Abstract
Objective
To evaluate the incidence and prevalence of juvenile idiopathic arthritis (JIA) in Olmsted County, Minnesota in 1994–2013 and trends in juvenile rheumatoid arthritis (JRA) in 1960–2013.
Methods
Cases of arthritis in 1994–2013 were identified by diagnosis code with medical chart review to confirm diagnosis separately for JIA and JRA. Overall incidence rates with 95% confidence intervals (95% CIs) were age and sex adjusted to the 2010 US white population. Comparisons were made with an earlier (1960–1993) cohort from this same population.
Results
Seventy-one incident cases of JIA in 1994–2013 were identified, with an overall age- and sex-adjusted incidence rate of 10.3 per 100,000 (95% CI 7.9–12.7). Forty-two (59%) were female, with an incidence of 12.4 per 100,000 (95% CI 8.6–16.2), as compared to 8.3 per 100,000 (95% CI 5.2–11.3) in males. The most common subtype was oligoarthritis (63%). The mean ± SD age at diagnosis was 8.2 ± 5.3 years. The prevalence of JIA on January 1, 2000 and January 1, 2010 was 51.0 per 100,000 (95% CI 25.2–76.8) and 57.6 per 100,000 (95% CI 31.0–94.5), respectively. When the annual incidence of JRA was compared over time from 1960 to 2013, there was no significant change in incidence overall; however, the incidence decreased among females (P = 0.003). A cyclic pattern of incidence was observed, with peaks approximately every 10 years. Similar to the findings with regard to incidence, prevalence did not change overall, but decreased among females (P = 0.048). There were 4 deaths in the cohort of JRA patients diagnosed in 1960–2013; the standardized mortality ratio was 1.50 (95% CI 0.41–3.83).
Conclusion
Incidence of juvenile arthritis overall in Olmsted County, Minnesota has not changed significantly in the past 53 years. A consistent cyclic pattern was noted.
Juvenile idiopathic arthritis (JIA) can have devastating consequences both during childhood and extending into adulthood (1–5). The joints can be impacted by prior damage or ongoing disease activity, which persists in a significant proportion of adults (1–3). Consequences even in the absence of ongoing disease activity are not limited to joint destruction but extend to effects on vision, effects on overall growth, osteoporosis, and potentially cardiovascular disease (6,7).
Published data on the epidemiology of JIA are very disparate, with one review noting a 50-fold difference between extremes of prevalence and a 28-fold difference in incidence between studies (8). There are several likely explanations for these divergent estimates. Part of the discrepancy stems from the changing classification systems used for juvenile arthritis. The original American College of Rheumatology (ACR) criteria for juvenile rheumatoid arthritis (JRA) identified 3 subtypes of the disease: pauciarticular, polyarticular, and systemic (9). The terminology and classification criteria have evolved, in the work of the International League of Associations for Rheumatology (ILAR), to encompass more categories, including psoriatic arthritis, enthesitisrelated arthritis, and undifferentiated arthritis, to acknowledge the heterogeneity of illness, and to prevent overlap of subgroups in order to better inform patient care and research design (10,11).
The source cohort is also a critical determinant of incidence and prevalence of JIA. Prevalence estimates from community-based studies are higher than those from studies using referral centers (12,13). Administrative data sets can provide helpful data on utilization but have limitations with regard to details of diagnosis (14). In addition, the training of the provider can affect diagnostic accuracy, further contributing to the disparate nature of epidemiologic data on JIA (15,16).
Geography has significant implications as well. More recent European studies using the ILAR criteria demonstrated higher rates of JIA in Nordic countries as compared to Spain (17,18). US data regarding the epidemiology of JIA based on the updated ILAR criteria are lacking. The timing of studies is also important because of year-to-year variability in disease incidence, which has been hypothesized to reflect infectious exposures (19,20).
The epidemiology of arthritis in juveniles in Olmsted County, Minnesota between 1960 and 1993 was previously evaluated utilizing the ACR criteria for JRA (20). In the present study we aimed to update understanding of the epidemiology of JIA as well as JRA over a long period, in a geographically defined US population with readily available pediatric rheumatologists and long-term followup.
PATIENTS AND METHODS
Identification of cases
Utilizing the resources of the Rochester Epidemiology Project and complete, comprehensive medical records of all residents, patients with JIA were identified from the population of Olmsted County, Minnesota (21). In 2000 in Olmsted County, the number of individuals under the age of 19 years was 38,943. For screening purposes, residents of Olmsted County age ≤18 years between January 1, 1993 and December 31, 2013 were identified with the following diagnoses as previously described (20): arthritis, JRA, rheumatoid arthritis, Still’s disease, rheumatic fever, systemic lupus erythematosus, infectious arthritis, inflammatory arthritis, palindromic rheumatism, degenerative joint disease, osteoarthritis, polyarthralgia, polyarthritis, acute arthritis, Felty’s syndrome, spondylitis, ankylosing spondylitis, collagen vascular and overall diseases, iritis, iridocyclitis, uveitis, Lyme disease, joint effusion, joint swelling, or traumatic arthritis. In addition, to take into account the new ILAR classification criteria, these diagnoses and terms were added: enthesitis, psoriatic arthritis, spondyloarthritis, sacroiliitis, HLA–B27, and ankylosing spondylitis (10,11).
The majority of patients with JIA have multiple different diagnosis codes from these lists over the duration of followup, and all individuals with >1 code were screened for the present study. However, some individuals had only a single qualifying diagnosis code during their entire followup. The records of all individuals with 1 code who had at least 1 evaluation by a trained rheumatologist were thoroughly reviewed by study investigators for potential inclusion. Patients with only a single diagnosis code, without any additional qualifying diagnoses, were excluded without further thorough chart review if the diagnosis code was one of the following: Lyme disease, hypopyon, Fuchs’ heterochromic cyclitis, rheumatic fever, sicca syndrome, pyogenic arthritis, osteoarthrosis, traumatic arthropathy, hemarthrosis, villonodular synovitis, pain in joint, iritis, uveitis, pars planitis, connective tissue laxity, fever of unknown origin, abnormal HLA positive, or sinus tarsi syndrome.
After patients were identified utilizing diagnosis codes, their medical charts were reviewed to ascertainment fulfillment of the ILAR criteria (11). Incident cases included all Olmsted County residents who first fulfilled the ILAR criteria between January 1, 1994 and December 31, 2013. Prevalent cases were included if juvenile arthritis was diagnosed prior to Olmsted County residency but the individual fulfilled ILAR criteria and was a resident of Olmsted County between January 1, 1994 and December 31, 2013. As described in the ILAR criteria, individuals needed to have evidence of symptoms lasting at least 6 weeks, and if there was no evidence of persistence beyond 6 weeks these individuals were excluded. Patients who met all criteria for polyarthritis—rheumatoid factor (RF) positive but had only a single RF test performed over the entire course of followup were classified as polyarthritis—RF positive.
Collection and recording of data
Demographic data, including date of birth, sex, self (or parent)–reported race/ethnicity, and date of last followup, were abstracted. If a death occurred, the cause and date of death were recorded. By review of the medical record, the subtype of JIA was classified using the ILAR criteria, as the full medical record allows for evaluation of laboratory studies, family history, and progression of JIA over time, all of which are needed to classify the subtype (11). Data were also collected incorporating the previous ACR criteria for JRA to permit comparison of the pre- and post-1993 cohorts (9). The date of symptom onset was recorded along with date of diagnosis, to calculate the time from symptom development to diagnosis. The occurrence of bony erosions and eye involvement was also abstracted.
Statistical analysis
Incidence and prevalence rates were estimated and age and/or sex adjusted to the 2010 US white population. Age- and sex-specific incidence and prevalence rates were calculated using the number of cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years. Because incident and prevalent cases in the 1960–1993 time period were identified among the Rochester, Minnesota population, estimates of the population size of Rochester, Minnesota were used as the denominator when estimating incidence and prevalence rates during this time interval.
For the 1994–2013 time period, incident and prevalent cases were identified among the population of Olmsted County, Minnesota (which is a larger geographic area that includes the city of Rochester), so Olmsted County population size was used as the denominator for incidence and prevalence rate calculations. In order to compute 95% confidence intervals (95% CIs) for incidence rates, it was assumed that the number of incident or prevalent cases followed a Poisson distribution. Trends in incidence rates were examined with Poisson regression methods using smoothing splines for age and calendar year. The annual incidence rates were graphically illustrated using a 3-year, centered, moving average to reduce random fluctuations over time.
Mortality rates were estimated using the Kaplan-Meier method and compared to expected mortality among persons of the same age, sex, and calendar year estimated using US population life tables. The standardized mortality rate (SMR) was estimated as the ratio of the observed to expected number of deaths. Ninety-five percent CIs for the SMR were calculated using the assumption that the expected rates were fixed and the observed rates followed a Poisson distribution.
RESULTS
Patient characteristics
The incidence cohort consisted of 71 patients who met the ILAR criteria for JIA between 1994 and 2013. Forty-two (59%) were female. Sixty-one (86%) reported their race/ethnicity as Caucasian, 3 (4%) reported as Asian, 3 (4%) reported as black, 1 (1%) reported as Hispanic, and 3 (4%) reported their race/ethnicity as unknown. The median length of followup was 7.5 years (interquartile range [IQR] 3.3–12.7). There were no differences in the length of followup between the different subtypes of JIA. No deaths occurred during the course of followup.
Of the incident cases, 45 (63%) had oligoarticular disease. Of these 45, persistent oligoarthritis (≤4 joints affected throughout the course) was most common (36 cases [80%]), followed by extended oligoarthritis (>4 joints involved 6 or more months after diagnosis) (7 cases [16%]). Two of the patients with oligoarthritis did not have followup beyond 6 months to enable further classification. Nine patients (13%) had polyarthritis. Two patients (3%) had systemic JIA. An additional 2 (3%) had psoriatic disease, 1 (1%) had enthesitis-related arthritis, and 12 (17%) had undifferentiated arthritis.
The mean ± SD age at diagnosis in the total group of incident cases was 8.2 ± 5.3 years; age at diagnosis among patients with oligoarthritis and patients with polyarthritis was 7.4 ± 5.4 years and 9.4 ± 5.0 years, respectively. There was no significant difference in age at diagnosis across subtypes of JIA. The mean ± SD time from symptom onset to diagnosis was 6.6 ± 8.7 months.
The knee was the most commonly involved joint at the time of diagnosis, affected as the initial joint in 69% of patients, with the ankle being the next most common, in 21%. Small joint involvement at diagnosis occurred at the proximal interphalangeal joint in 20%, metacarpophalangeal joint in 13%, toe interphalangeal joint in 13%, and metatarsophalangeal joint in 10%. In 14% of patients both the elbow and the wrist were involved at the time of diagnosis. Hip or shoulder involvement at initial diagnosis was very uncommon (6% and 3%, respectively).
Fever was an uncommon presenting symptom, affecting only 1 patient (1%). Over the course of followup, erosions were documented radiographically in 17%. This occurred most frequently in patients with polyarthritis (5 of 9 [56%]). Erosions occurred uncommonly in patients with oligoarthritis (4 of 45 [9%]). Eye involvement was documented in 5 patients (7%), 4 of whom had oligoarthritis.
Incidence of JIA, 1994–2013
The overall age- and sex-adjusted incidence of JIA was 10.3 per 100,000 (95% CI 7.9–12.7) (Table 1). Incidence among females was 12.4 per 100,000 (95% CI 8.6–16.2), and incidence among males was 8.3 per 100,000 (95% CI 5.2–11.3).
Table 1.
Incidence of juvenile idiopathic arthritis in Olmsted County, Minnesota by disease subtype, 1994–2013*
| Female | Male | Total | ||||
|---|---|---|---|---|---|---|
| Subtype | No. | Incidence per 100,000 (95% CI)† |
No. | Incidence per 100,000 (95% CI)† |
No. | Incidence per 100,000 (95% CI)‡ |
| Overall | 42 | 12.4 (8.6–16.2) | 29 | 8.3 (5.2–11.3) | 71 | 10.3 (7.9–12.7) |
| Systemic | 2 | 0.6 (0.0–1.3) | 0 | 0.0 | 2 | 0.3 (0.0–0.7) |
| Oligoarthritis | 29 | 8.5 (5.4–11.6) | 16 | 4.5 (2.3–6.7) | 45 | 6.5 (4.6–8.4) |
| Polyarthritis | 6 | 1.8 (0.4–3.2) | 3 | 0.9 (0.0–1.9) | 9 | 1.3 (0.5–2.2) |
| Psoriatic | 1 | 0.3 (0.0–0.9) | 1 | 0.3 (0.0–0.9) | 2 | 0.3 (0.0–0.7) |
| Enthesitis | 0 | 0.0 | 1 | 0.3 (0.0–0.9) | 1 | 0.2 (0.0–0.5) |
| Undifferentiated | 4 | 1.2 (0.0–2.4) | 8 | 2.3 (0.7–3.8) | 12 | 1.8 (0.8–2.7) |
95% CI = 95% confidence interval.
Age-adjusted to the 2010 US white population.
Age- and sex-adjusted to the 2010 US white 2010 population.
The most common subtype of JIA was oligoarticular disease, occurring at an age- and sex-adjusted rate of 6.5 per 100,000 (95% CI 4.6–8.4) (Table 1). The next most common subtype was undifferentiated arthritis, at 1.8 per 100,000 (95% CI 0.8–2.7). This was followed by polyarticular disease, with an incidence rate of 1.3 per 100,000 (95% CI 0.5–2.2). Systemic JIA and psoriatic arthritis each occurred at the same rate, 0.3 per 100,000 (95% CI 0.0–0.7). The least common subtype was enthesitis-related arthritis, at 0.2 per 100,000 (95% CI 0.0–0.5).
Prevalence of JIA
The prevalence of JIA on January 1, 2000 was 51.0 per 100,000 (95% CI 25.2–76.8). Among females, prevalence was 77.1 per 100,000 (95% CI 31.5–122.8); among males, it was lower at 26.3 per 100,000 (95% CI 0.5–52.0). On January 1, 2010, the prevalence of JIA was 57.6 per 100,000 (95% CI 31.0–84.5), with sex-specific prevalence rates of 78.4 per 100,000 (95% CI 33.9–122.8) among females and 38.2 per 100,000 (95% CI 7.6– 68.8) among males.
Incidence of JRA, 1960–2013
Between 1994 and 2013 there were 59 incident cases of JRA that fulfilled the ACR criteria. In the earlier study of incidence in 1960–1993 (20), there were also 59 incident cases of JRA. There was no significant difference in sex distribution between the earlier and later cohorts (71% female and 61% female, respectively) (Table 2). The mean ± SD time from symptom onset to diagnosis was 7.5 ± 12.7 months in the 1960–1993 cohort, compared to 4.3 ± 4.7 months in the 1994–2013 cohort (P not significant). There were no significant differences in incidence of different JRA subtypes as described in the ACR criteria. Pauciarticular disease was the most common in both time periods (41 cases [69%] in the earlier cohort and 48 [81%] in the later cohort). This was followed by polyarticular disease, with 11 cases (19%) and 9 cases (15%) in the earlier and later cohorts, respectively. There were 7 patients (12%) with systemic disease in the earlier cohort and only 2 (3%) in the later cohort.
Table 2.
Comparison of the 1960–1993 and 1994–2013 juvenile rheumatoid arthritis cohorts*
| 1960–1993 (n = 59) |
1994–2013 (n = 59) |
Total (n = 118) |
|
|---|---|---|---|
| Age at diagnosis, mean ± SD years | 8.3 ± 4.8 | 7.2 ± 5.0 | 7.8 ± 4.9 |
| Female | 42 (71) | 36 (61) | 78 (66) |
| Race/ethnicity† | |||
| Caucasian | 33 (56) | 51 (86) | 84 (71) |
| Black | 0 (0) | 3 (5) | 3 (3) |
| Asian | 0 (0) | 3 (5) | 3 (3) |
| Other | 1 (2) | 0 (0) | 1 (1) |
| Unknown | 25 (42) | 2 (3) | 27 (23) |
| Time from symptom onset to diagnosis, mean ± SD years |
7.5 ± 12.7 | 4.3 ± 4.7 | 5.9 ± 9.7 |
| ACR classification | |||
| Pauciarticular | 41 (69) | 48 (81) | 89 (75) |
| Polyarticular | 11 (19) | 9 (15) | 20 (17) |
| Systemic | 7 (12) | 2 (3) | 9 (8) |
Except where indicated otherwise, values are the number (%). ACR = American College of Rheumatology.
P for trend < 0.001, 1960–1993 cohort versus 1994–2013 cohort.
The age- and sex-adjusted incidence of JRA in 1960–1993 was 11.8 per 100,000 (95% CI 8.8–14.8). The age-adjusted rate for females in this same time period was 16.9 per 100,000 (95% CI 11.8–22.0), and for males it was 6.9 per 100,000 (95% CI 3.6–10.2). By comparison, in the 1994–2013 cohort, the age- and sex-adjusted incidence rate was numerically lower at 9.6 per 100,000 (95% CI 7.1–12.0). The incidence rate during this later period was 11.9 per 100,000 (95% CI 8.0–15.8) among females and 7.3 per 100,000 (95% CI 4.3–10.3) among males.
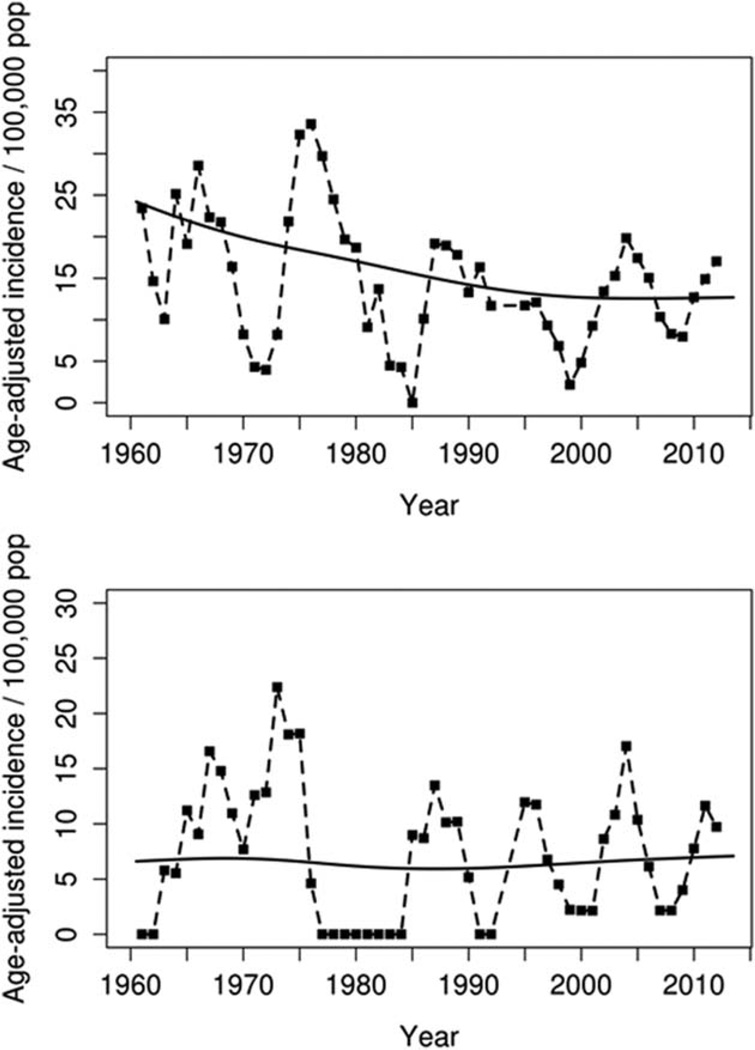
Age- and sex-adjusted incidence rates of JRA among both females and males fluctuated over the entire period of 1960–2013 (Figure 1). Cyclic changes in incidence were seen for both females and males, with peaks approximately every 10 years. The incidence of JRA declined significantly among females (P = 0.003), but not males (P = 0.69), between the 1960–1993 time period and the 1994–2013 time period. However, time trends in incidence within the 1994–2013 period remained stable (P = 0.71 for females and P = 0.89 for males). Further, there were no time trends in incidence of the subtypes of JRA (systemic, pauciarticular, or polyarticular) during 1994–2013.
Figure 1.
Annual incidence of juvenile rheumatoid arthritis (age adjusted to the 2010 US white population) among female residents (top) and male residents (bottom) of Rochester, Minnesota in 1960–1993 and of Olmsted County, Minnesota in 1994–2013.
Prevalence of JRA
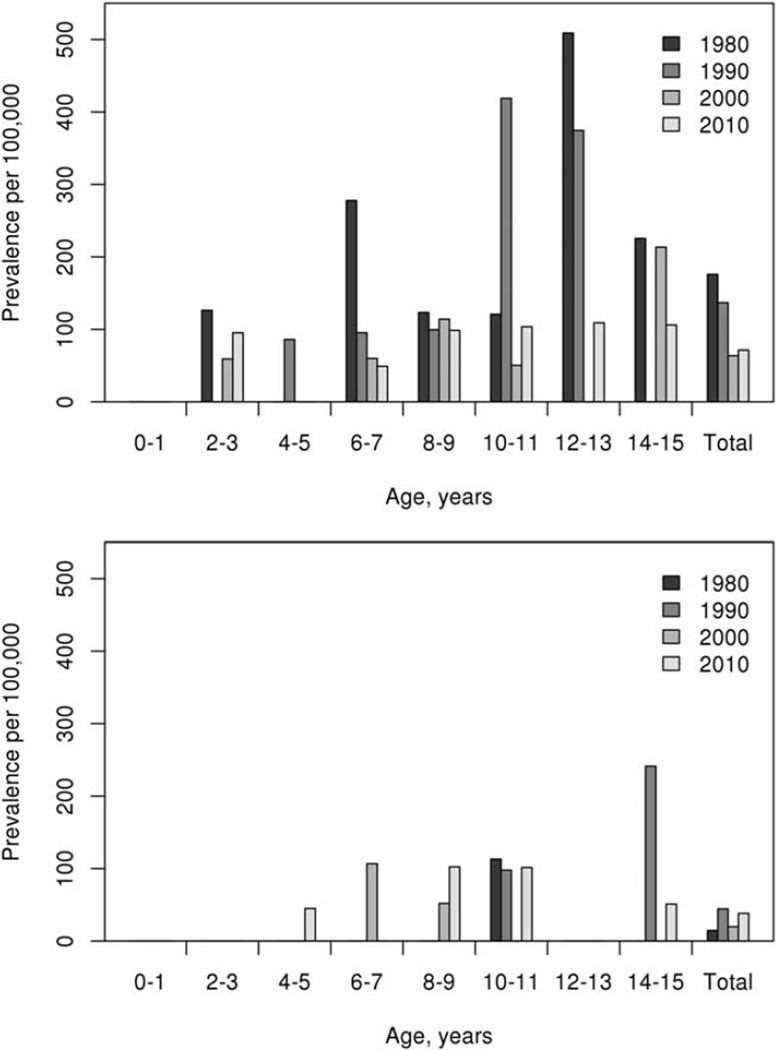
The prevalence of JRA on January 1, 1980 was 93.1 per 100,000 (95% CI 40.4–145.9). On January 1, 1990 the prevalence was 89.4 per 100,000 (95% CI 40.4–138.5), and on January 1, 2000 it was 41.0 per 100,000 (95% CI 17.8–64.2). Among females, prevalence was as high as 63.5 per 100,000 (95% CI 22.0–105.1) on this date and among males, it was 19.8 per 100,000 (95% CI 0–42.2). On January 1, 2010 the prevalence of JRA was 54.4 per 100,000 (95% CI 28.5–80.2), with prevalence rates of 71.4 (95% CI 29.1–113.7) among females and 38.2 (95% CI 7.6–68.8) among males. Prevalence data by age group and sex at the 4 different time points are shown in Figure 2. There were no significant changes in the prevalence of JRA overall (P = 0.42) or among males (P = 0.22), but the prevalence among females decreased significantly over time (P = 0.048).
Figure 2.
Prevalence of juvenile rheumatoid arthritis (adjusted to the 2010 US white population) among female residents (top) and male residents (bottom) of Rochester, Minnesota on January 1, 1980 and January 1, 1990 and of Olmsted County, Minnesota on January 1, 2000 and January 1, 2010, by age group.
Survival
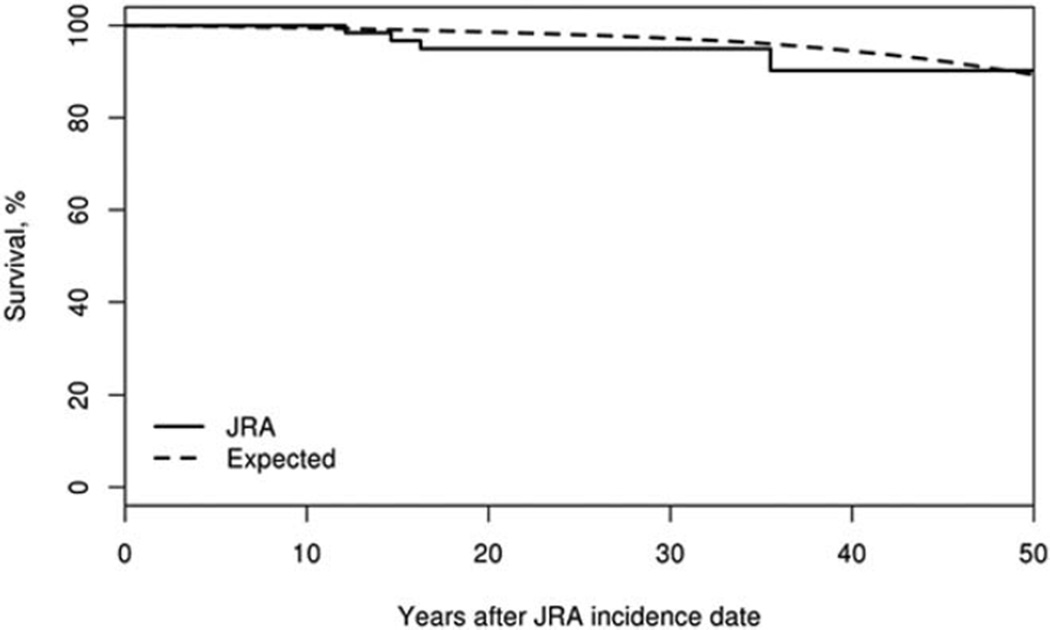
Among incident JRA cases that occurred between 1960 and 2013, there were 4 deaths over 2,180.7 person-years of followup (Figure 3). This was marginally higher than expected (based on age-, sex-, and calendar year–specific life tables for the US population, 2.7 deaths were expected); however, the absolute numbers were small. The SMR was 1.50 (95% CI 0.41–3.83).
Figure 3.
Survival among Olmsted County, Minnesota residents first diagnosed as having juvenile rheumatoid arthritis (JRA) between January 1, 1960 and December 31, 2013 (n = 118; solid line) compared to expected survival (dashed line). The standardized mortality ratio was 1.50 (95% confidence interval 0.41–3.83).
DISCUSSION
This was a population-based retrospective cohort study that evaluated incidence and prevalence of juvenile arthritis, as defined by the updated ILAR criteria (for 1994–2013) and by the ACR criteria (for 1960–2013). JRA incidence exhibited a fluctuating pattern, as has been observed in earlier studies (19,20). Previously, cyclic incidence of JRA in the province of Manitoba, Canada was found to coincide with increased rates of Mycoplasma pneumoniae infection (19). The finding of recurrent peaks suggests a role of some environmental exposure in the etiology in juvenile arthritis. Findings of cyclic changes in incidence have also been noted in rheumatoid arthritis (22).
Overall, during the 53-year span there was no significant change in the incidence of JRA. However, subanalysis by sex revealed that, whereas the incidence did not change significantly among males, it declined among females. It is unclear what biologic factor is mediating this change. Further understanding of this sex difference may help in elucidation of the underlying pathogenesis of juvenile arthritis.
The incidence rates obtained in this study are similar to those observed in other studies from Europe, and are slightly higher than reported in Catalonia, Spain (6.9 per 100,000) and slightly lower than in northern Europe (overall incidence 15 per 100,000) (17,18). In a prospective cohort consisting of the catchment populations of 20 medical practices throughout northern Europe there was significant variation depending on the geographic area, with the lowest rates in Iceland and highest rates in the Helsinki region of Finland; the incidence rate in our study falls within the range recorded from this cohort (17). This suggests that differences in the incidence of juvenile arthritis are potentially related to genetic factors, infectious exposures, or ascertainment differences.
Subtype distribution of JIA in the Nordic population (17) is similar to that in our cohort. Oligoarthritis was the most common in the Nordic population (46%, as compared to 63% in the Olmsted County cohort). The rate of polyarticular disease was higher in the Nordic cohort (21%, versus 12% in the Olmsted County cohort). Psoriatic arthritis, enthesitis-related arthritis, and systemic arthritis occurred at similar, very low rates (17). Enthesitis-related arthritis was more common in the Catalonian cohort (12.4%) (18) compared to our cohort (1%). Classification of enthesitis-related arthritis may be particularly difficult to compare across studies. To classify this subtype in our study the findings of enthesitis would have to be documented in the chart, and in some individuals in this cohort, the overall diagnosis predated the development of this subcategory. There is potential that some individuals with enthesitis-related arthritis were classified as having oligoarthritis. Further, 3 individuals whose clinical findings were consistent with enthesitis-related arthritis had to be classified as having undifferentiated arthritis due to either family history of psoriasis (2 patients) or RF positivity (1 patient).
It is difficult to compare the epidemiology of JRA across studies as there are significant differences in study methodology, including patient/case detection techniques, geographic location of patients, and timing of the studies (8). Our is by far the longest-studied and oldest cohort in terms of period of observation, during which no significant change in the incidence of juvenile arthritis has occurred (20).
No deaths occurred in our more recent cohort (1994–2013). When the findings from the recent cohort and the earlier cohort were combined (1960–2013), there was no significant increase in mortality compared to expected. The finding of no elevated risk of mortality is similar to the results in other contemporary cohorts (23). While our earlier cohort did exhibit a small elevation in mortality compared to expected (SMR 4.0), the deaths were the result of other comorbidities, as opposed to juvenile arthritis (24).
This study was retrospective, using medical record data that were not collected according to juvenile arthritis classification features. Further, some of the patients were evaluated prior to the development of the ILAR criteria. However, each chart was individually reviewed and since the medical record is comprehensive, including laboratory results and family history, individuals could be classified into ILAR categories.
Our first step of case identification via the use of diagnostic codes raises the possibility of missed cases since this was not a prospective registry, as have been used in other studies (17). However, the same diagnosis search strategy was used as for the earlier cohort (20) with the exception of additional diagnostic codes to capture the additional patients now included in the ILAR criteria (e.g., those with psoriatic arthritis and enthesitis-related arthritis). Diagnosis codes for psoriasis alone were not included and potentially some children with psoriatic arthritis could have been missed, but for this to have occurred they must have not had any diagnosis code associated with joint symptoms. The similarity of incidence rates between the earlier cohort and the more recent cohort suggests reliability of the case identification.
While many studies from the Rochester Epidemiology Project are thought to lack generalizability due to the predominance of persons of white race in the Olmsted County population, this may be less of an issue for our study since the juvenile population of the area is more racially diverse than the population overall. In addition, results of studies from this population have nevertheless been found to be generalizable to the overall population of the US (25).
This study has the advantage of being population based with long-term followup. The single medical record used by a broad spectrum of medical providers and in a wide range of medical scenarios, ranging from acute-care visits to subspecialty consultations, and broad diagnosis codes should have captured cases of interest even if active symptoms were shortlived.
Juvenile arthritis has significant effects that are not confined to the pediatric years. Further, it is a disease not just of joints, but rather a systemic illness with wide-ranging organ impact. Understanding the epidemiology of JIA as well as the frequency of long-term consequences is necessary to provide support for the resources, both clinical and research, that are needed to address the needs of patients with this diagnosis.
Acknowledgments
The content herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the NIH (National Institute on Aging award R01-AG-034676 and National Center for Advancing Translational Sciences CTSA grant UL1-TR-000135). Dr. Wampler Muskardin’s work was supported by a Mayo Clinic Career Development Award.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Krause had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Krause, Crowson, Mason, Wampler Muskardin, Matteson.
Acquisition of data. Krause, Crowson, Michet, Matteson.
Analysis and interpretation of data. Krause, Crowson, Mason, Wampler Muskardin, Matteson.
REFERENCES
- 1.Foster HE, Marshall N, Myers A, Dunkley P, Griffiths ID. Outcome in adults with juvenile idiopathic arthritis: a quality of life study. Arthritis Rheum. 2003;48:767–775. doi: 10.1002/art.10863. [DOI] [PubMed] [Google Scholar]
- 2.Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. for the Nordic Study Group of Pediatric Rheumatology. Ongoing disease activity and changing categories in a long-term Nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:2809–2818. doi: 10.1002/art.30426. [DOI] [PubMed] [Google Scholar]
- 3.Haverman L, Grootenhuis MA, van den Berg JM, van Veenendaal M, Dolman KM, Swart JF, et al. Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: results from a Web-based survey. Arthritis Care Res (Hoboken) 2012;64:694–703. doi: 10.1002/acr.21609. [DOI] [PubMed] [Google Scholar]
- 4.Selvaag AM, Aulie HA, Lilleby V, Flato B. Disease progression into adulthood and predictors of long-term active disease in juvenile idiopathic arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-206034. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. for the ReACCh-Out Investigators. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;74:1854–1860. doi: 10.1136/annrheumdis-2014-205372. [DOI] [PubMed] [Google Scholar]
- 6.French AR, Mason T, Nelson AM, Crowson CS, O’Fallon WM, Khosla S, et al. Osteopenia in adults with a history of juvenile rheumatoid arthritis: a population based study. J Rheumatol. 2002;29:1065–1070. [PubMed] [Google Scholar]
- 7.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 8.Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol. 2002;29:1520–1530. [PubMed] [Google Scholar]
- 9.Brewer EJ, Jr, Bass J, Baum J, Cassidy JT, Fink C, Jacobs J, et al. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of the Arthritis Foundation. Current proposed revision of JRA criteria. Arthritis Rheum. 1977;20(Suppl):195–199. [PubMed] [Google Scholar]
- 10.Fink CW. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J Rheumatol. 1995;22:1566–1569. [PubMed] [Google Scholar]
- 11.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 12.Manners PJ, Diepeveen DA. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics. 1996;98:84–90. [PubMed] [Google Scholar]
- 13.Mielants H, Veys EM, Maertens M, Goemaere S, De Clercq L, Castro S, et al. Prevalence of inflammatory rheumatic diseases in an adolescent urban student population, age 12 to 18, in Belgium. Clin Exp Rheumatol. 1993;11:563–567. [PubMed] [Google Scholar]
- 14.Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum. 2007;57:1439–1445. doi: 10.1002/art.23087. [DOI] [PubMed] [Google Scholar]
- 15.Arguedas O, Fasth A, Andersson-Gare B, Porras O. Juvenile chronic arthritis in urban San Jose, Costa Rica: a 2 year prospective study. J Rheumatol. 1998;25:1844–1850. [PubMed] [Google Scholar]
- 16.Kiessling U, Doring E, Listing J, Meincke J, Schontube M, Strangfeld A, et al. Incidence and prevalence of juvenile chronic arthritis in East Berlin 1980–88. J Rheumatol. 1998;25:1837–1843. [PubMed] [Google Scholar]
- 17.Berntson L, Andersson Gare B, Fasth A, Herlin T, Kristinsson J, Lahdenne P, et al. Incidence of juvenile idiopathic arthritis in the Nordic countries: a population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol. 2003;30:2275–2282. [PubMed] [Google Scholar]
- 18.Modesto C, Anton J, Rodriguez B, Bou R, Arnal C, Ros J, et al. Incidence and prevalence of juvenile idiopathic arthritis in Catalonia (Spain) Scand J Rheumatol. 2010;39:472–479. doi: 10.3109/03009741003742722. [DOI] [PubMed] [Google Scholar]
- 19.Oen K, Fast M, Postl B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975–92: cycles in incidence. J Rheumatol. 1995;22:745–750. [PubMed] [Google Scholar]
- 20.Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Juvenile rheumatoid arthritis in Rochester, Minnesota 1960– 1993: is the epidemiology changing? Arthritis Rheum. 1996;39:1385–1390. doi: 10.1002/art.1780390817. [DOI] [PubMed] [Google Scholar]
- 21.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–834. doi: 10.1016/j.rdc.2004.07.010. vii. [DOI] [PubMed] [Google Scholar]
- 22.Myasoedova E, Crowson CS, Maradit Kremers H, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashkes PJ, Wright BM, Lauer MS, Worley SE, Tang AS, Roettcher PA, et al. Mortality outcomes in pediatric rheumatology in the US. Arthritis Rheum. 2010;62:599–608. doi: 10.1002/art.27218. [DOI] [PubMed] [Google Scholar]
- 24.French AR, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Increased mortality in adults with a history of juvenile rheumatoid arthritis: a population-based study. Arthritis Rheum. 2001;44:523–527. doi: 10.1002/1529-0131(200103)44:3<523::AID-ANR99>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]