Abstract
Purpose
We investigated the incremental diagnostic yield of S-MRCP in a population with high prevalence of small pancreatic cysts.
Methods
Standard MRCP protocol was performed with and without secretin using 1.5 T unites in subjects undergoing pancreatic screening because of a strong family history of pancreatic cancer as part of the multicenter Cancer of the Pancreas Screening-3 trial (CAPS 3). All studies were reviewed prospectively by two independent readers who recorded the presence and number of pancreatic cysts, the presence of visualized ductal communication before and after secretin, and the degree of confidence in the diagnoses.
Result
Of 202 individuals enrolled (mean age 56 years, 46% males), 93 (46%) had pancreatic cysts detected by MRCP, and 64 of the 93 had pre-and post-secretin MRCP images available for comparison. Data from the 128 readings show that 6 (6/128 = 4.7%) had ductal communication visualized only on the secretin studies compared to pre-secretin studies (odds ratio 1.28, p = 0.04). In addition, there was a statistically significant increase in confidence in reporting ductal communication after secretin compared to before secretin (p <0.0005).
Conclusion
At 1.5 T MRI, the use of secretin can improve the visualization of ductal communication of cystic pancreatic lesions.
Keywords: Cyst, Magnetic resonance imaging, Neoplasm, Pancreas, Screening, Secretin
Introduction
The majority of pancreatic cancer cases are sporadic, however up to 10% are considered to be inherited (1,2). Pancreatic cancer is the fourth leading cause of cancer death in USA (1,3), and its incidence has increased over the last 8 years. It is associated with a high mortality rate and about 38,460 deaths are estimated in 2013 (4). Individuals with multiple first-degree relatives with pancreatic cancer as well as subjects with hereditary pancreatitis, carriers of germline mutations in BRCA2, ATM, p16, PALB2 and mismatch repair genes and those with Peutz–Jeghers syndrome (PJS) have a higher risk of developing pancreatic cancer than the general population (5). Pancreatic screening studies of high risk individuals identified a high prevalence of pancreatic lesions, particularly pancreatic cysts in these individuals (6–12). Pancreatic screening of high risk individuals was also recommended at a recent consensus conference, even though the method and frequency of screening were not defined (1).
Pancreatic intraepithelial neoplasia (PanIN) is by far the most common precursor lesion of pancreatic ductal adenocarcinoma, followed by two cystic precursor lesions, intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) (6,13). PanIN can only be diagnosed microscopically in the resected pancreas. IPMNs and MCNs can be identified radiologically. Given that MCN lesions are rare, the main diagnostic challenge is to accurately distinguish IPMN from benign cystic neoplasms of the pancreas such as serous cystadenomas. In this scenario, visualizing the communication of the lesion with the pancreatic duct is a key diagnostic feature, since it is essentially only observed in cases of side branch IPMNs (SB-IPMN) (14).
The majority of pancreatic cystic lesions can be detected with magnetic resonance (MR) imaging with high spatial resolution and soft-tissue contrast. Furthermore, magnetic resonance cholangiopancreatography (MRCP) is a non-invasive and an accurate modality in detecting, localizing and classifying IPMNs (14–16).
Some centers have recommended the use of intravenous secretin stimulation as part of their MR protocol to evaluate pancreatic cystic lesions (14–16). Secretin is a 27-amino acid polypeptide hormone secreted by the duodenal mucosa in response to luminal acid (17). One of its numerous physiological effects is to stimulate pancreatic duct epithelial cells to produce bicarbonate-rich fluid. Due to this mechanism of action, secretin is frequently used as part of the MR protocol to improve visualization of the pancreatic duct. Secretin increases the caliber of the pancreatic duct, permitting better assessment of ductal strictures, irregularities and anatomic variants. It has also been recently demonstrated to be of value in patients with pancreatitis (3) Secretin-enhanced MRCP may also delineate to a better degree the communication of a cystic lesion with the ductal system, allowing accurate diagnosis of IPMNs (15,16). The purpose of our study was, therefore, to evaluate the incremental value of secretin-enhanced MRCP (S-MRCP) in detecting ductal communication in a population with high prevalence of small pancreatic cysts.
Material and Methods
This was a prospective multicenter study performed at five tertiary academic medical centers (_________________________). The study was undertaken in compliance with the HIPAA and Institutional Review Board approval was obtained from all participating sites. All subjects provided an informed consent.
Patient Population
Data collected in this study were part of the American Cancer of the Pancreas Screening (CAPS) 3 study (1). Three groups of asymptomatic high-risk individuals were included in the study. They were identified by each site either through their participation in pancreatic cancer family registries such as the National Familial Pancreas Tumor Registry (NFPTR), or from referrals generated by notices of the study through various websites (1,18–21). Risk categories included PJS patients, germline BRCA2 mutation carriers with at least 1 affected first- or second-degree relative with pancreatic cancer, and first degree relatives of patients with familial pancreatic cancer (FPC). FPC was defined as kindred with at least two first-degree relatives with pancreatic cancer. None of these patients had diseases that could affect pancreatic exocrine function including chronic liver diseases, renal diseases, pancreato-biliary diseases, or diabetes mellitus.
MRI Acquisition technique and contrast
A standardized protocol for MRI was performed at 1.5 Tesla unites (Siemens Avanto and GE Signa HDx) using a phased-array torso coil. The protocol included axial T2 weighted fat saturated images (4–6 mm, TR 4000–6000, TE 90–100), at least 6 thick slab (40–50 mm, TR 4500, TE 500–700) heavily T2 weighted magnetic resonance cholangiopancreatography (MRCP) images obtained in the straight coronal plane, one before and every minute for five minutes after intravenous administration of human synthetic secretin (0.2 ug/kg; ChiRhoClin, Inc, Burtonsville, MD). The sequence was repeated in the coronal oblique planes. Thin section (1–3 mm, TE 150–200, overlap 0–50%) respiratory triggered or navigator coronal 3D FS TSE images are also obtained before and 5 minutes after secretin administration. Breath-hold unenhanced and contrast-enhanced (0.1 mmol/kg intravenous gadopentetate; Magnevist; Bayer, Wayne, NJ) T1-weighted (T1W) three-dimensional fat suppressed spoiled gradient-echo images (field of view, 320–400 mm; matrix, 192 × 160; slice thickness, 2.5 mm; repetition time, 5.77 ms; echo time, 2.77 ms; received bandwidth, 64 kHz; flip angle, 10°) in the arterial (20 s), portal venous (70 s), and delayed phase (3 min) were also obtained. Two subjects reported adverse events related to the use of secretin in this study. One patient had a mild reaction in the form of itching, and the other patient had nausea.
Image Analysis
The MRI studies were de-identified and prospectively reviewed at (________). Initial review was by a single MR radiologist (___) with 14 years of experience in abdominal imaging who was blinded to clinical findings. The presence and number of pancreatic cysts was recorded as well as the size of the largest cyst. Subsequently, all MRI studies with cystic lesions of the pancreas that included thin section MRCP pre and post-secretin were reviewed by 2 radiologists specialized in abdominal imaging, with 3 years (____) and 14 years (___) of experience. Both radiologists, who were blinded to the diagnosis, reviewed the largest (dominant) pancreatic cystic lesion and independently recorded the presence of communication of the lesion with the main pancreatic duct (MPD) and confidence in the presence or absence of ductal communication. These 2 features were analyzed first on the pre-secretin images alone. Post secretin images were analyzed separately after 2 weeks to reduce recall bias. The degree of confidence in the presence or absence of ductal communication was scored on a 5 –point scale: 1 (≤20%), 2 (>20% – ≤40%), 3 (>40%– ≤60%), 4 (>60%– ≤80%) and 5 (>80%).
Statistical Analysis
Continuous sociodemographic variables are presented as mean ± standard deviation (SD) and categorical variables as proportions or percentages. Continuous and categorical sociodemographic variables were compared using the Student t- test and chi- squared tests, respectively.
The relationship between visualization of ductal communication and the presence of secretin was examined with a logistic regression model. The model was adjusted for potential inter-reader differences, statistical interaction between reader and secretin administration, and statistical correlation arising from having both readers interpret the same case set. The relationship between interpretation confidence and secretin administration was examined with an ordered logistic regression model having the same covariates as the preceding logistic regression model. All statistical analyses were performed using the Stata statistical software package (version 10, Stata Corp., College Station, Texas). A p-value of <0.05 indicated statistical significance.
Results
Demographic Information
Of 202 asymptomatic HRIs enrolled and imaged, 93 (46%) were found to have at least one pancreatic cyst. Demographic information of the entire cohort and the subjects with and without pancreatic cysts is shown in Tables 1 and 2. Average cyst size was 7 ± SD 4 mm (2–20 mm). The presence of cystic lesions of the pancreas increased with age (p<0.001) in our population. No association was found between the presence of pancreatic cysts and gender, ethnicity or risk group (Table 2). Forty-nine (52.7%) of the 93 subjects with a cyst had one or two cystic lesions, while 6 (6.5%) subjects had 12 or more lesions.
Table 1.
Demographics information of the entire cohort
| Age (yrs) | |
| Mean ± SD | 56 +/− 10 |
| Range | 27–79 |
| Gender (%) | |
| Male | 93 (46.0) |
| Female | 109 (54.0) |
| Ethinicity (%) | |
| Caucasian | 198 (98.0) |
| Black/African American | 2 (1.0) |
| Hispanic | 1 (0.5) |
| Indian | 1 (0.5) |
| Medical Center (%) | |
| --- | 121 (59.9) |
| --- | 46 (22.8) |
| --- | 20 (9.9) |
| --- | 15 (7.4) |
| Risk Categories (%) | |
| FPC relative (3 affected, 1 FDR, 2 SDR minimum) | 108 (53.5) |
| FPC relative (2 affected FDR) | 70 (34.7) |
| Germline mutation carrier BRCA2 (with close relative with PC) | 20 (9.9) |
| Germline mutation carrier p16 (FAMMM) | 3 (1.5) |
| Peutz-Jegher syndrome | 1 (0.5) |
Table 2.
Difference in demographic information for patients with and without pancreatic cysts
| Presence of Pancreatic Cystic Lesion |
|||
|---|---|---|---|
| No | Yes | p value | |
| Age (yrs) | |||
| Mean ± SD | 53 ± 9 | 60 ± 9 | <0.001 |
| Gender | |||
| Male | 52 | 41 | 0.607 |
| Female | 57 | 52 | |
| Ethnicity | |||
| Caucasian | 107 | 91 | 0.564 |
| Black/African American | 1 | 1 | |
| Hispanic | 1 | 0 | |
| Indian | 0 | 1 | |
| Risk Categories | |||
| FPC relative (3 affected, 1 FDR, 2 SDR minimum) | 61 | 47 | 0.676 |
| FPC relative (2 affected FDR) | 37 | 33 | |
| Germline mutation carrier BRCA2 (with close relative with PC) | 9 | 11 | |
| Germline mutation carrier p16 (FAMMM) | 2 | 1 | |
| Peutz-Jegher syndrome | 0 | 1 | |
Of the 93 patients with cystic lesion of the pancreas identified by S-MRCP, 64 were included in the analysis that assessed the incremental value of S- MRCP in the screening of asymptomatic individuals with high risk of pancreatic cancer. 29 patients were excluded from this analysis either because their MR study quality was classified as poor (3 patients) or because thin section MRCP either pre or post-secretin were not acquired (26 patients).
Value of Secretin in Visualizing Ductal Communication
On the 64 pre-secretin images Reader 1 (with 3 year experience) visualized communication between the largest cyst and the MPD in 31 cases (48.4%), while no communication was observed in the remaining 33 lesions (51.6%). Reader 2 (with 14 years experience) detected communication between the largest cyst and the MPD in 33 of the 64 cases (51.6%) on the pre-secretin images, while no communication was visualized in the remaining 31 lesions. Analysis of images after the administration of secretin allowed Reader 1 and Reader 2 to visualize ductal communication which was not identified on pre-secretin images in an additional 4 (6.3%) and 2 (3.1%) exams, respectively (Table 2, Figure 1). Data from both readers demonstrated ductal communication between the largest cyst and the MPD in 6 additional cases out of their 128 interpretations (4.7%). Post-secretin imaging was associated with a greater likelihood of visualizing ductal communication compared to pre-secretin imaging (odds ratio 1.28, p = 0.04). Overall, there was no statistically significant difference between readers for visualizing ductal communication (p = 0.2). No case had better demonstration of ductal communication on the pre-secretin dataset compared to the post-secretin images.
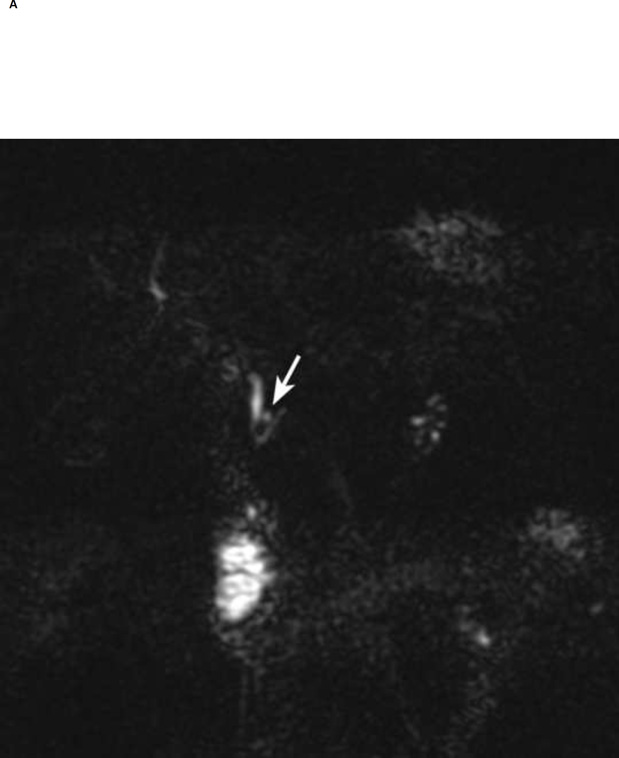
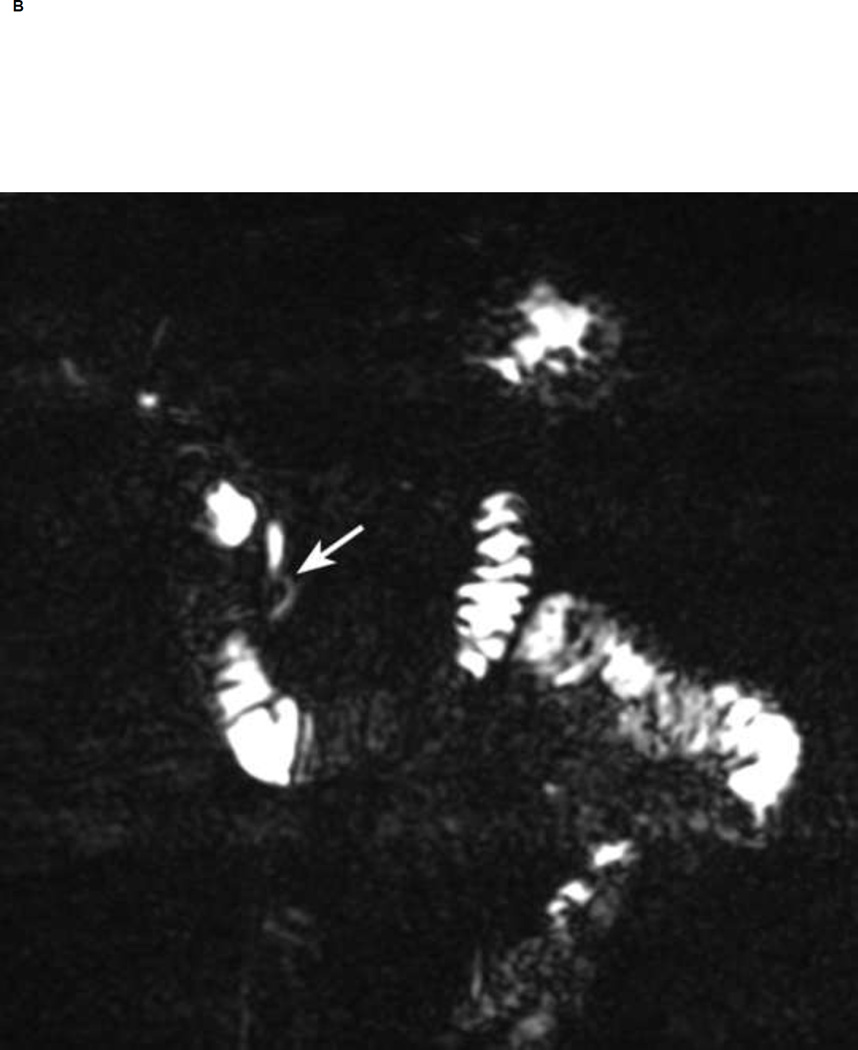
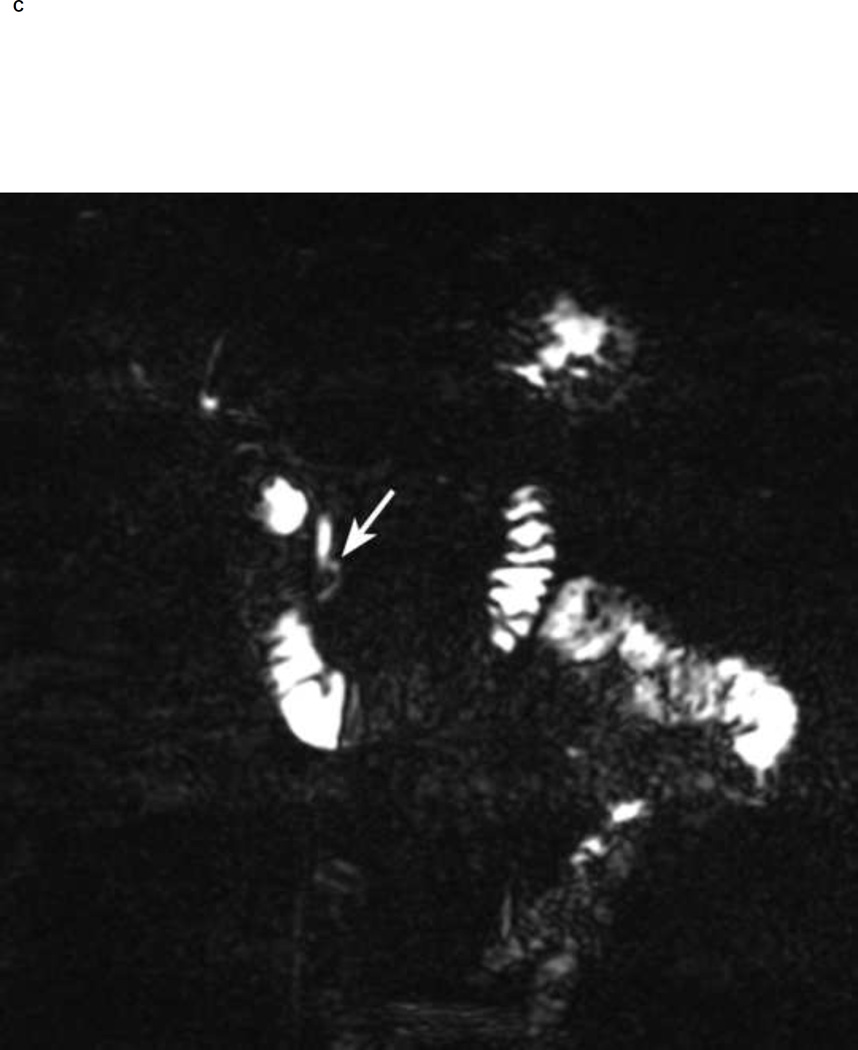
Figure 1.
Thin section (1-mm) MRCP before (A) and 5 min after secretin (B, C). Notice a 5 mm cyst in the region of the head of the pancreas (arrow in A). Neither of the readers visualized communication of the cyst with the pancreatic duct before secretin. After secretin both readers had high confidence (score of 5) in visualizing ductal communication (arrow in B). Notice increasing pancreatic secretions in duodenum and small bowel post secretin.
Value of Secretin in Improving Confidence in Diagnosis
The effect of secretin on the degree of confidence on the diagnosis of visualizing ductal communication between the lesion and MPD was tested. Reader 1 scored ductal communication on pre-secretin images with a confidence level of 3, 4 and 5 in 5 (7.8%), 22 (34.4%) and 37 lesions (57.8%), respectively. Reader 2 scored ductal communication on pre-secretin images scored a confidence level of 3, 4 and 5 in 8 (12.5%), 14 (21.9%) and 41 lesions (64.1%), respectively. No case was scored a confidence level of 1 or 2.
After secretin, 50 (78.1%) and 49 (76.6%) cases were diagnosed with the greater level of confidence by Reader 1 and 2, respectively. Neither of the readers scored the post-secretin images with a level 1 or 2 confidence. In the ordered logistic regression model, interpretation of post-secretin images was associated with a much greater level of confidence than pre-secretin imaging (p < 0.0005). There was no statistically significant difference in confidence ratings between the two readers.
Discussion
Pancreatic cysts have become an increasingly common diagnosis over the last decade in part thanks to increase in the use of high resolution abdominal imaging. Individuals with multiple first-degree relatives with pancreatic cancer have a very high incidence of pancreatic cysts (13,22). The goal of screening is to detect high grade benign precursor lesions such as high risk IPMNs, that can be operatively treated. The radiological assessment of pancreatic cysts can be challenging, particularly the differentiation of IPMNs from those pancreatic cysts with little malignant potential such as serous cystadenomas. Visualizing ductal communication of a pancreatic cyst is very helpful since it supports the diagnosis of IPMN. The objective of this study was to quantify the incremental value of S-MRCP in detecting ductal communication in a population with high prevalence of small pancreatic cysts. Only high risk subjects were included in this study.
This study has several interesting findings. First, the presence of pancreatic cystic lesions increased with patient age. This is similar to what was reported in a prior study by Lee et al, where the incidence of pancreatic cysts increased form 7.9% below the age of 70 to 40.2% above 70 years of age (23). Second, we found no association between the presence of pancreatic cysts and gender, ethnicity or type of high risk group (p-value = 0.67). Third, we found that secretin improved visualization of ductal communication and also increased the reader’s confidence in making a diagnosis of IPMN.
Secretin stimulates pancreatic exocrine secretions causing a temporary dilatation of the pancreatic ducts. This allows for better visualization of the ducts at MRCP. Few prior reports have demonstrated the added value of secretin in diagnosing pancreatic ductal anatomy and pancreatic pathology (16). A recent phase 3 multicenter studies demonstrated that the use of secretin in patients with pancreatitis resulted in significantly improved sensitivity in detecting ductal abnormalities, with minimal loss of specificity (3). However, the diagnostic utility of secretin in the diagnosis of pancreatic cysts remains debated in part because there have not been controlled studies that have assessed the added diagnostic value of secretin. Our study demonstrated that the administration of secretin improved visualization of ductal communication of a cystic lesion in 4.6% of patients (p=0.04) with pancreatic cysts. In addition, both readers regardless of their experience had higher confidence in making the diagnosis of IPMN using secretin compared to their confidence without using secretin. A recent study by Purysko et al. evaluated the value of secretin in SB-IPMN patients and non-IPMN subjects (control) (24). They reported that Secretin administration did not improve the demonstration of communication between the SBIPMN and the MPD. The difference between their study and our study is that the time of acquisition of the post-secretin HASTE images was beyond the time of maximal effect of secretin on the pancreatic ductal system (10 minutes in their study compared to 5 minutes in the current study) and most of the secreted fluid has already passed in the duodenum. This potentially affects the visualization of duct communication on the post-secretin HASTE images and explains the lack of significant difference in duct communication and the lesion size on the pre- and post-secretin HASTE images.
In spite of the added value of using secretin there are several disadvantages to using it routinely in MRI. The added cost, the potential for side effects of secretin and the extended scanning time should be considered when considering S-MRCP. These potential disadvantages should be balanced with the usefulness of detecting a curable precursor lesion. The majority of small pancreatic cysts remanin stable and do not develop cancer (9,25–27). However, when an adenocarcinoma arises in association with an IPMN, the IPMN and the invasive carcinoma almost always harbor the same genetic alterations, supporting the hypothesis that IPMNs are a precursor to invasive adenocarcinomas (28–30). Detection of early precursor lesions could lead to decreased morbidity and mortality from pancreatic adenocarcinoma. Therefore, regardless of the cost and time, it may be useful to use secretin in high-risk patients to detect early-stage pancreatic cancer or high-grade benign noninvasive precursor neoplastic lesions such as IPMNs (31). In addition, it may be advantageous to use secretin in patients based on their symptoms, risk factors and previous imaging findings. Clinicians should also be aware that, especially in females, small non communicating pancreatic cysts can be mucinous cystic neoplasm, which are considered premalignant lesions. Thus, in high risk patients small non-communicating cysts may still be followed-up (32).
There are several limitations to this study. First, the gold standard for diagnosing an IPMN is surgical pathology but most patients with small IPMNs such as those in this study do not undergo surgical resection of their cyst(s). These patients do not undergo ERCP routinely either, unless there is a need for diagnostic or therapeutic intervention. This is due to the high risk of pancreatitis associated with ERCP (1). However, we considered the non S-MRCP sequences as the standard of reference in this study, and we compared visualization of ductal communication to S-MRCP. In addition, routine utilization of S-MRCP in high risk patients has been recommended by Salvia, et al (33), who reported high reliability of S-MRCP in correctly diagnosing branch duct IPMN. Endoscopic ultrasound, without or cyst aspiration analysis can help determine the nature of the pancreatic cyst but this is not performed in the current study due to the small size of cysts (mean 7 mm). Also, endoscopic ultrasound is more invasive than SMRCP, is operator dependent, and maybe associated with false positive results (33). Our study was performed using 1.5T units for this multi-institutional study to standardize the MRCP protocol using the most widely available magnet. Future studies should be performed to assess the validity of our results at 3T units.
Conclusion
With 1.5 T MRI, the use of secretin improved visualization of ductal communication of a cystic lesion in 4.7% of patients with cysts. This incremental increase in visualizing ductal communication was also associated with increasing the reader’s confidence in making a diagnosis of IPMN.
The incremental value of secretin in screening subjects for IPMN could potentially offset the added cost and time for additional sequences. Radiologists should decide on the cost/benefit ratio of using secretin in such cases.
Highlights.
Secretin improved visualization of ductal communication of a cystic pancreatic lesion.
No association between cysts and gender, ethnicity or type of high risk.
Incremental value of secretin could offset the added cost and time.
Acknowledgments
Research funding:
National Cancer Institute (NCI) SPORE (CA62924)
Role of funding source:
Provided funds to recruit, image, and follow up high risk subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013 Mar;62(3):339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman S, Freeman ML, Tarnasky PR, Wilcox CM, Kulkarni A, Aisen AM, et al. Secretin (RG1068) Administration Increases Sensitivity of Detection of Duct Abnormalities by Magnetic Resonance Cholangiopancreatography in Patients with Pancreatitis. Gastroenterology. 2014 Jun 3; doi: 10.1053/j.gastro.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 4. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2013/index. [Google Scholar]
- 5.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012 Jan;51(1):14–24. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012 Apr;142(4):796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010 Oct 15;16(20):5028–5037. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 8.Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009 Oct;58(10):1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 9.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004 Jul;2(7):606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 10.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006 Jun;4(6):766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 11.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999 Aug 17;131(4):247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 12.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009 Sep;104(9):2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 13.Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013 May;42(4):717–721. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 14.Tirkes T, Sandrasegaran K, Sanyal R, Sherman S, Schmidt CM, Cote GA, et al. Secretin-enhanced MR Cholangiopancreatography: Spectrum of Findings. Radiographics. 2013 Nov-Dec;33(7):1889–1906. doi: 10.1148/rg.337125014. [DOI] [PubMed] [Google Scholar]
- 15.Carbognin G, Pinali L, Girardi V, Casarin A, Mansueto G, Mucelli RP. Collateral branches IPMTs: secretin-enhanced MRCP. Abdom Imaging. 2007 May-Jun;32(3):374–380. doi: 10.1007/s00261-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 16.Akisik MF, Sandrasegaran K, Aisen AA, Maglinte DD, Sherman S, Lehman GA. Dynamic secretin-enhanced MR cholangiopancreatography. Radiographics. 2006 May-Jun;26(3):665–677. doi: 10.1148/rg.263055077. [DOI] [PubMed] [Google Scholar]
- 17.Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol. 2003;38(11):1025–1035. doi: 10.1007/s00535-003-1235-3. [DOI] [PubMed] [Google Scholar]
- 18. http://pathology.jhu.edu/pc/. [Google Scholar]
- 19. http://www.lustgarten.org. [Google Scholar]
- 20. http://www.nih.gov.
- 21. http://www.clinicaltrials.gov.
- 22.Schmid RM, Siveke JT. Approach to cystic lesions of the pancreas. Wien Med Wochenschr. 2013 Nov 20; doi: 10.1007/s10354-013-0244-y. [DOI] [PubMed] [Google Scholar]
- 23.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010 Sep;105(9):2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 24.Purysko AS, Gandhi NS, Walsh RM, Obuchowski NA, Veniero JC. Does secretin stimulation add to magnetic resonance cholangiopancreatography in characterising pancreatic cystic lesions as side-branch intraductal papillary mucinous neoplasm? Eur Radiol. 2014 Aug 7; doi: 10.1007/s00330-014-3355-y. [DOI] [PubMed] [Google Scholar]
- 25.Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006 Oct;244(4):572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rautou PE, Levy P, Vullierme MP, O'Toole D, Couvelard A, Cazals-Hatem D, et al. Morphologic changes in branch duct intraductal papillary mucinous neoplasms of the pancreas: a midterm follow-up study. Clin Gastroenterol Hepatol. 2008 Jul;6(7):807–814. doi: 10.1016/j.cgh.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008 Mar;57(3):339–343. doi: 10.1136/gut.2007.129684. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011 Jul 20;3(92):92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012 Jan;43(1):1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg WM, Barkin JS, Bradley EL, 3rd, DiMagno E, Layer P, Canto MI, et al. Should patients with a strong family history of pancreatic cancer be screened on a periodic basis for cancer of the pancreas? Pancreas. 2009 Jul;38(5):e137–e150. doi: 10.1097/MPA.0b013e3181a86b2c. [DOI] [PubMed] [Google Scholar]
- 32.Tada M, Kawabe T, Arizumi M, Togawa O, Matsubara S, Yamamoto N, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006 Oct;4(10):1265–1270. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007 Aug;56(8):1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]