reference value (p < 0.05), ↔ Study mean not different to reference value (p < 0.05), ↓ Study mean lower than reference value (p < 0.05)
The results show that the mean reported biomarker values in the majority of studies suggested that young women’s nutritional status was sufficient, with the exception of markers of iron and selenium status. Results for haematocrit and plasma ferritin were mixed, with results being more likely to be below the cut off in the third trimester and at delivery. Measures of serum selenium were reported to be less than the cut off in the majority of studies.
Examination of pooled, weighted means showed that only mean selenium concentration was below the reference value. The weighted means for all biological markers where there were two or more valid results are shown in Table 8.
Table 8.
Pooled weighted means of biological markers
| Biological Marker | Pooled Mean | Confidence Interval | Target Value | Number of studies |
|---|---|---|---|---|
| Haemoglobin (g/dL) | 112.4 | 95.4–133.8 | 110 | 9 |
| 25_OH_D (nmol/L) | 55.9 | 6.2–105.7 | 25 | 6 |
| Ferritin (μg/L) | 18.2 | 0–48.3 | 15 | 4 |
| Haematocrit (g/L) | 32.5 | 28.0–39.4 | 33 | 4 |
| Zinc (μmol/L-1) | 11.5 | 5.9–17.1 | 6.12 | 3 |
| B12 (pmol/L) | 181.6 | 0–271.1 | 150 | 3 |
| Serum Folate (nmol/L) | 12.8 | 0–26.8 | 10 | 2 |
| Selenium (μg/L) | 61.8* | 39.2–84.4 | 90 | 2 |
| Homocystine (μmol/L) | 7.6 | 0–17.0 | Less than 13 | 2 |
* Significant at the p<0.005 level
The sub-group analysis by country of origin was only possible by US vs. non-US studies as there was only one UK based study reporting biological markers. The analysis failed to detect any differences by study country of origin. Analysis by stage of pregnancy suggests a decline in iron status markers haemoglobin, haematocrit and ferritin as pregnancy progresses; the levels observed however do not necessarily reflect iron deficiency. Detailed results of the sub-group analysis of biological markers are available in Additional file 1: Table S4 and Additional file 1: Table S5.
Discussion
Compared with reviews of the nutritional status of pregnant adolescents published in 2007, this review identified a further 13 studies that reported data on nutritional intakes and biomarkers of status. The summary results show that there may be areas of concern in adolescent’s nutritional intake during pregnancy, particularly compared to US recommendations, with regard to calcium, vitamin D, vitamin E, folate, potassium and magnesium. The evidence also suggests that overall energy intake may be lower than recommended.
In terms of comparison with dietary reference values, combined analysis of the individual study means showed very few statistically significant results, with the exception of vitamin D. One possible explanation for this is that there was a high level of variance between participants in the majority of studies resulting in very wide confidence intervals. This suggests that there may be sub-groups of young women within the total population who are at higher risk of poor nutritional status which this review has failed to detect. Differences in micronutrient intake were observed between UK and USA based studies which may in part be explained by routine fortification of food products in the USA compared to the UK. Only one study reported that supplements were included in the reported intake values, and the compliance rates for supplements in this study were low, meaning that the impact of supplement use on intakes data is marginal.
Macronutrient contributions to energy were found to be roughly in line with recommendations, however there was a significant lack of data for carbohydrates (including sugars and fibre) and fats (including saturated fat) meaning these results should be interpreted with caution. Further research into macronutrient intakes in this population, particularly with regard to types of carbohydrates and fats, is needed.
The methods used to assess dietary intake varied across the included studies. The two methods reported by the included studies were 24 h dietary recalls (single and multiple) and food diaries. While these are validated and accepted methods of nutritional surveillance [45], it is acknowledged that underreporting biases may exist [46] and so results should be considered with this in mind.
Four of the studies reporting energy intake also reported mean gestational weight gain which ranged from 14 kg to 17 kg, consistently higher than the required pregnancy weight gain [47]. Mean percentage intake of the EAR for energy was higher in the studies reporting weight gain than those which did not report this measure, but not significantly so. This is potentially contradictory of the finding that energy intake was low in individual studies and suggests further work is needed regarding the potential level of under reporting in this population and the relationship between dietary patterns, overall energy consumption and gestational weight gain.
Inadequacies in nutrient intakes did not necessarily translate to systemic deficiencies as measured by mean values of biological markers, with the possible exceptions of markers of iron and selenium status. One possible explanation for this is that food intake may have been under reported therefore suggesting that intake was insufficient when this was not the case. A further possible explanation is that measures of biological markers were elevated by dietary supplements. Details regarding the type, dose, duration and number of participants taking supplements were inconsistent in the included papers meaning that detailed analysis of the impact of supplement use on nutritional status was not possible, however 10 out of the 15 included studies reporting biomarkers did report some level of supplementation. This finding does suggest that supplements may play an important role in ensuring young women do not experience nutrient insufficiency, however attention to clear reporting of supplement use in research papers is essential to allow a better understanding of the impact of supplementation on nutritional status.
The participants in all of the included studies where supplements were provided may also have been more compliant with taking supplements due to the very fact that they were taking part in a research study than might be expected outside of a study environment. A systematic review [48] of the effect of dietary interventions in adolescent pregnancies found some evidence to suggest that nutritional supplements may reduce the likelihood of low birth weight; however the review also reported a serious lack of good quality research papers in this area. Further work to establish the extent to which pregnant young women in the general population suffer more from nutrient deficiencies and the impact of supplement use would be advantageous.
There is significant evidence in the literature regarding the role of nutrition in supporting healthy pregnancies and allowing the foetus to achieve its full potential. Adolescent girls are at particular risk of iron deficiency anaemia due in part to rapid growth during adolescence [49] combined with the onset of menarche. This coupled with the increased demand for iron in pregnancy for expansion of maternal tissues and foetal growth, makes pregnant adolescents a particularly vulnerable group. There is some evidence to suggest that iron deficiency is implicated in the risk of adverse birth outcomes such as prematurity and low birth weight [50], meaning this is potentially an important factor in improving maternal and infant health. While analysis of mean values for markers of iron deficiency in this review did not indicate a significant issue, consideration of the reported prevalence of iron deficiency anaemia in the included studies suggests that this may be a concern for this population.
Vitamin D and calcium have an essential role in the mineralization of the developing foetal skeleton and insufficient intake of these nutrients may impact on foetal bone growth. The interaction between these two nutrients has been shown to be key to maximising foetal bone growth in pregnant adolescents and that growth is adversely affected when either of the two nutrients were lacking [30]. The pooled mean for vitamin D status as reflected in blood 25 (OH)D in this review was significantly below recommended levels across all trimesters. It was not possible to conduct analysis of vitamin D status by ethnicity or exposure to sunlight, however, which are factors known to have a significant impact on vitamin D status [51–53].
The role of folate in the prevention of neural tube defects in early pregnancy has been well documented [54]. There is a lack of data collected in early pregnancy in the papers included in the current review, however the observed failure to meet recommendations for folate intake in later pregnancy reported may suggest that the participants were unlikely to have been meeting recommendations prior to taking part in a research study. A systematic review of the impact of folate intake over the course of pregnancy [55] also found a significant effect in the second and third trimesters on infant birth weight, suggesting that the importance of folate for a healthy pregnancy extends beyond the first trimester.
Selenium status has been identified as a potential area of concern in this review. Selenium is a trace element which has an anti-oxidative effect and protects cell membranes [56]. The target value used to assess selenium status was based on the intake necessary for maximisation of plasma glutathione peroxidase activity, which is the criteria used in the derivation of the US RDA [18]. Selenium status has been shown to be associated with a number of adverse outcomes for both mother and child including neural tube defects [57], lower birth weights [58], cholestasis [59] and gestational diabetes [60].
While demographic characteristics of participants were reported in the included studies data was generally reported for the study population as a whole, meaning that sub-group analysis was not possible. This is significant in that evidence suggests that younger adolescents, those who smoke and those from more deprived backgrounds may be at higher risk of nutritional issues [3, 61, 62].
There is also evidence to suggest that the nutritional status of adult pregnant women may raise similar concerns to those identified within this review. A systematic review of micronutrient intakes in pregnancy found that intakes of folate, vitamin D and iron were sub-optimal [63]. A further review focusing on energy and macronutrient intake in this population found that intakes of energy and fibre were also below recommendations [64]. These results are consistent with the findings of this review suggesting that maternal age alone may not be the most important factor in sub-optimal nutritional status during pregnancy. Further work to identify the characteristics of those most at risk, particularly within the adolescent population, and the nature of that risk is needed.
Limitations
There were some significant limitations which impact on the conclusions of this review. The majority of the included papers used convenience samples meaning that there is likely to be an element of bias in the reported outcomes. The majority of participants in the included studies were aged 16 and over meaning that the results may not be generalisable to younger adolescents, who may also be at greater nutritional risk compared to older adolescents due to competing growth needs [3]. The lack of detail regarding participant’s supplement use meant that it was not possible to evaluate the impact of supplements on biological markers of nutritional status. It is therefore likely that these results may have been biased by supplement use in some participants.
There was significant heterogeneity in the included papers in terms of study design. Measurements of dietary intake differed between papers however the majority of studies used 24 h recall methods to assess nutrient intake. This has been shown to have limitations in terms of both participants reporting their intake accurately and the likelihood that the recorded intake is representative of the usual diet, particularly in adolescents [46]. Three studies used multiple 24 h recalls in order to produce more reliable estimates of intakes; however this approach was not consistent across the included studies using this method.
There were also considerable differences in the number of nutritional indicators represented. Pooled means were calculated wherever two or more data points were available in order to maximise the results available from the review. This however means that some estimates will be more robust than others depending on the number of data points on which they are based. There was a large degree of variation in the amount of data available for different nutrients, for example assessment of serum selenium was based on data from only two papers whereas nine independent studies contributed data on haemoglobin concentration.
The pooling of study means gives a useful indication of potential inadequacies across the population as a whole; however this approach lacks the sensitivity to draw conclusions regarding the prevalence of nutrient deficiencies in the population. Examination of the reported prevalence of deficiency in the included studies shows results which are inconsistent with the analysis based on study means. The prevalence of iron deficiency anaemia measured by haemoglobin concentration reportedly ranged from 1.2 to 63.5 %, with prevalence in the third trimester ranging from 29 to 63.5 %. Other markers of iron status followed a similar pattern with higher prevalence of deficiency occurring in the third trimester. The prevalence of vitamin D deficiency in many of these studies was also higher than suggested by the analysis of study means. This suggests, similarly to the data regarding nutrient intakes, that there are substantial skews within the data and sub-groups of young women who may be more at risk of deficiencies.
Conclusion
This review identifies some concerns in the nutritional status of pregnant adolescents which may impact on maternal and infant outcomes. Intake of vitamin D and serum selenium status were identified as being significantly low in pooled analysis of included studies. Fibre intake was also below recommendations.. This said there are some significant limitations meaning these results should be interpreted with caution. No analysis of the effect of demographic characteristics on either nutritional intake or biological markers was possible, nor was it possible to examine the impact of supplement use on biological markers.
Patterns in this population are also similar to those reported in the adult population during pregnancy. These findings suggest that targeted work to identify those most at risk, and the nature of that risk, is needed. Recommendations for other areas of further research include the macronutrient composition of adolescent’s diet during pregnancy, the relationship between nutrient intakes and birth outcomes and the role of nutritional supplements in this population.
Acknowledgments
The authors would like to thank Dr Karen Kilner for her assistance with the statistical methods used in this review.
We would also like to thank the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Yorkshire and Humber (NIHR CLAHRC YH) for supporting us in conducing this review. Further details about the new NIHR CLAHRC YH can be found at www.clahrc-yh.nihr.ac.uk. The views and opinions expressed are those of the authors, and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
This review has been carried out as part of a PhD project supported by Sheffield Hallam University and the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Yorkshire and Humber. Therefore no additional funding was required for this work.
Availability of data and materials
All data on which the paper is based has been made available in either the main tables or Additional file 1: Tables S1-S5.
Authors’ contributions
KM-D Primary responsibility for development of research question and study design, extraction and analysis of data and writing the main body of the article. VB Advisory role in development of research question and study design, revisions to and approval of final article. HS Advisory role in development of research question and study design, cross-checking of all extracted data, revisions to and approval of final article. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was not required for this systematic review due to only studies previously published in the academic literature being eligible for inclusion.
Abbreviations
- CASP
Critical appraisal and skills program
- DRV
Dietary reference value
- EAR
Estimated average requirement
- RDA
Recommended daily allowance
- RNI
Reference nutrient intake
- WHO
World Health Organisation
Additional file
Table S1. Characteristics of Excluded Studies. Table S2. Pooled, Weighted Mean Nutrient Intakes Expressed as Percentages of Dietary Reference Values by Stage of Pregnancy. Table S3. Pooled, Weighted Mean Nutrient Intakes Expressed as Percentages of Dietary Reference Values by Study Country of Origin. Table S4. Pooled, Weighted Mean Biological Markers of Nutritional Status by Stage of Pregnancy. Table S5. Pooled, Weighted Mean Biological Markers of Nutritional Status by Study Country of Origin. (DOCX 57 kb)
Contributor Information
Katie Marvin-Dowle, Email: katie.marvin-dowle@student.shu.ac.uk.
Victoria Jane Burley, Email: v.j.burley@leeds.ac.uk.
Hora Soltani, Phone: 0114 2255444, Email: H.Soltani@shu.ac.uk.
References
- 1.Cook SM, Cameron ST. Social issues of teenage pregnancy. Obstetrics Gynaecol Rep Med. 2015;25(9):243–248. doi: 10.1016/j.ogrm.2015.06.001. [DOI] [Google Scholar]
- 2.Office for National Statistics. Census data. http://www.ons.gov.uk/ons/guide-method/census/2011/census-data/index.html. Updated 2014. Accessed 1 Mar 2016.
- 3.Gibbs CM, Wendt A, Peters S, et al. The impact of early age at first childbirth on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26(s1):259–284. doi: 10.1111/j.1365-3016.2012.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke BS, Harding VV, Stuart HC. Nutrition studies during pregnancy: IV. Relation of protein content of mother’s diet during pregnancy to birth length, birth weight, and condition of infant at birth. J Pediatr. 1943;23(5):506–515. doi: 10.1016/S0022-3476(43)80253-5. [DOI] [Google Scholar]
- 5.Oliver MH, Jaquiery AL, Bloomfield FH, et al. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc Rep Fertil suppl. 2007;64:397. doi: 10.5661/rdr-vi-397. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SP, Guenther PM, Kretsch MJ. Using the dietary reference intakes to assess intakes of groups: pitfalls to avoid. J Am Diet Assoc. 2006;106(10):1550–1553. doi: 10.1016/j.jada.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr. 2003;133(6):1997S–2002S. doi: 10.1093/jn/133.6.1997S. [DOI] [PubMed] [Google Scholar]
- 8.Bates, B., Lennox, A., Prentice, A., et.al (Eds.). National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009-2011/2012): A Survey Carried Out on Behalf of Public Health England and the Food Standards Agency. 2014
- 9.Kearney J. Food consumption trends and drivers. Philo Trans Royal Soc London B. 2010;365(1554):2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran VH. A systematic review of dietary assessments of pregnant adolescents in industrialised countries. Br J Nutr. 2007;97(03):411–425. doi: 10.1017/S0007114507381373. [DOI] [PubMed] [Google Scholar]
- 11.Hall Moran V. Nutritional status in pregnant adolescents: a systematic review of biochemical markers. Maternal child nutri. 2007;3(2):74–93. doi: 10.1111/j.1740-8709.2007.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik K. Human development report 2014. New York: New York United Nations Development Programme; 2014.
- 13.Onis MD, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Critical Appraisal Skills Programme. CASP checklists. http://www.casp-uk.net/#!casp-tools-checklists/c18f8. Updated 2013. Accessed 7 Dec 2015.
- 15.Department of Health (DoH). Dietary reference values for food energy and nutrients for the United Kingdom. Committee on Medical Aspects of Food Policy. Report on Health and Social Subjects. 1991;41:xxii-xxiv. [PubMed]
- 16.Otten JJ, Hellwig JP, Meyers LD. (Eds.). Dietary reference intakes: the essential guide to nutrient requirements. Washington DC: National Academies Press; 2006.
- 17.Scientific Advisory Committee for Nutrition . Dietary reference values for energy. London: HMSO; 2011. [Google Scholar]
- 18.Duffield AJ, Thomson CD, Hill KE, et al. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70(5):896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 19.Leffler D. BMJ Best Practice Zinc deficiency. 2015 http://bestpractice.bmj.com Accessed 1 Jun 2016
- 20.Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998;67(5):952S–959S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 21.Romani A. Assessment of magnesium deficiency. 2015 http://bestpractice.bmj.com Accessed 1 Jun 2016
- 22.Walker HK, Hall WD, Hurst JW, et al. Chapter 198. In: Walker HK, Hall WD, Hurst JW, et al., editors. Serum inorganic phosphorus in Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 23.Baker PN, Wheeler SJ, Sanders TA, et al. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr. 2009;89(4):1114–1124. doi: 10.3945/ajcn.2008.27097. [DOI] [PubMed] [Google Scholar]
- 24.Chan GM, McElligott K, McNaught T, et al. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet Gynecol. 2006;108(3, Part 1):565–571. doi: 10.1097/01.AOG.0000231721.42823.9e. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S., Young, B. E., Cooper, E. M., et.al. Nutrient inadequacy is prevalent in pregnant adolescents, and prenatal supplement use may not fully compensate for dietary deficiencies. ICAN: Infant, Child, & Adolescent Nutrition. 2014. 1941406414525993
- 26.Pope JF, Skinner JD, Carruth BR. Adolescents’ self-reported motivations for dietary changes during pregnancy. J Nutr Educ. 1997;29(3):137–144. doi: 10.1016/S0022-3182(97)70178-X. [DOI] [Google Scholar]
- 27.Giddens JB, Krug SK, Tsang RC, et al. Pregnant adolescent and adult women have similarly low intakes of selected nutrients. J Am Diet Assoc. 2000;100(11):1334–1340. doi: 10.1016/S0002-8223(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez YM. Cultural factors affecting diet and pregnancy outcome of Mexican American adolescents. J Adolesc Health. 1999;25(3):227–237. doi: 10.1016/S1054-139X(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 29.Young MF, Pressman E, Foehr ML, et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31(11):1010–1014. doi: 10.1016/j.placenta.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Young BE, McNanley TJ, Cooper EM, et al. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr. 2012;95(5):1103–1112. doi: 10.3945/ajcn.111.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire Davis L, Chang SC, Mancini J, et al. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Ginde AA, Sullivan AF, Mansbach JM, et al. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436–e1. doi: 10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien KO, Nathanson MS, Mancini J, et al. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr. 2003;78(6):1188–1193. doi: 10.1093/ajcn/78.6.1188. [DOI] [PubMed] [Google Scholar]
- 34.Meier PR, Nickerson HJ, Olson KA, et al. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin Med Res. 2003;1(1):29–36. doi: 10.3121/cmr.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson EB, Albers JH, McGanity WJ. The apparent effect of iron supplementation on serum selenium levels in teenage pregnancy. Biol Trace Elem Res. 2000;77(3):209–217. doi: 10.1385/BTER:77:3:209. [DOI] [PubMed] [Google Scholar]
- 36.Iannotti LL, O’Brien KO, Chang SC, et al. Iron deficiency anemia and depleted body iron reserves are prevalent among pregnant African-American adolescents. J Nutr. 2005;135(11):2572–2577. doi: 10.1093/jn/135.11.2572. [DOI] [PubMed] [Google Scholar]
- 37.Chang SC, O’Brien KO, Nathanson MS, et al. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr. 2003;133(7):2348–2355. doi: 10.1093/jn/133.7.2348. [DOI] [PubMed] [Google Scholar]
- 38.Pobocik RS, Benavente JC, Boudreau NS, et al. Pregnant adolescents in Guam consume diets low in calcium and other micronutrients. J Am Diet Assoc. 2003;103(5):611–614. doi: 10.1053/jada.2003.50114. [DOI] [PubMed] [Google Scholar]
- 39.Derbyshire E. Dietary habits of pregnant and non-pregnant adolescents. Nutri Food Sci. 2009;39(5):490–498. doi: 10.1108/00346650910992141. [DOI] [Google Scholar]
- 40.Mistry HD, Kurlak LO, Young SD, et al. Maternal selenium, copper and zinc concentrations in pregnancy associated with small‐for‐gestational‐age infants. Maternal child nutri. 2014;10(3):327–334. doi: 10.1111/j.1740-8709.2012.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Job, J., Capra, S., & Ash, S. Nutritional assessment of pregnant teenagers attending a metropolitan, public, and maternity hospital in Brisbane. I. Nutrient intakes. Australian j nutri diet. 1995
- 42.Gadowsky SL, Gale K, Wolfe SA, et al. Biochemical folate, B 12, and iron status of a group of pregnant adolescents accessed through the Public Health System in Southern Ontario. J Adolesc Health. 1995;16(6):465–474. doi: 10.1016/1054-139X(94)00001-U. [DOI] [PubMed] [Google Scholar]
- 43.Castillo-Durán C, Marín VB, Alcázar LS, et al. Controlled trial of zinc supplementation in Chilean pregnant adolescents. Nutr Res. 2001;21(5):715–724. doi: 10.1016/S0271-5317(01)00285-8. [DOI] [Google Scholar]
- 44.Rycel M, Sobala W, Wilczynski J, et al. Hemoglobin and hematocrit concentrations influence birth outcome in pregnant polish adolescents. Arch Perinatal Med. 2009;15(2):101–105. [Google Scholar]
- 45.Rockett HR, Colditz GA. Assessing diets of children and adolescents. Am J Clin Nutr. 1997;65(4):1116S–1122S. doi: 10.1093/ajcn/65.4.1116S. [DOI] [PubMed] [Google Scholar]
- 46.Livingstone MBE, Robson PJ, Wallace JMW. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92(S2):S213–S222. doi: 10.1079/BJN20041169. [DOI] [PubMed] [Google Scholar]
- 47.Luke B. Nutrition During Pregnancy: Part I, Weight Gain; Part II, Nutrient Supplements. JAMA. 1991;265(2):281–282. doi: 10.1001/jama.1991.03460020139044. [DOI] [Google Scholar]
- 48.Soltani H, Duxbury A, Rundle R, et al. A systematic review of the effects of dietary interventions on neonatal outcomes in adolescent pregnancy. Evidence Based Midwifery. 2015;13(1):29–34. [Google Scholar]
- 49.Scientific Advisory Committee for Nutrition . Iron and health. London: HMSO; 2010. [Google Scholar]
- 50.Scholl TO, Hediger ML, Fischer RL, et al. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. 1992;55(5):985–988. doi: 10.1093/ajcn/55.5.985. [DOI] [PubMed] [Google Scholar]
- 51.Webb AR, Kline L, Holick MF. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D3: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D3 Synthesis in Human Skin*. j clin endocrinol metabol. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Meer IM, Karamali NS, Boeke AJP, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84(2):350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- 53.Scientific Advisory Committee for Nutrition . Draft vitamin D and health report. London: HMSO; 2015. [Google Scholar]
- 54.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- 55.Fekete K, Berti C, Trovato M, et al. Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J. 2012;11(1):1. doi: 10.1186/1475-2891-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pieczyńska J, Grajeta H. The role of selenium in human conception and pregnancy. J Trace Elem Med Biol. 2015;29:31–38. doi: 10.1016/j.jtemb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Cengiz B, Söylemez F, Öztürk E, Çavdar AO. Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects. Biol Trace Elem Res. 2004;97(3):225–235. doi: 10.1385/BTER:97:3:225. [DOI] [PubMed] [Google Scholar]
- 58.Bogden JD, Kemp FW, Chen X, Stagnaro-Green A, Stein TP, Scholl TO. Low-normal serum selenium early in human pregnancy predicts lower birth weight. Nutr Res. 2006;26(10):497–502. doi: 10.1016/j.nutres.2006.08.008. [DOI] [Google Scholar]
- 59.Reyes H, Báez ME, González MC, Hernández I, Palma J, Ribalta J, Zapata R. Selenium, zinc and copper plasma levels in intrahepatic cholestasis of pregnancy, in normal pregnancies and in healthy individuals, in Chile. J Hepatol. 2000;32(4):542–549. doi: 10.1016/S0168-8278(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 60.Al-Saleh E, Nandakumaran M, Al-Shammari M, Makhseed M, Sadan T, Harouny A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in insulin-dependent diabetic pregnancies. Arch Gynecol Obstet. 2005;271(3):212–217. doi: 10.1007/s00404-004-0636-8. [DOI] [PubMed] [Google Scholar]
- 61.Golley RK, Hendrie GA, McNaughton SA. Scores on the dietary guideline index for children and adolescents are associated with nutrient intake and socio-economic position but not adiposity. J Nutr. 2011;141(7):1340–1347. doi: 10.3945/jn.110.136879. [DOI] [PubMed] [Google Scholar]
- 62.Giannakopoulos G, Panagiotakos D, Mihas C, et al. Adolescent smoking and health‐related behaviours: Interrelations in a Greek school‐based sample. Child Care Health Dev. 2009;35(2):164–170. doi: 10.1111/j.1365-2214.2008.00906.x. [DOI] [PubMed] [Google Scholar]
- 63.Blumfield ML, Hure AJ, Macdonald‐Wicks L, et al. A systematic review and meta‐analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. 2013;71(2):118–132. doi: 10.1111/nure.12003. [DOI] [PubMed] [Google Scholar]
- 64.Blumfield ML, Hure AJ, Macdonald-Wicks L, et al. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr Rev. 2012;70(6):322–336. doi: 10.1111/j.1753-4887.2012.00481.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data on which the paper is based has been made available in either the main tables or Additional file 1: Tables S1-S5.


