Abstract
Aims
To date, studies on sleep disturbances in type 1 diabetes (T1D) have been limited to youth and/or small samples. We therefore assessed the prevalence of subjective sleep disturbances and their associations with glycemia and estimated insulin sensitivity in individuals with longstanding T1D.
Methods
We conducted a cross-sectional study including 222 participants of the Epidemiology of Diabetes Complications study of childhood-onset T1D attending the 25-year examination (mean age=52 years, diabetes duration=43 years). The Berlin Questionnaire (risk of obstructive sleep apnea, OSA), the Epworth Sleepiness Scale (daytime sleepiness), and the Pittsburgh Sleep Quality Index (sleep quality, bad dreams presence, and sleep duration) were completed. Associations between sleep disturbances and poor glycemic control (HbA1c ≥7.5%/58 mmol/mol), log-transformed HbA1c, and estimated insulin sensitivity (estimated glucose disposal rate, eGDR, squared) were assessed in multivariable regression.
Results
The prevalences of high OSA risk, excessive daytime sleepiness, poor sleep quality, and bad dreams were 23%, 13%, 41%, and 26%, respectively, with more women (51%) reporting poor sleep quality than men (30%, p=0.004). Participants under poor glycemic control were twice as likely to report bad dreams (p=0.03), but not independently (p=0.07) of depressive symptomatology. Sleep duration was directly associated with HbA1c among individuals with poor glycemic control, but inversely in their counterparts (interaction p=0.002), and inversely associated with eGDR (p=0.002).
Conclusions
These findings suggest important interrelationships between sleep, gender, depressive symptomatology, and glycemic control, which may have important clinical implications. Further research is warranted to examine the mechanism of the interaction between sleep duration and glycemic control.
Keywords: Type 1 diabetes, Subjective sleep, Sleep disturbances, Sleep duration, Glycemic control, Insulin sensitivity
1. Introduction
Two related chronic conditions, diabetes and sleep disorders, have increased over the past few decades [1–3]. Disturbed sleep is associated with burdensome public health conditions including incident hypertension [4], coronary heart disease [5, 6], stroke [5, 7], and even all-cause mortality [5]. Importantly, the association between sleep disturbance and diabetes appears to be bidirectional, e.g. poor sleep quality can worsen diabetes control, while diabetes complications can impair sleep quality [8]. The majority of studies linking sleep disturbances and diabetes have focused on type 2 diabetes [9–11] and obesity risk factors. Sleep disturbances in type 1 diabetes (T1D) have been studied, however, most research has been limited to youth [12–14] and/or samples of less than 50 individuals [12–15]. As obesity has been increasing among persons with T1D [16], studying the role of disturbed sleep in this population has now become even more relevant.
The prevalence of sleep-disordered breathing in T1D [17–20] appears somewhat similar to that in the general population, however, without a male preponderance [21]. For instance, polysomnography-measured moderate-to-severe obstructive sleep apnea (OSA, apnea hypopnea index (AHI) >10 events/hour) in a T1D study by Manin et al. was present among 46%, while the prevalence of severe OSA (AHI•30 events/hour) was 19% [20]. No gender difference was present in this study [20]. In another T1D cohort, Schober and colleagues observed a lower moderate-to-severe OSA prevalence (10.3%), although defined as AHI ≥15 events/hour, but did not find differences by gender [19]. In a small T1D pilot study (n=40), Borel et al. observed a high prevalence of OSA (40%) defined as AHI >15 events/per hour or OSA treatment, but did not assess gender differences [18]. In contrast to the T1D studies by Manin et al. [20] and Schober et al. [19], a profound gender difference in moderate-to-severe sleep-disordered breathing defined by the current 2012 American Academy of Sleep Medicine criterion [22] (AHI ≥ 15 events/hour) has been recently reported in the general population (i.e. 23.4% in women; 49.7% in men) [21].
The variability in the prevalence of OSA/sleep-disordered breathing in T1D mainly stems from differences in methodologies and definitions used to characterize disturbed sleep [18–20]. Although polysomnography is the gold standard for diagnosing sleep disorders, it is rarely feasible to implement in large epidemiological studies. Additionally, screening tools for different sleep disturbances are widely available and easy to implement. However, to the best of our knowledge, only one previous study measured various sleep disturbances in T1D adults using several validated sleep questionnaires, but did not assess gender differences [17].
The relationship between disturbed sleep and glycemic control among adults with T1D is inconsistent. While some studies observed non-significant associations [17, 18, 20] between sleep disturbances (e.g. poor sleep quality, excessive daytime sleepiness, high-risk OSA) and hemoglobin A1c (HbA1c), positive associations have also been reported (i.e. with excessive daytime sleepiness, shorter sleep duration, and shorter deep sleep time) [19, 23, 24]. The effect of disturbed sleep on insulin resistance/sensitivity in T1D is also unclear. Nevertheless, a previous T1D study demonstrated a decrease in peripheral insulin sensitivity in seven participants after only one night of restricting sleep duration to four hours [15].
The objectives of our study were therefore to determine the overall and gender-specific prevalence of subjective sleep disturbances using three validated sleep questionnaires and to assess cross-sectional relationships between sleep disturbances and both glycemic control and estimated insulin sensitivity in a well-characterized cohort of adults with long-standing, childhood-onset T1D.
2. Materials and Methods
The study population comprised participants from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study - an ongoing, 25-year prospective cohort of childhood-onset (<17 years) T1D [25, 26]. The EDC was based on individuals diagnosed with incident T1D, or seen within one year of diagnosis, at Children’s Hospital of Pittsburgh between 1950 and 1980. In order to participate in EDC, participants had to reside within a two hours’ drive or 100 miles from the EDC clinic. This cohort was previously shown to be representative of the T1D population in Allegheny County, Pennsylvania [27]. At baseline (1986-1988), participants (n=658) were on average 28 years old and had a mean diabetes duration of 19 years. They were re-examined or surveyed every two years post baseline. The study protocol was approved by the University of Pittsburgh Institutional Review Board and a written informed consent was provided prior to any study procedure.
During the most recent, 25-year clinical examination (2011–2014), three validated sleep questionnaires, the Pittsburgh Sleep Quality Index [28] (PSQI), the Epworth Sleepiness Scale [29] (ESS), and the Berlin Questionnaire [30] (BQ), were used for the first time to assess sleep quality, excessive daytime sleepiness, and OSA risk, respectively. The PSQI was self-administered prior to the clinical examination. During the clinical exam, the BQ and ESS were administered by a trained research specialist who also inquired about a history of diagnosed OSA. Out of 376 in-area, exam-eligible participants, 222 (59%) completed the BQ and the ESS, while 196 (52%) fully completed the PSQI. One participant attended the exam but did not complete the sleep questionnaires.
2.1. Subjective sleep disturbances
The PSQI assesses sleep quality and disturbance during the past month [28]. It consists of 19 self-rated questions and five questions rated by a roommate or bed partner which are not calculated into the total score. The 19 questions are grouped into seven component scores (i.e. sleep quality, latency, duration, efficiency, disturbances, sleep medication use, and daytime dysfunctions), weighted equally from 0 to 3. Higher scores indicate worse sleep quality. A global PSQI score is a sum of all component scores (range, 0–21). We used the standard cut-off point to define poor sleep quality (PSQI global score >5). As a proxy for disturbed rapid eye movement (REM) sleep, which has been linked to poor glycemic control in individuals with T1D [24] and type 2 diabetes [31], we defined the presence of bad dreams (any versus none) by utilizing the following PSQI item: “During the past month, how often have you had trouble sleeping because you had bad dreams?” Sleep duration in hours was quantified from PSQI, where participants reported self-perceived duration of actual sleep per night. We also categorized sleep duration by tertiles and by the National Sleep Foundation’s (NSF) [32] adult sleep guidelines (<7 hours=short sleep, 7–9 hours=normal sleep, and >9 hours=long sleep).
The ESS is a widely used and validated method [33, 34] of assessing one’s average level of daytime sleepiness during different daily life situations like sitting, reading, or watching television [29]. Participants are asked to rate eight daily situations on a scale from 0, no chance of dozing, to 3, high chance of dozing. We defined excessive daytime sleepiness using the standard cut-off point (ESS score >10) [35].
The BQ is a validated [30] screening instrument used for classifying individuals as being at high or low OSA risk. The questionnaire is divided into three categories, 1) snoring and breathing cessation during sleep, 2) tiredness and fatigue, and 3) hypertension history and/or body mass index (BMI) >30 kg/m2, for a total of 10 questions. Individuals screening positively on two or more categories are considered to be at high-risk for OSA. We classified our participants as being at high risk for OSA if they screened positively on the BQ and/or self-reported previous OSA diagnosis.
2.2. Glycemic control and estimated insulin sensitivity
All participants had HbA1c measured via the DCA 2000 Analyzer (Bayer, Tarrytown, NY, USA) during the 25-year clinic visit. Poor glycemic control was defined as HbA1c ≥7.5%/58 mmol/mol for consistency with van Dijk et al. [17]. Insulin sensitivity was determined by a previously validated estimated glucose disposal rate (eGDR) regression equation [36].
2.3. Covariates
Demographic and lifestyle factors (i.e. age, gender, race, education, smoking status, alcohol intake, and current medication use) were assessed through self-administered questionnaires prior to the clinic visit. Depressive symptomatology was measured using the self-administered Beck Depression Inventory (BDI) [37].
Clinical and laboratory assessments were performed by a study physician and trained research specialists, as previously described [25, 26], and included weight, height, waist circumference, and hip circumference. BMI was defined as weight in kilograms divided by height in meters squared. Waist to hip ratio (WHR) was calculated by dividing waist by hip circumference. Blood pressure was measured after a five-minute rest according to the Hypertension Detection and Follow-up Program Protocol [38]. Hypertension was defined as blood pressure ≥140/90 mm Hg or antihypertensive medication use. Cholesterol and triglycerides were measured enzymatically by the Cholestech LDX System (Alere, Hayward, CA, USA). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation [39].
2.4. Statistical analysis
Participant characteristics were described displaying means (SD) for normally-distributed variables (age, WHR, LDL), medians (IQR) for non-normally distributed variables (alcohol intake, BMI, diabetes duration, HbA1c, eGDR, systolic blood pressure, diastolic blood pressure, high-density lipoprotein (HDL) cholesterol, triglycerides, and BDI score), and number (percentage) for categorical variables. We assessed normality using the Shapiro-Wilk test. Two-sided, nonparametric Wilcoxon Rank-Sum tests were used to test the differences in continuous variables by the categories of three sleep questionnaires and glycemic control, while two-sided χ2 or Fisher’s Exact tests, where appropriate, were used for categorical variables. Relationships between continuous variables were assessed through Spearman’s correlations.
Logistic regression assessed the relationships between each subjective sleep characteristic/disturbance (i.e. poor sleep quality, excessive daytime sleepiness, high risk for OSA, bad dreams presence, continuous sleep duration, sleep duration tertiles and the NSF categories) and poor glycemic control (i.e. HbA1c ≥7.5%/58 mmol/mol) separately. Variables which were univariately associated with the exposure and the outcome at the conservative p-value of <0.2, but were not considered to be in the causal pathway between the two, were selected as potential confounders. Simple and multivariable logistic regression analyses, adjusting for all potential confounders, were performed. Linear regression models were constructed in the same fashion using natural log-transformed HbA1c (lnHbA1c) as the outcome. The associations between each separate sleep exposure and estimated insulin sensitivity were also modeled via linear regression with eGDR-squared as the outcome. Traditional confounders (i.e. gender and diabetes duration) were also considered in all multivariable regression analyses. We assessed multicollinearity in regression models using the criterion of variance inflation factor <10 as acceptable. In a sensitivity analysis, each significant association between a sleep exposure and poor glycemic control/eGDR-squared was re-assessed, excluding individuals who reported taking sleep medications regularly or as needed.
Statistical analyses were completed in SAS version 9.3 (SAS Institute, Cary, NC). A p-value of less than 0.05 was considered significant.
3. Results
Eligible participants who attended the 25-year EDC exam and completed the sleep questionnaires (n=222) were compared to the exam non-attendees (n=153) with respect to their baseline characteristics. Attendees had lower baseline median HbA1c (8.4%/68 mmol/mol versus 8.7%/72 mmol/mol, p=0.04) and systolic blood pressure (108 mmHg versus 110 mmHg, p=0.03), though no further differences were observed.
Table 1 displays participant overall characteristics. At the 25-year follow-up, when mean participant age was 51.8 years and median diabetes duration was 42.5 years, about half (45.5%) were male and the majority were non-Hispanic white (98.2%). Median HbA1c was 7.5% and 51.8% of the participants had poor glycemic control. Only 6.3% reported taking sleep medications.
Table 1.
Participant characteristics at the 25-year EDC follow-up
| Demographic factors | N | %, Mean ± SD or Median (IQR) |
|---|---|---|
| Age (years) | 222 | 51.8 ± 7.4 |
| Male gender | 101/222 | 45.5% |
| Non-Hispanic white race | 218/222 | 98.2% |
| Education above high school | 170/220 | 77.3% |
|
| ||
| Behavioral factors | ||
| Smoking history | 76/216 | 35.2% |
| Current smokers | 20/214 | 9.3% |
| Alcoholic drinks per week | 209 | 0 (0–3) |
|
| ||
| Diabetes factors | ||
| Diabetes duration (years) | 222 | 42.5 (37.8–48.0) |
| HbA1c (%) | 222 | 7.5 (6.9–8.4) |
| HbA1c (mmol/mol) | 222 | 58 (52–68) |
| HbA1c ≥7.5%/58 mmol/mol | 115/222 | 51.8% |
| eGDR (mg*kg−1*min−1) | 213 | 7.5 (5.8–9.0) |
|
| ||
| Clinical factors | ||
| BMI (kg/m2) | 220 | 27.2 (23.8–31.6) |
| Waist to hip ratio | 215 | 0.87 ± 0.09 |
| SBP (mmHg) | 221 | 115 (106–127) |
| DBP (mmHg) | 219 | 65.5 (59–72) |
| Hypertension presence | 69/220 | 31.4% |
| LDL cholesterol (mg/dL) | 199 | 99.2 ± 30.4 |
| HDL cholesterol (mg/dL) | 222 | 59 (47–73) |
| Triglycerides (mg/dL) | 221 | 70 (49–102) |
| BDI score | 206 | 5 (2–11) |
|
| ||
| Current medications | ||
| Antihypertensives | 54/212 | 25.5% |
| Antidepressants | 52/212 | 24.5% |
| Sleep medications | 14/222 | 6.3% |
Abbreviations: EDC = Epidemiology of Diabetes Complications study, HbA1c = hemoglobin A1c, eGDR=estimated glucose disposal rate, BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, LDL = low-density lipoprotein, HDL = high-density lipoprotein, BDI = Beck Depression Inventory.
3.1. Subjective sleep disturbances
More than one-fourth of participants (26.6%) were at high risk for OSA, with 22.7% screening positively on the BQ and 9.5% reporting past OSA diagnosis (Table 2). While OSA risk and positive BQ screening did not differ by gender, men had a significantly higher prevalence of self-reported OSA diagnosis (14.9% men, 5.0% women, p=0.01). Excessive daytime sleepiness (12.6% prevalence) did not significantly differ by gender. The overall prevalence (41.3%) of poor sleep quality (PSQI >5) was higher in women (51%) than men (30.4%), p=0.004. The prevalence of poor sleep quality was two times higher in women regardless of BDI score, antidepressant use, and sleep medication use (OR=2.11, 95% CI: 1.07–4.17, p=0.03). In addition, women also had higher PSQI component scores measuring sleep disturbance, latency, and efficiency. The presence of bad dreams (26.3% prevalence) did not significantly differ by gender. One hundred (52.1%) participants fell in the NSF’s category of normal sleep, while three individuals (1.6%) reported sleeping longer than nine hours. Only 5 (2.5 %) participants experienced poor sleep quality, high risk for OSA, and excessive daytime sleepiness.
Table 2.
Subjective sleep disturbances among the EDC participants, overall and by gender
| Sleep characteristic | Overall (n=222) | Men (n=101) | Women (n=121) |
|---|---|---|---|
| Berlin Questionnaire | |||
| Habitual snoring | 128 (58.2) | 64 (64.0) | 64 (53.3) |
| Tiredness and fatigue | 53 (24.1) | 19 (19.0) | 34 (28.3) |
| BMI >30 kg/m2 and/or high blood pressure | 44 (20.0) | 16 (16.0) | 28 (23.3) |
| Positive BQ screen (high-risk OSA) | 50 (22.7) | 19 (19.0) | 31 (25.8) |
|
| |||
| Self-reported OSA history | 21 (9.5) | 15 (14.9) | 6 (5.0)* |
| High risk for OSA (positive BQ screen and/or self-report of previous OSA diagnosis) | 59 (26.6) | 26 (25.7) | 33 (27.3) |
|
| |||
| Epworth Sleepiness Scale (ESS) | |||
| Total score | 6 (3–9) | 5 (3–8) | 6 (3–9) |
| Excessive daytime sleepiness (ESS>10) | 28 (12.6) | 11 (10.9) | 17 (14.1) |
|
| |||
| Pittsburgh Sleep Quality Index (PSQI) | |||
| Global score | 5 (3–8) | 4 (2–6) | 9 (6–12)* |
| Poor sleep quality (PSQI >5) | 81 (41.3) | 28 (30.4) | 53 (51.0)† |
| Component scores (0–3 points) | |||
| Subjective duration | 0.69±0.90 | 0.66±0.85 | 0.73±0.95 |
| Sleep disturbance | 1.23±0.57 | 1.10±0.54 | 1.35±0.59* |
| Sleep latency | 0.88±0.95 | 0.72±0.86 | 1.03±1.01† |
| Daytime dysfunction | 0.75±0.72 | 0.67±0.69 | 0.82±0.74 |
| Sleep efficiency | 0.61±0.93 | 0.42±0.79 | 1.02±0.86† |
| Overall sleep quality | 1.00 ±0.80 | 0.89±0.76 | 1.11±0.82 |
| Need medications to sleep | 0.55±1.08 | 0.49±1.05 | 0.59±1.12 |
| Presence of bad dreams | 51 (26.3) | 21 (22.8) | 30 (29.4) |
|
| |||
| Sleep duration | |||
| Self-reported sleep duration (hours) | 7 (6.0–7.5) | 7 (6–8) | 7 (6–7) |
| EDC tertiles of sleep duration | |||
| <6 hours | 31 (16.2) | 14 (15.6) | 17 (16.7) |
| 6–7 hours | 113 (58.9) | 53 (58.9) | 60 (58.8) |
| >7 hours | 48 (25.0) | 23 (25.6) | 25 (24.5) |
| NSF categories of sleep duration | |||
| <7 hours (short sleep) | 89 (46.3) | 41 (45.6) | 48 (47.1) |
| 7–9 hours (normal sleep) | 100 (52.1) | 48 (53.3) | 52 (51.0) |
| >9 hours (long sleep) | 3 (1.6) | 1 (1.1) | 2 (1.96) |
|
| |||
| Self-reported sleep medication use | 14 (6.3) | 5 (5.0) | 9 (7.4) |
Data are n (%), mean ± SD, or median (IQR)
p<0.05
p <0.005
Abbreviations: EDC = Epidemiology of Diabetes Complications study, BMI = body mass index, BQ = Berlin Questionnaire, OSA= obstructive sleep apnea, NSF = National Sleep Foundation.
3.2. Subjective sleep disturbances and glycemic control
Table 3 depicts participant characteristics by their level of glycemic control. In comparison to participants with good glycemic control, those in poor glycemic control were slightly younger, had shorter diabetes duration, worse insulin sensitivity, slightly lower systolic blood pressure, slightly higher LDL cholesterol, triglycerides, and continuous BDI score, and were slightly more likely to use antidepressants. Results from the logistic regression analysis with poor glycemic control as the outcome are displayed in Table 4. Poor sleep quality, high risk for OSA, excessive daytime sleepiness and sleep duration were not associated with poor glycemic control. Conversely, the presence of bad dreams was twice as likely to be present among individuals with poor glycemic control as in those with better control (OR=2.08, 95% CI: 1.08, 4.01, p=0.03). This association was attenuated after adjustment for continuous BDI score and antidepressant use (OR=1.88, 95% CI: 0.95, 3.71, p=0.07). In the sensitivity analysis, the crude relationship between the presence of bad dreams and poor glycemic control remained after excluding participants who reported taking sleep medications (OR=2.08, 95% CI: 1.06, 4.12, p=0.03). Traditional risk factors such as diabetes duration and gender were allowed in the models as potential confounders, but the results were not altered (data not shown). None of the models showed multicollinearity (all variance inflation factors <3).
Table 3.
Participant characteristics associated with glycemic control and estimated glucose disposal rate
| Demographic factors | N | HbA1c<7.5% | HbA1c≥7.5% | eGDR | |
|---|---|---|---|---|---|
| Median (IQR) or n (%) | Median (IQR) or n (%) | N | Spearman ρ or Median (IQR) | ||
| Age (years) | 222 | 52.0 (47.7–58.1) | 50.5 (46.1–55.9)* | 213 | −0.03 |
| Male gender | 101 | 50 (46.7) | 51 (44.4) | 97 | 6.5 (4.8–8.0)** |
| Female gender | 121 | 57 (53.3) | 64 (55.6) | 116 | 8.6 (6.9−9.5) |
| Non-Hispanic white race | 218 | 105 (98.1) | 113 (98.3) | 210 | 7.5 (5.8–8.9) |
| Non-Hispanic black race | 4 | 2 (1.9) | 2 (1.7) | 3 | 9.0 (7.7–10.4) |
| Education above high school | 170 | 85 (79.4) | 85 (75.2) | 163 | 7.6 (5.9–9.0) |
| Education below high school | 50 | 22 (20.6) | 28 (24.8) | 48 | 7.4 (5.4–9.0) |
|
| |||||
| Behavioral factors | |||||
| Smoking history (positive) | 76 | 36 (34.0) | 40 (36.4) | 73 | 6.9 (5.3–8.7)* |
| (negative) | 140 | 70 (66.0) | 70 (63.6) | 134 | 7.7 (5.9–9.2) |
| Current smokers | 20 | 8 (7.6) | 12 (11.0) | 19 | 7.6 (5.9–8.9) |
| Not currently smoking | 194 | 97 (92.4) | 97 (89.0) | 186 | 7.6 (5.8–9.0) |
| Alcoholic drinks per week | 209 | 0 (0–3) | 0 (0–2.5) | 200 | −0.01 |
|
| |||||
| Diabetes factors | |||||
| Diabetes duration (years) | 222 | 43.1 (39.2–49.2) | 41.9 (36.4–47.3)** | 213 | −0.07 |
| HbA1c (%) | 222 | 6.8 (6.3–7.2) | 8.4 (7.9–9.0)** | — | n/a† |
| HbA1c (mmol/mol) | 222 | 51 (45–55) | 68 (63–75) | — | n/a† |
| eGDR (mg* kg−1* min−1) | 213 | 8.3 (6.3–9.2) | 7.0 (5.0–8.5)** | — | –– |
|
| |||||
| Clinical factors | |||||
| BMI (kg/m2) | 220 | 26.5 (24.2–30.4) | 27.8 (23.3–32.0) | 212 | −0.37** |
| Waist to hip ratio | 215 | 0.86 (0.81–0.93) | 0.88 (0.80–0.96) | — | n/a† |
| SBP (mmHg) | 221 | 117 (108–129) | 114 (104–124)* | — | n/a† |
| DBP (mmHg) | 219 | 66 (60–72) | 64 (58–73) | — | n/a† |
| Hypertension presence | 69/220 | 37 (34.6) | 32 (28.3) | — | n/a† |
| LDL cholesterol (mg/dL) | 199 | 93 (74–116) | 99 (81–120)* | 191 | −0.08 |
| HDL cholesterol (mg/dL) | 222 | 59 (47–74) | 59 (46–73) | 213 | 0.30** |
| Triglycerides (mg/dL) | 221 | 69 (44–87) | 70.5 (52–117)* | 212 | −0.29** |
| BDI score | 206 | 5 (2–9) | 7 (2–12)* | 197 | −0.03 |
|
| |||||
| Current medications | |||||
| Antihypertensives | 54/212 | 28 (26.7) | 26 (24.3) | — | n/a† |
| Antidepressants (Yes) | 52 | 20 (19.1) | 32 (30.0)* | 49 | 6.7 (4.6–8.4)** |
| (No) | 160 | 85 (80.9) | 75 (70.0) | 154 | 7.7 (6.1–9.1) |
| Sleep medications (Yes) | 14 | 5 (4.7) | 9 (7.8) | 12 | 6.7 (4.6–8.0) |
| (No) | 208 | 102 (95.3) | 106 (92.2) | 201 | 7.6 (5.8–9.0) |
0.05≤ p<0.1
p<0.05
Because the eGDR equation comprises waist to hip ratio, hypertension and HbA1c, correlations between eGDR and: HbA1c, waist to hip ratio, SBP, DBP, hypertension presence, and antihypertensive medications were not estimated.
Abbreviations: HbA1c = hemoglobin A1c, eGDR=estimated glucose disposal rate, BMI= body mass index, SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL = low-density lipoprotein, HDL = high-density lipoprotein, BDI=Beck Depression Inventory, n/a = not applicable.
Table 4.
Regression associations between subjective sleep disturbances and: poor glycemic control and estimated glucose disposal rate (squared)
| Sleep exposure | Poor glycemic control (HbA1c ≥ 7.5%/58 mmol/mol) | Estimated glucose disposal rate (mg* kg−1* min−1) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Crude models | Fully adjusted models* | Crude models | Fully adjusted models† | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | B (95% CI) | P-value | β (95% CI) | P-value | |
| Poor sleep quality | 1.08 (0.61, 1.91) | 0.79 | 0.89 (0.47, 1.69) | 0.72 | 2.41 (−1.83, 3.86) | 0.21 | 2.53 (−1.50, 3.89) | 0.15 |
| High risk for OSA | 0.87 (0.48, 1.57) | 0.63 | 0.64 (0.30, 1.37) | 0.25 | −3.19 (−4.42, −0.89) | 0.03 | −2.33 (−3.95, 2.17) | 0.29 |
| EDS (ESS >10) | 1.28 (0.58, 2.85) | 0.54 | 0.77 (0.29, 2.02) | 0.59 | −3.29 (−4.84, −1.35) | 0.09 | −2.57 (−4.46, 2.57) | 0.32 |
| Presence of bad dreams | 2.08 (1.08, 4.01) | 0.03 | 1.88 (0.95, 3.71) | 0.07 | −2.50 (−4.06, 2.02) | 0.23 | −1.79 (−3.66, 2.65) | 0.54 |
| Sleep duration (hours) | 1.11 (0.90, 1.36) | 0.35 | 1.11 (0.88,1.40) | 0.36 | −2.20 (−2.85, −1.23) | 0.005 | –– | –– |
| Sleep duration tertiles | ||||||||
| <6 hours | 1.02 (0.46, 2.27) | 0.79 | 0.95 (0.41, 2.19) | 0.56 | 3.62 (0.73, 5.06) | 0.04 | 3.89 (1.62, 5.26) | 0.02 |
| 6–7 hours | Referent | –– | Referent | –– | Referent | –– | Referent | –– |
| >7 hours | 1.29 (0.66, 2.54) | 0.49 | 1.48 (0.72, 3.01) | 0.27 | −2.68 (−4.25, 1.92) | 0.19 | −2.23 (−3.99, 2.43) | 0.37 |
| Sleep duration (NSF) | ||||||||
| <7 hours (short) | 0.61 (0.34, 1.08) | 0.09 | 0.70 (0.37, 1.34) | 0.28 | 2.49 (−1.73, 3.92) | 0.19 | –– | –– |
| 7–9 hours (normal) | Referent | –– | Referent | –– | Referent | –– | –– | –– |
Poor sleep quality adjusted for continuous BDI score and antidepressant use; High risk for OSA adjusted for diabetes duration, LDL cholesterol, triglycerides, continuous BDI score, antidepressant use, and presence of bad dreams; EDS adjusted for diabetes duration, SBP, continuous BDI score, and presence of bad dreams; Presence of bad dreams adjusted for continuous BDI score and antidepressant use; Continuous sleep duration adjusted for age, SBP, LDL cholesterol, and presence of bad dreams; Sleep duration tertiles adjusted for SBP and continuous BDI score; NSF sleep duration adjusted for LDL cholesterol, continuous BDI score, and presence of bad dreams.
Poor sleep quality adjusted for gender, smoking history, antidepressant use, high risk for OSA, and EDS; High risk for OSA adjusted for smoking history, antidepressant use, and EDS; EDS adjusted for smoking history and high risk for OSA; Presence of bad dreams adjusted for antidepressant use, high risk for OSA, EDS, and continuous sleep duration; Sleep duration tertiles adjusted for smoking history.
Abbreviations: HbA1c = hemoglobin A1c, OSA= obstructive sleep apnea, EDS = excessive daytime sleepiness, ESS = Epworth Sleepiness Scale, NSF = National Sleep Foundation, BDI = Beck Depression Inventory, LDL = low-density lipoprotein, SBP = systolic blood pressure.
In the linear regression analysis, none of the sleep characteristics were significantly associated with lnHbA1c (data not shown). However, the relationship between sleep duration and lnHbA1c was modified by the level of glycemic control (interaction p=0.002, Figure 1). While sleep duration was directly associated with lnHbA1c in individuals with poor glycemic control (β=0.022, 95% CI: 0.006, 0.038, p=0.007) regardless of age, systolic blood pressure, LDL cholesterol, and bad dreams presence, an inverse association was observed among well-controlled individuals independent of the same confounders (β= −0.020, 95% CI: −0.036, −0.005, p=0.01). The interaction between continuous sleep duration and glycemic control persisted upon exclusion of three individuals who reported sleeping longer than nine hours (p=0.02). The relationships between sleep duration tertiles and lnHbA1c and the NSF’s categories of sleep duration and lnHbA1c were also modified by the level of glycemic control (interaction p-values 0.02 and 0.05, respectively).
Figure 1. Regression line plot with 95% confidence bands: Sleep duration versus HbA1c per level of glycemic control.
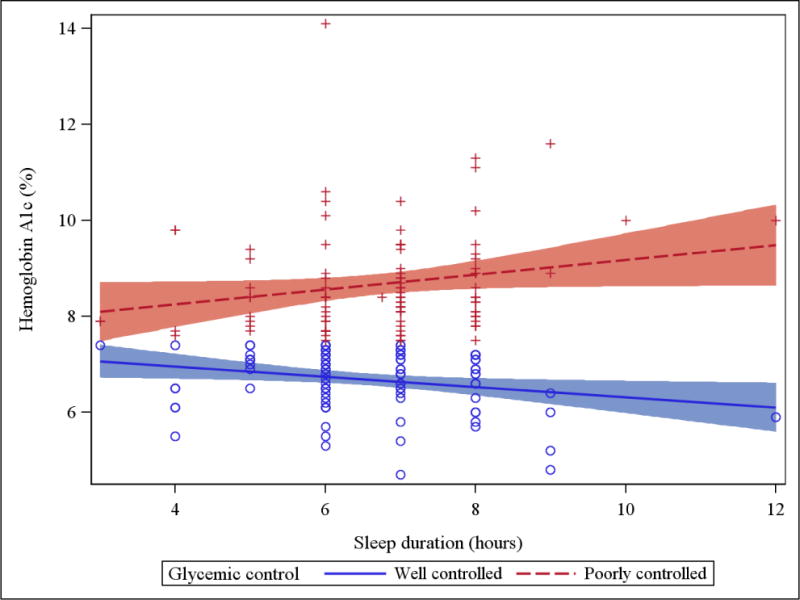
(Well controlled = HbA1c <7.5%/58 mmol/mol; Poorly controlled = HbA1c ≥7.5%/58 mmol/mol)
3.3. Subjective sleep disturbances and estimated insulin sensitivity
Men, current smokers and antidepressant users had lower eGDR (i.e. worse insulin sensitivity, Table 3). eGDR was also directly associated with HDL cholesterol and inversely associated with BMI and triglycerides (Table 3). Back transformed (square rooted) beta estimates and 95% CIs from the linear regression models of subjective sleep disturbances and eGDR-squared are displayed in Table 4. Individuals at high risk for OSA had 3.19 units lower eGDR than their counterparts (95% CI: −4.42, −0.89, p=0.03). However, this association was no longer significant after adjustment for smoking history, antidepressant use, and excessive daytime sleepiness (Table 4) or upon exclusion of individuals taking sleep medications (β=−2.66, 95% CI: −4.11, 1.63, p=0.15). Participants with excessive daytime sleepiness had marginally lower eGDR than their counterparts (β =−3.29, 95% CI: −4.84, −1.35, p=0.09). This association attenuated after adjusting for smoking history and high risk for OSA (Table 4). For every one hour increase in sleep duration, eGDR decreased by 2.20 units (95% CI: −2.85, −1.23, p=0.005), even upon exclusion of those taking sleep medications (β=−1.96, 95% CI: −2.77, −0.14, p=0.049). Compared to the second tertile of sleep (6–7 hours) duration, those who reported sleeping less than 6 hours per night had 3.62 units higher eGDR (95% CI: 0.73, 5.06, p=0.04). The association remained significant independent of smoking history (Table 4), but not after excluding participants taking sleep medications (β=3.50, 95% CI: −1.61, 5.21, p=0.10). In contrast, eGDR levels did not differ between individuals who reported sleeping longer than 7 hours and those sleeping 6 to 7 hours. When sleep duration was categorized by the NSF’s guidelines, we were unable to model long sleep (>9 hours) compared to normal sleep (7–9 hours) due to the small sample size of long sleepers (n=3). However, normal sleepers did not differ from short sleepers with regard to eGDR. We also allowed for traditional risk factors (i.e. diabetes duration and gender) as potential confounders, but the associations between sleep disturbances and eGDR were not altered (data not shown). None of the linear regression models showed multicollinearity. When a sensitivity analysis was performed including 156 participants with complete exposure, covariate, and outcome data, none of the regression results were affected (data not shown).
4. Discussion
In this cross-sectional study of 222 middle-aged adults with long-standing T1D, we found that approximately one-fourth were at high risk for OSA and that one-eight exhibited excessive daytime sleepiness regardless of gender. Poor sleep quality was observed in half of the women and in one-third of the men, and approximately one-fourth of the participants reported trouble sleeping due to bad dreams. Poor sleep quality, high risk for OSA, excessive daytime sleepiness, and sleep duration were not associated with poor glycemic control. Participants with poor glycemic control were, nonetheless, twice as likely to report having trouble sleeping because of bad dreams, although this was not independent of depressive symptomatology. The relationship between sleep duration and HbA1c was modified by the level of glycemic control, i.e. we observed a direct sleep duration–HbA1c association in those with poor glycemic control and an inverse association among well-controlled individuals. To our surprise, longer, but not shorter, sleep duration was independently associated with worse insulin sensitivity.
To our knowledge, there has only been one other study which examined the prevalences of poor sleep quality, excessive daytime sleepiness, and high-risk OSA, through the three sleep questionnaires, in a sample of adults with long-standing T1D (n=99, 55% men, mean age=44 years, mean diabetes duration=27 years, mean BMI=24.5 kg/m2, and mean HbA1c=7.8%) [17]. Van Dijk and colleagues observed a higher prevalence of excessive daytime sleepiness (19.2% versus 12.5% in our study). However, this higher estimate was likely attributable to the use of a lower cut-off value (ESS ≥10) to define excessive daytime sleepiness in that study [17]. When we used the same cut-off value, the prevalence of excessive daytime sleepiness in our cohort (18.5%) was very similar to that in the van Dijk’s study (19.2%). In fact, the average total Epworth scores were similar in both studies (5.9 ± 0.4 in the van Dijk study; 6.1 ± 3.9 in our study), but slightly higher than in sleepers without any evidence of a sleep disorder (4.6 ± 2.8) [40]. In comparison to the van Dijk study, poor sleep quality and high-risk OSA were slightly higher in our cohort (41.3% versus 35.4% and 22.7% versus 17.2%, respectively), possibly due to the longer diabetes duration and higher BMI among our participants. Unfortunately, van Dijk and colleagues did not assess gender differences in any of the subjective sleep disturbance measures.
Compared to our study, Borel et al. [18] and Manin et al. [20] observed a higher OSA prevalence (40% and 46%, respectively), but a similar severe OSA prevalence (27% and 19%, respectively) in adults with T1D. The differences in the OSA prevalence may have resulted from the use of polysomnography [20] and oximetry [18] for measuring sleep in these studies, since these methods yield higher rates of OSA than the BQ [30, 41]. Despite the BQ being a screening, rather than a diagnostic, tool, which prohibited any conclusions regarding the prevalence of an OSA diagnosis among EDC participants, the prevalence of severe OSA in the two studies corresponds closely to our BQ-determined OSA-risk prevalence. Nevertheless, it is important to recognize that a previous validation of the BQ against polysomnography in a primary care setting yielded a specificity of 86%, a sensitivity of 77%, and a positive predictive value of 89% [30]. Misclassification of true OSA cases for non-OSA cases could have, therefore, occurred in our study, leading to an underestimation of the true OSA prevalence, as previously also reported in the general population [41].
The presence of self-reported sleep disturbances in the EDC study appears somewhat similar to the general population, although with the opposite gender preponderance. Namely, Hiestand et al. examined BQ-determined OSA risk among 1,506 nationally-representative, middle-aged adults who completed the NSF’s Sleep in America 2005 Poll and found that 31% of men fell in the high-risk category compared to 21% of women (p<0.001) [42]. Almost identical figures were recently reported in a general population sample from Switzerland (31% in men; 19% in women) [21]. Although we did not observe a statistically significant gender difference, the prevalence of the BQ-determined high-risk OSA in our cohort was higher in women (26%) than in men (19%). Additionally, our participants reported excessive daytime sleepiness at a somewhat similar rate (12.5%) than the nationally-representative adults from the National Health and Nutrition Examination Survey 2007–2008 (15.1%) [43]. However, a single question regarding feeling excessively sleepy during the day, and not the ESS, was used in this sample [43]. The Swiss general population sample experienced excessive daytime sleepiness (ESS >10) at an almost identical rate as the EDC cohort (12%) [21]. While excessive daytime sleepiness was significantly higher in Swiss men (14%) compared to Swiss women (10%), we did not observe significantly higher prevalence rates in EDC women (14.1%) compared to EDC men (10.9%), likely due to the smaller sample size in our population. We further observed a strong female excess in poor sleep quality (PSQI >5), sleep disturbance, latency, and efficiency; however, the prevalences of these sleep disturbances were not reported in the Swiss study [21]. Nonetheless, the global PSQI score in the Swiss general population was not as profoundly higher among women (women: median 5 IQR (3–7); men: 4 (3–6)) as it was in our cohort (women: 9 (6–12); men: 5 (3–8)). While Swiss men were heavier, had a bigger neck circumference, larger WHR, and higher frequencies of smoking, drinking, snoring, hypertension, diabetes, and metabolic syndrome [21] in comparison to Swiss women, adjusting for anthropometric measures, smoking, drinking and hypertension status did not alter the higher prevalence of poor sleep quality in the EDC women, who had a higher prevalence of poor sleep quality even after accounting for depressive symptomatology and sleep medication use.
We did not observe significant relationships between sleep duration, or any of the questionnaire-determined sleep disturbances, and poor glycemic control. These findings are consistent with the previous study by van Dijk et al. [17]. In a study by Schober and colleagues [19], participants with ESS-defined excessive daytime sleepiness had higher HbA1c values (p=0.02). However, no distinction was made on diabetes type in this assessment and adjustment for potential confounding factors was also not performed.
Nevertheless, we did observe a significant independent relationship between sleep duration and continuous HbA1c, but this association was modified by the level of glycemic control. Similarly, Barone and colleagues showed that glycemic control modified the relationship between actimeter-measured sleep duration and glycemia in a small T1D study (n=18) [44]. Specifically, among individuals with T1D and HbA1c<7%/53 mmol/mol, a strong negative correlation between glycemia and the night rest duration was observed (r= −0.90, p=0.04). While the authors did report the overall correlation (r=0.54, p=0.07), the correlation among individuals with HbA1c≥7.0%/53 mmol/mol was not reported. The mechanisms behind the interaction in our study and in the Barone et al. study are unclear and warrant attention of future research. A potential explanation may be that longer sleep duration among individuals under poor glycemic control is driven by poor sleep quality (i.e. someone who does not sleep well seeks more sleep). However, in the present study, no differences in sleep quality were observed by glucose control and individuals reporting poor sleep quality also reported shorter sleep duration.
In a recent pilot study of 17 adults with T1D, longer time in deep, REM sleep measured by a wireless sleep monitor was associated with lower HbA1c levels (R2=0.42, p<0.01) [24]. Because nightmares most likely occur during REM sleep and interrupt the deep sleep cycle, we used “trouble sleeping due to bad dreams” as the closest alternative for disturbed REM sleep. We observed that bad dreams were, in fact, associated with poor glycemic control, even upon exclusion of individuals who reported taking sleep medications, which may promote or disturb REM sleep. Although this association was attenuated after adjustment for depressive symptoms and antidepressant use, we believe that this finding merits further investigation. Because depressive symptomatology could be in the causal pathway between disturbed sleep and poor glycemic control, or a consequence of dysglycemia, we suggest that the adjustment for BDI score and antidepressant use should be interpreted with caution. To our knowledge, no previous study has examined the single PSQI item (i.e. trouble sleeping due to bad dreams) as a proxy for disturbed REM sleep. Our findings relating to its association with glycemic control should, therefore, be confirmed in future studies.
Contrary to the previous studies which observed short sleep duration impairing insulin sensitivity in both healthy [45] and T1D individuals [15], we observed worse estimated insulin sensitivity as the sleep time increased irrespective of confounders. While Donga and colleagues measured insulin sensitivity objectively and after only one night of restricted sleep [15, 45], we assessed self-perceived sleep duration during the past month. Sleep duration may have different effects on insulin sensitivity acutely and chronically, but our study was not suited to disentangle such effects. Further, our measure of insulin sensitivity was calculated from an equation consisting of HbA1c, WHR, and hypertension which was derived from previous hyperinsulinemic-euglycemic clamp studies in which eGDR was highly related to glucose disposal during the clamp (R2=0.63) [36]. It is, therefore, possible that longer sleep duration may be associated with impaired clinical or metabolic states captured by lower eGDR values.
Both short and long sleep duration have been associated with increased HbA1c in type 2 diabetes, suggesting that an optimal level of sleep for glycemic control may exist [45]. However, we did not observe a U-shaped relationship between sleep duration and either HbA1c or eGDR, likely because only three of our participants reported sleeping longer than nine hours. Nevertheless, our study is among the first to thoroughly examine the association between subjective sleep duration and estimated insulin sensitivity in adults with long-standing T1D. Our findings should, however, be confirmed in future studies.
The main strength of our study lies in the use of three validated sleep questionnaires to assess sleep disturbance in a population of adults with long-standing T1D, as previous sleep research has been mostly limited to youth and smaller sample sizes. We are also among the first to assess gender differences in sleep disturbance in T1D.
Nevertheless, this study has several limitations. Primarily, due to the cross-sectional design of our analyses, it is impossible to establish temporality between sleep disturbances and glycemia. Precaution should therefore be taken when interpreting our findings as they should be validated against future prospective data. Furthermore, since our study population has survived T1D for over 40 years, considerable survival bias is present in the current study. This type of bias may have resulted in an underestimation of the estimated prevalences of sleep disturbances. Low participation rates for the 25-year exam could have also resulted in selection bias. Participants attending the 25-year exam were more likely to have had a better risk factor profile compared to non-attendees at study entry, suggesting that the observed associations between sleep disturbances and glycemic control could have been underestimated. Further, all of our sleep disturbances were obtained through self-report. While other objective sleep measurement tools can be more sensitive/accurate, we did utilize widely-used, validated sleep questionnaires. Nonetheless, this study is among the first to thoroughly evaluate relationships between subjective sleep disturbances and glycemia beyond traditional risk factors in a well-phenotyped, relatively large T1D adult population.
In conclusion, the present study demonstrated a high burden of self-reported sleep disturbances among adult women with long-standing T1D, indicating that this group may represent a potential target for monitoring sleep. The overall, non-gender-specific prevalence of sleep disturbance in T1D did not differ greatly from that of the general population. Longer sleep duration and bad dreams were associated with reduced insulin sensitivity and poor glycemic control, respectively. The relationship between sleep duration and HbA1c was modified by the level of glycemic control. Future research should focus on the mechanisms underlying this effect modification. Should our findings be confirmed in future prospective studies, improving sleep hygiene may have the potential to impact glycemic control, or vice versa, in individuals with type 1 diabetes.
Acknowledgments
The Pittsburgh EDC Study was supported by a grant from the National Institutes of Health DK34818. We are extremely thankful to the participants of the EDC study who have tirelessly volunteered their time for longer than 25 years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors disclose no conflict of interest relevant to this manuscript.
Author contributions
H.D. wrote the manuscript, researched data, and performed data analysis. T.C. researched data, contributed to the data analysis, and reviewed/edited the manuscript. T.J.O. researched data, contributed to the data analysis, and reviewed/edited the manuscript. All authors have read and approved the final version of the manuscript. T.J.O. is the guarantor of this work and, as such, had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Tina Costacou, Email: costacouT@edc.pitt.edu.
Trevor J. Orchard, Email: tjo@pitt.edu.
References
- 1.Centers for Disease Control and Prevention National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey Statistical analysis by the Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation.
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2014. 2014. [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep medicine. 2013;14:324–32. doi: 10.1016/j.sleep.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. International journal of cardiology. 2013;169:207–14. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. International journal of cardiology. 2014;172:466–9. doi: 10.1016/j.ijcard.2013.12.230. [DOI] [PubMed] [Google Scholar]
- 8.Taub LF, Redeker NS. Sleep disorders, glucose regulation, and type 2 diabetes. Biological research for nursing. 2008;9:231–43. doi: 10.1177/1099800407311016. [DOI] [PubMed] [Google Scholar]
- 9.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 10.Skomro RP, Ludwig S, Salamon E, Kryger MH. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep medicine. 2001;2:417–22. doi: 10.1016/s1389-9457(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 11.Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. The Clinical journal of pain. 2006;22:681–5. doi: 10.1097/01.ajp.0000210910.49923.09. [DOI] [PubMed] [Google Scholar]
- 12.Matyka KA, Crawford C, Wiggs L, Dunger DB, Stores G. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. The Journal of pediatrics. 2000;137:233–8. doi: 10.1067/mpd.2000.107186. [DOI] [PubMed] [Google Scholar]
- 13.Villa MP, Multari G, Montesano M, Pagani J, Cervoni M, Midulla F, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696–702. doi: 10.1007/s001250051365. [DOI] [PubMed] [Google Scholar]
- 14.Perfect MM, Patel PG, Scott RE, Wheeler MD, Patel C, Griffin K, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35:81–8. doi: 10.5665/sleep.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes care. 2010;33:1573–7. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2010;27:398–404. doi: 10.1111/j.1464-5491.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk M, Donga E, van Dijk JG, Lammers GJ, van Kralingen KW, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967–76. doi: 10.1007/s00125-011-2184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borel AL, Benhamou PY, Baguet JP, Halimi S, Levy P, Mallion JM, et al. High prevalence of obstructive sleep apnoea syndrome in a Type 1 diabetic adult population: a pilot study. Diabetic medicine: a journal of the British Diabetic Association. 2010;27:1328–9. doi: 10.1111/j.1464-5491.2010.03096.x. [DOI] [PubMed] [Google Scholar]
- 19.Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. The clinical respiratory journal. 2011;5:165–72. doi: 10.1111/j.1752-699X.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- 20.Manin G, Pons A, Baltzinger P, Moreau F, Iamandi C, Wilhelm JM, et al. Obstructive sleep apnoea in people with Type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabetic medicine: a journal of the British Diabetic Association. 2015;32:90–6. doi: 10.1111/dme.12582. [DOI] [PubMed] [Google Scholar]
- 21.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet Respiratory medicine. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borel AL, Pepin JL, Nasse L, Baguet JP, Netter S, Benhamou PY. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes care. 2013;36:2902–8. doi: 10.2337/dc12-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feupe SF, Frias PF, Mednick SC, McDevitt EA, Heintzman ND. Nocturnal continuous glucose and sleep stage data in adults with type 1 diabetes in real-world conditions. Journal of diabetes science and technology. 2013;7:1337–45. doi: 10.1177/193229681300700525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–24. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 26.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Ellis D, LaPorte RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes care. 1990;13:741–7. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 27.Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes. 1982;31:136–44. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of internal medicine. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Yoda K, Inaba M, Hamamoto K, Yoda M, Tsuda A, Mori K, et al. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PloS one. 2015;10:e0122521. doi: 10.1371/journal.pone.0122521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Parkes JD, Chen SY, Clift SJ, Dahlitz MJ, Dunn G. The clinical diagnosis of the narcoleptic syndrome. Journal of sleep research. 1998;7:41–52. doi: 10.1046/j.1365-2869.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. Journal of sleep research. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 35.Johns MW. What is excessive daytime sleepiness? In: Fulke P, Vaughan S, editors. Sleep Deprivation: Causes, Effects and Treatment. New York: 2009. pp. 59–94. [Google Scholar]
- 36.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–32. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 38.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Preventive medicine. 1976;5:207–15. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 39.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 40.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 41.Simpson L, Hillman DR, Cooper MN, Ward KL, Hunter M, Cullen S, et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep & breathing = Schlaf & Atmung. 2013;17:967–73. doi: 10.1007/s11325-012-0785-0. [DOI] [PubMed] [Google Scholar]
- 42.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 43.Chakravorty S, Jackson N, Chaudhary N, Kozak PJ, Perlis ML, Shue HR, et al. Daytime sleepiness: associations with alcohol use and sleep duration in americans. Sleep disorders. 2014;2014:959152. doi: 10.1155/2014/959152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barone MT, Wey D, Schorr F, Franco DR, Carra MK, Lorenzi Filho G, et al. Sleep and glycemic control in type 1 diabetes. Archives of endocrinology and metabolism. 2015;59:71–8. doi: 10.1590/2359-3997000000013. [DOI] [PubMed] [Google Scholar]
- 45.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. The Journal of clinical endocrinology and metabolism. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 46.Ohkuma T, Fujii H, Iwase M, Kikuchi Y, Ogata S, Idewaki Y, et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes care. 2013;36:611–7. doi: 10.2337/dc12-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]


