Abstract
Evidence has emerged for macrophages in the perivascular niche of tumors regulating important processes like angiogenesis, various steps in the metastatic cascade, the recruitment and activity of other tumor-promoting leukocytes, and tumor responses to frontline therapies like irradiation and chemotherapy. Understanding the mechanisms controlling the recruitment, retention, and function of these cells could identify important targets for anti-cancer therapeutics.
Introduction
Tumor-associated macrophages (TAMs) are a major cellular component of both mouse and human tumors (Pollard, 2004; Lewis and Pollard, 2006). High numbers of TAMs in human tumors usually correlate with reduced patient survival (Bingle et al., 2002; Zhang et al., 2012), and studies in mice have shown that TAMs promote important steps in tumor progression including tumor angiogenesis, cancer cell invasion, metastasis, and the suppression of adaptive anti-tumor immunity (Noy and Pollard, 2014). TAMs also mediate or limit the efficacy of various forms of anti-cancer therapies (De Palma and Lewis, 2013).
Recent fate-mapping experiments have shown that macrophages in steady-state tissues can originate from at least three different sources. In the adult brain, skin, lung, and liver, they arise from the local proliferation of embryonic yolk sac or fetal liver precursors; whereas in such tissues as the intestines and mammary gland they largely derive from blood monocytes (Perdiguero and Geissmann, 2015). Studies using fluorescently labeled bone marrow (BM) transplants or the intravenous injection of labeled monocytes have shown that TAMs in mouse mammary, lung, or brain tumors are also derived from blood monocytes (De Palma et al., 2003, 2005; Franklin and Li, 2014; Movahedi et al., 2010). Similar results were found at lung metastatic sites, where metastasis-associated macrophages (MAMs) were shown to be derived from a subset of monocytes (Qian et al., 2009, 2011). Circulating monocytes are derived mainly from hematopoietic stem cells in the BM, and are recruited across the vasculature into tumors by tumor-derived chemoattractants such as CSF1, CCL2, VEGFA, or CXCL12 (Murdoch et al., 2008; Noy and Pollard, 2014). Alternatively, TAMs in some mouse tumors can expand within tumors, in part, via proliferation (Franklin et al., 2014; Tymoszuk et al., 2014).
Irrespective of their origins, TAMs tend to gather in distinct tumor microenvironments including the invasive tumor edge, cancer cell/stromal border, central tumor mass, hypoxic/necrotic regions, and perivascular (PV) areas. Moreover, TAM distribution can vary between different types of tumor, and between primary or metastatic tumors (Lewis and Pollard, 2006). Importantly, across several types of human tumor, the abundance of a distinct subset of PV macrophages has been shown to correlate with increased tumor angiogenesis, distant metastasis, poor prognosis, and/or the recurrence of tumors after chemotherapy in various forms of cancer (Kurahara et al., 2012; Matsubara et al., 2013; Robinson et al., 2009).
As discussed in later sections, the markers expressed by PV macrophages vary between primary and metastatic tumors. However, a unifying feature is their proximity to blood vessels (sometimes making direct contact with endothelial cells or pericytes), or their preference for highly vascularized tumor areas. This contrasts with the majority of TAMs found elsewhere in tumors (Lewis and Pollard, 2006). This review discusses recent studies demonstrating an array of tumor-promoting functions for PV macrophages in both primary and secondary tumors (summarized in Figure 1).
Figure 1. The Roles of Perivascular Macrophages in Tumor Progression.
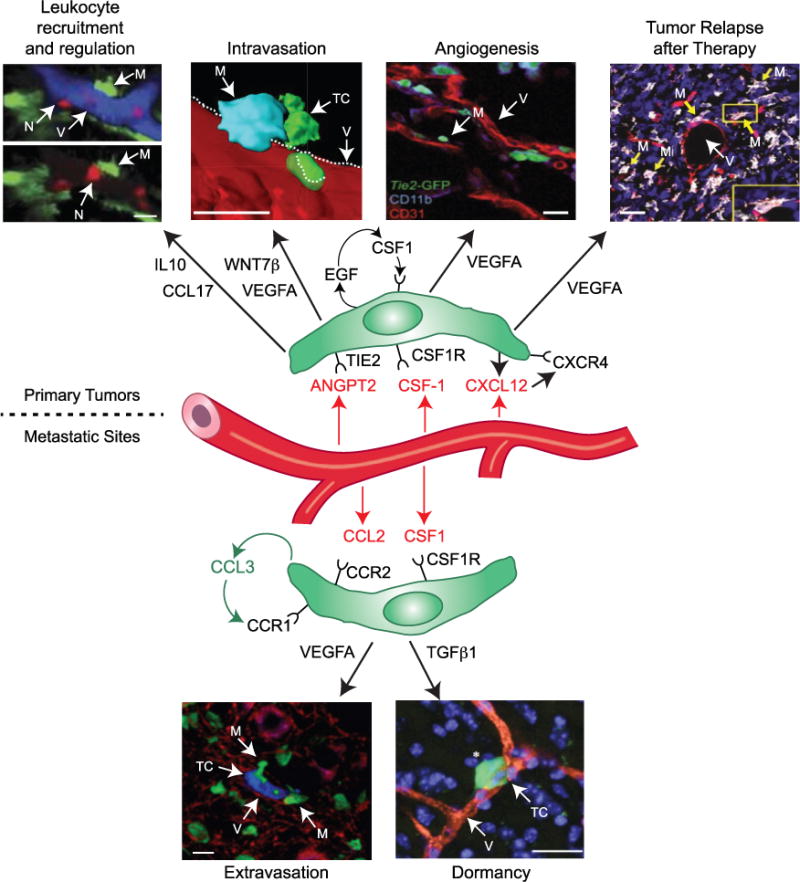
In primary tumors. Recruitment and regulation of other tumor-promoting leukocytes – the two images, with and without vessels (blue) included, show that neutrophils (red, N) extravasate in inflamed tissues in close proximity to perivascular (PV) macrophages (green, M). [Reprinted with permission: Abtin et al., 2014.] Intravasation of tumor cells: the images show a triad of a PV TIE2+VEGFA+ TAM (blue, M), cancer cells (green, TC), and endothelial cells (red). [Reprinted with permission: Harney et al., 2015.] Angiogenesis stimulation: the image shows TIE2+ TAMs (green, M) located near blood vessels (red, V) in tumors. [Reprinted with permission: De Palma et al., 2003.] Relapse of tumors after therapy: the images show a subcutaneous Lewis lung carcinoma after treatment with cyclophosphamide (TIE2+ blood vessels [red, V]; TIE2+MRC1+ TAMs [white/pink, M]; and cell nuclei [blue]). Inset: a single, TIE2+MRC1+ TAM (white/red). [Reprinted with permission: Hughes et al., 2015.] In metastatic sites. Extravasation of cancer cells: the image shows a cancer cell (blue, TC), PV macrophages (green, M), and blood vessels (red, V) in the lungs of mice. [Reprinted with permission: Qian et al., 2009.] Dormancy: the image shows a dormant cancer cell (green, TC; white asterisk) located close to a blood vessel (red, V) in the brain. Cell nuclei are shown in blue. [Reprinted with permission: Ghajar et al., 2013.] Many of the above functions involve the release of soluble factors by PV macrophages (green), and often activated by factors expressed by neighboring endothelial cells (red). Scale bars, 20 μm.
Tumor Angiogenesis
A distinct subset of PV TAMs has been shown to regulate angiogenesis in various mouse primary tumor models and to correlate with microvessel density in human tumors. BM transplant experiments conducted by De Palma et al. (2003, 2005) identified a subset of BM-derived TAMs that cluster around tumor blood vessels and express higher levels of the angiopoietin receptor, TIE2, and the mannose receptor, MRC1, than macrophages elsewhere in tumors, or circulating monocytes (Figure 1). Subsequent gene expression profiling of TIE2+ TAMs isolated from mouse mammary (N202) tumors revealed their higher expression of a number of tumor-promoting genes including Mmp9, Vegfa, Cxcl12, Tlr4, Nrp1, and Pdgfb than was seen in TIE2− TAMs from the same tumors (Pucci et al., 2009). Although TIE2-expressing monocytes appear to be pre-programmed to be proangiogenic in the circulation, this function can be further upregulated by exposure to tumor-derived factors like angiopoietin-2 (ANGPT2), expressed mainly by activated endothelial cells (Coffelt et al., 2010).
De Palma et al. (2005) compared the proangiogenic function of mouse TIE2− and TIE2+ TAMs in vivo. First, they showed that the specific elimination of TIE2+ TAMs using a conditional suicide strategy impaired vascularization and growth of several tumor types including gliomas, insulinomas, and mammary cancers in mice. They then isolated TIE2− and TIE2+ TAMs from mouse mammary (N202) tumors and coinjected them with N202 cells. TIE2+ TAMs resulted in significantly more vascularized tumors than N202 tumor cells injected alone or with TIE2− TAMs. These data demonstrate a distinct functional difference between the proangiogenic functions of TIE2− and TIE2+ TAMs in mouse tumors. While such experimental approaches are invaluable, live imaging of these two TAM subsets in vivo may also prove useful in elucidating their role specifically in the PV niche.
An interesting parallel occurs during development, suggesting that tumors may co-opt some physiological functions of macrophages. In mouse embryos, TIE2+ macrophages are known to associate with adjacent vascular sprouts and to “bridge” between endothelial tip cells facilitating vascular anastomosis (Fantin et al., 2010; Baer et al., 2013). They also release factors like VEGFC (Tammela et al., 2011) and soluble VEGFR 1 (Stefater et al., 2011) to regulate angiogenic vessel branching. In tumors, the direct association of these PV TAMs with blood vessels was also found to be essential for their pro-angiogenic role. Tumor angiogenesis was markedly reduced when this association was impeded using either a conditional, hematopoietic-specific, knockdown strategy to silence their TIE2 expression, or the pharmacological blockade of ANGPT2 (Mazzieri et al., 2011).
Finally, these TIE2+ PV TAMs may also have a proangiogenic role in human cancers as their frequency has been reported to correlate with the density of tumor microvessels, as well as lymph node status, tumor grade, and distant metastasis (Ji et al., 2013; Matsubara et al., 2013).
Intravasation of Cancer Cells in Primary Mammary Tumors
Genetic and inhibitor experiments coupled with high-resolution intravital imaging have shown that TAMs in autochthonous and xenografted mammary tumors engage with tumor cells in an obligate epidermal growth factor (EGF)-CSF1 paracrine signaling loop (Wyckoff et al., 2004). This engagement results in tumor cells streaming toward blood vessels where they then undergo intravasation at clusters of PV macrophages; the first step in the metastatic cascade (Wyckoff et al., 2007). A recent intravital imaging study showed that these PV TAMs are TIE2+MRC1+VEGFA+ and are an essential component of a micro-anatomical site regulating tumor cell escape from primary tumors called the tumor microenvironment of metastasis (TMEM) (Harney et al., 2015). This consists of a PV TAM in direct contact with both an endothelial cell and a tumor cell expressing a splice variant of mammalian-enabled protein, an actin regulatory protein involved in tumor cell motility and heightened sensitivity to EGF chemotaxis (Robinson et al., 2009; Rohan et al., 2014) (Figure 1). The clinical relevance of these three-cell structures was confirmed in breast cancer when it was found that high TMEM density in breast carcinomas correlates with increased metastatic recurrence, independently of other clinical prognostic indicators (Robinson et al., 2009; Rohan et al., 2014).
Intravital imaging has revealed that dynamic vascular permeability occurs concurrently with cancer cell intravasation at these TMEM sites, and genetic deletion of Vegfa in TAMs prevents both events (Harney et al., 2015). Two vascular junction proteins that limit vascular leakiness, ZO-1 and vascular endothelial (VE)-cadherin, were frequently disrupted at these hyper-permeable sites, suggesting that VEGFA signaling directly affects vascular permeability through vascular junction stability (Harney et al., 2015). In both mouse mammary tumors and human breast carcinomas, VE-cadherin is reduced in endothelial cells in and around TMEMs (Harney et al., 2015). These data suggest that VEGFA released by TIE2+ macrophages in TMEMs causes the local dissolution of vascular junction proteins, resulting in a transient increase in vascular permeability and tumor cell intravasation.
It is not known whether ANGPT2, a proangiogenic factor and chemokine for TIE2-expressing macrophages (Murdoch et al., 2007; Venneri et al., 2007), plays a part in regulating the recruitment and/or activation of PV macrophages in TMEMs. Although pharmacologic blockade of ANGPT2 markedly reduced lung metastases in mouse bearing mammary tumors, this may have been due to inhibition of the direct proangiogenic effect of ANGPT2 on tumor blood vessels (Mazzieri et al., 2011). So, the relative contribution of these two ANGPT2-driven events in metastasis remains unclear. Further characterization of the prometastatic subset of PV TAMs in TMEMs could lead to effective ways to selectively target them, and thus reduce the spread of breast cancer cells from the primary to distant sites. It also remains to be seen whether such PV TAMs (and TMEMs generally) exist in other types of primary tumor than just mammary cancers. This may prove to be the case as TAMs have also recently been reported to promote vascular permeability in a mouse ovarian tumor model (Moughon et al., 2015).
Seeding of Distant Metastatic Sites
In an experimental metastasis model, PV MAMs have been shown to interact closely with mouse mammary cancer cells as the latter extravasate across the lung vasculature (Figure 1). These seeding events were strongly inhibited when MAMs were depleted either by genetic ablation or by inhibition of their recruitment with a neutralizing antibody to the chemokine, CCL2, the ligand for CCR2 expressed on circulating Ly6C+ monocytes and MAMs (Qian et al., 2009; 2011). In early metastatic lesions, CCL2 is thought to be secreted by both cancer cells (Qian et al., 2011) and the endothelium (Srivastava et al., 2014), and has been shown to stimulate the expression of another important chemokine, CCL3, by MAMs. This chemokine acts via CCR1 to retain CCR2+ macrophages in the metastatic site by promoting their adhesion to cancer cells, to which they deliver a survival signal (Kitamura et al., 2015). This interaction between tumor cells and PV MAMs results in a VEGFA-induced increase in vessel permeability and cancer cell escape from the vessel (Qian et al., 2011), in a manner reminiscent of the TMEM-driven intravasation at the primary tumor site. In contrast to these results, a population of intravascular Ly6Clo monocytes present in the lung inhibits metastatic seeding by recruiting natural killer cells, which are known to be anti-metastatic and able to kill circulating tumor cells before their seeding (Hanna et al., 2015).
In some metastatic sites like the lungs, liver or bone, disseminated cancer cells (DCCs) are known to remain dormant for long periods before proliferating to form metastases. These dormant cells usually reside close to microvessels in such tissues (Figure 1), where local factors like endothelial-derived thrombospondin-1 suppress their proliferation. This inhibitory cue is lost around sprouting neovessels and replaced by a mitogen for DCCs, transforming growth factor β1 (TGFβ1), which is released by endothelial tip cells and stimulates the outgrowth of micrometastases (Ghajar et al., 2013). It is tempting to speculate that PV macrophages may also help DCCs exit from dormancy. As mentioned previously, PV macrophages interact with cancer cells as they extravasate into metastatic sites like the lungs (Qian et al., 2009, 2011) and are present in established metastatic tumors (Hughes et al., 2015). It is possible that monocytes recruited from the circulation are retained in the PV niche due to tip cells in neovessels expressing high levels of macrophage migratory inhibitory factor (Ghajar et al., 2013), a cytokine known to immobilize macrophages. Exposure to high local levels of TGFβ1 could then activate them (Gong et al., 2012), upregulating their own expression of TGFβ1. This would further elevate TGFβ1 levels around blood vessels, adding to the stimulation of DCC proliferation.
Once metastases form in tissues like the lung or bones, MRC1+ PV macrophages are abundant, especially after exposure to cytotoxic agents (Hughes et al., 2015) (Figure 1; see next section). Whether they congregate into TMEM-like structures in such metastatic tumors has yet to be elucidated. But if so, this could then promote the escape of cancer cells from metastatic tumors into the circulation and contribute to the phenomena of both “metastasis-to-metastasis” seeding reported recently in human prostate tumors (Gundem et al., 2015) and “self-seeding” back to the primary site (Kim et al., 2009).
Tumor Relapse after Therapy
PV TAMs have also been implicated in the relapse of mouse tumors after various frontline therapies. For example, in orthotopic gliomas, subcutaneous lung xenografts, and mouse mammary tumors, a CXCL12-driven increase in the recruitment of CD11b+ BM-derived cells was a requisite for tumor regrowth after the cessation of local irradiation (Kioi et al., 2010; Kozin et al., 2010). A high proportion (>50%) of these myeloid cells were found to be TIE2+ macrophages, and to often take up a PV location in relapsing tumors (Kioi et al., 2010; Kozin et al., 2010). This raised the possibility that such an increase in the numbers of these proangiogenic cells around blood vessels could drive tumor revascularization and regrowth.
A similar role for PV TIE2+ TAMs has emerged in mouse tumors recovering from exposure to chemotherapeutic drugs. Initial studies used CSF1R inhibitors showed that CSF1-dependent TAMs limit the cytotoxic effects of the chemotherapeutic agent, paclitaxel, on tumors, in part, by suppressing cytotoxic T cells (DeNardo et al., 2011; Mitchem et al., 2013; Shree et al., 2011). More recently, TIE2+MRC1+CXCR4+ TAMs have been shown to accumulate in PV areas of mouse subcutaneous lung and orthotopic mammary tumors after treatment with cytotoxic agents and then promote tumor relapse. The accumulation of these cells around blood vessels in treated tumors was found not to be due to TAM proliferation but rather their recruitment by CXCL12 upregulated in PV tumor areas after chemotherapy. These cells then stimulated tumor revascularization and regrowth, in part, via their release of VEGFA. Depletion of these PV TAMs using an inhibitor of CXCR4 (the receptor for CXCL12) dramatically reduced vascular density in the re-emergent tumor and extended the therapeutic response to the cytotoxic agent (Hughes et al., 2015).
Tumor-initiating cells (TICs) have been reported in PV areas of brain tumors in mice (Calabrese et al., 2007; Charles et al., 2010, 2011) which are rich in TAMs (Yi et al., 2011). It is, therefore, possible that PV TAMs may contribute to the maintenance of this stem cell niche. As TICs could potentially be the cells from which recurrent tumors originate, this may be a further way in which PV TAMs promote tumor recurrence post-therapy.
The dendritic cell, another myeloid cell type, has recently been shown to display an intrinsic resistance to tumor irradiation in subcutaneous B16 melanoma and to then increase regulatory T cells (Tregs) in treated tumors (Price et al., 2015). It remains to be seen whether PV TAMs respond to tumor irradiation in a similar manner, but TIE2+ TAMs can express the Treg chemokine, CCL17, in progressing mouse tumors when exposed to vascular-derived signals like ANGPT2 (Coffelt et al., 2011). They may do so in the PV niche of tumors after irradiation or cytotoxic therapies, thereby contributing to an immunosuppressive environment.
Interestingly, TIE2+CXCR4hI TAMs also promote the relapse of mouse mammary tumors after exposure to the vascular damaging agent, combretastatin-A-4P (Welford et al., 2011). Furthermore, TIE2+ TAMs have been implicated in the recurrence of gliomas in mice after treatment with anti-angiogenic therapies targeting VEGFA or VEGF receptor 2 (VEGFR2). Although the recruitment of this TAM subset was shown to be dependent on ANGPT2 upregulated in tumors after these treatments (Cortes-Santiago et al., 2016), the role of this cytokine in the recruitment and/or activation of PV TIE2+ TAMs after tumor irradiation or exposure to cytotoxic agents remains to be elucidated.
The above studies prompt speculation that therapy-induced increase in PV TIE2+VEGFAhi TAMs could result in greater TMEM abundance and thus increased shedding of tumor cells into the circulation. Further studies on the effects of irradiation or chemotherapy on TMEM formation could provide additional insights into the role of PV TIE2+ macrophages in cancer dissemination and metastasis after such treatments.
Regulation of Other Leukocyte Subsets in Tumors by PV TAMs
As mentioned earlier, PV TIE2+ TAMs may also play an immunosuppressive role in primary and metastatic tumors. Their ability to upregulate CCL17 near ANGPT2-expressing tumor vessels (Coffelt et al., 2010, 2011), could explain why Foxp3+ Tregs are retained in a PV location in mouse mammary tumors (Egeblad et al., 2008). ANGPT2 also stimulates TIE2+ macrophages to upregulate interleukin 10 (IL10) (Coffelt et al., 2011), which likely suppresses cytotoxic T cells in PV tumor areas (Boissonnas et al., 2007; Mrass et al., 2006). TAM-derived IL10 has also been shown to suppress Tregs in mouse mammary tumors after paclitaxel and carboplatin treatment, in this case by suppressing intratumoral dendritic cell expression of IL12 (Ruffell et al., 2014).
Furthermore, PV TAMs could help recruit neutrophils into tumors, as intravital imaging has shown that neutrophils extravasate through post-capillary venules in inflamed tissues in response to chemoattractants released by PV macrophages (Abtin et al., 2014) (Figure 1).
Origin of PV Macrophages
The precursors of PV macrophages in tumors have yet to be fully defined. Peripheral blood monocytes consist of two main populations: classical and non-classical monocytes (defined respectively as Ly6ChiCX3CR1loCCR2hi or Ly6CloCX3CR1hiCCR2lo in mice, and CD14hiCD16−CCR2hiCX3CR1lo or CD14mid CD16hiCCR2loCX3CR1hi in humans). A minor subset of circulating monocytes expresses TIE2 in both species and could be the precursors of TIE2+ PV macrophages in tumors (De Palma et al., 2003; Murdoch et al., 2007; Venneri et al., 2007). Interestingly, the frequency of circulating TIE2+ monocytes in patients with hepatocellular carcinoma (HCC) correlates positively with that of TIE2+ TAMs in the PV areas of their tumors. Moreover, high numbers of these TIE2+ monocytes correlate with shorter recurrence-free survival of HCC patients after tumor resection and radiofrequency ablation therapy (Matsubara et al., 2013). These data suggest that either a subset of TIE2+ circulating monocytes are the precursors of PV TAMs in progressing and therapy-treated HCCs, or tumor-derived signals trigger similar, parallel changes in a subset of circulating monocytes and PV TAMs. The similarity in the phenotype of non-classical monocytes and TIE2+ PV TAMs prompted some to suggest that they may represent different stages of the same cell lineage (Pucci et al., 2009; Venneri et al., 2007); however, non-classical monocytes rarely extravasate (Carlin et al., 2013), so this now appears unlikely.
Alternatively, PV TAMs may differentiate from the classical Ly6C+CCR2hi monocytic population as has been shown for the MAMs promoting cancer cell extravasation and seeding in the lungs (Qian et al., 2009, 2011). Interestingly, CX3CR1 loss on monocytes causes accumulation of Ly6Chi inflammatory monocytes in PV sites of mouse glioblastomas (Feng et al., 2015). As mentioned earlier, TAM proliferation may occur in some tumor types (Franklin et al., 2014), so it remains a possibility that PV macrophages may have proliferative potential in primary and/or metastatic tumors and be derived, at least in part, from resident yolk sac-derived progenitors. Cell-lineage experiments are now needed to identify the circulating monocytic precursors and/or the local origins of these distinct subsets of PV macrophages in both primary and metastatic tumors. Mechanistic studies of the regulators of these populations will require new genetic models to disrupt gene function in specific monocytic subsets and their macrophage progeny.
Regulation of Macrophages by the PV Niche
The fact that the abluminal surface of tumor vessels provides such an attractive niche for macrophages suggests that there are chemoattractants, retention signals, survival factors, and/or docking proteins for these cells present within this niche. Several possible chemoattractants for monocytes/macrophages are released by endothelial cells in tumors including CSF1, CXCL12, ANGPT2, and CCL2 (He et al., 2012; Hughes et al., 2015; Murdoch et al., 2007; Ryan et al., 2001; Srivastava et al., 2014; Venneri et al., 2007). The recruitment of monocytes and their subsequent differentiation into PV macrophages may also involve specific adhesion molecules. For example, L-selectin (CD62L) regulates the recruitment of macrophages into PV regions during inflammation (Hickey et al., 2000; León and Ardavín, 2008), and metastasis is decreased in CD62L-deficient mice (Borsig et al., 2002). In the metastatic site, monocytes recruited by CCL2 express CCL3, which, through CCR1, activates monocyte αvβ integrin binding to VCAM1 expressed on tumor cells. This anchors the monocyte to tumor cells and provides a survival signal for tumor cells as they extravasate through the endothelial barrier (Kitamura et al., 2015). Monocytes also need to adhere to vessels in order to extravasate, take up their PV location, and differentiate into PV macrophages. These steps have recently been shown to involve VEGFA and VEGFR1 autocrine signaling (Qian et al., 2015).
Furthermore, local factors may also regulate gene expression in PV TAMs. For example, CSF1 stimulates the expression of both TIE2 and VEGFA by human monocytes/macrophages (Eubank et al., 2003; Forget et al., 2014) as well as their chemotactic responses to ANGPT2 and proangiogenic activity in vitro and in tumors (Forget et al., 2014). Also, as mentioned previously, endothelial-derived ANGPT2 regulates the close association of TIE2+ TAMs with blood vessels in primary tumors (Mazzieri et al., 2011), and stimulates their expression of proangiogenic and immunosuppressive genes like VEGFA, IL10, CCL17, and MMP9 (Coffelt et al., 2010, 2011).
Other PV cell types may also regulate PV TAMs. Expression of CXCL12 by fibroblasts has been shown to retain CXCR4+ myeloid cells in close proximity to blood vessels in healthy tissues (Grunewald et al., 2006). However, this was not the case in MMTV-PyMT mammary tumors (Lin et al., 2007), suggesting the involvement of alternative factors in this tumor model. PV TAMs can themselves upregulate CXCL12 when close to blood vessels in some tumor types like metastatic melanoma (Sánchez-Martín et al., 2011). As CXCL12 is known to stimulate macrophage expression of VEGFA, an elevation in PV CXCL12 levels could upregulate this in PV TAMs (Sánchez-Martín et al., 2011). Finally, CCL2 upregulation by the endothelium in metastatic lung tumors both recruits and polarizes CCR2+ macrophages (Srivastava et al., 2014).
Concluding Remarks
A picture is now emerging of the multiple ways in which PV TAMs promote tumor progression and response to therapy. PV TAMs expressing TIE2 and VEGFA stimulate tumor angiogenesis, tumor cell escape into the circulation, and tumor relapse after frontline therapies. Similar PV cells are also present in metastatic tumors but their functions have yet to be determined. Alternatively, another PV macrophage subset, those expressing CCR2 and VEGFA (but not TIE2), also exist in metastatic sites like the lungs and promotes cancer cell seeding through direct interactions with cancer cells at the vessel wall and subsequent promotion of colonization (Entenberg et al., 2015; Srivastava et al., 2014; Qian et al., 2011).
Studies are now needed to identify the origin of these PV macrophage subsets, and the key factors recruiting them and/or regulating their various tumor-promoting functions. Such insights could lead to the development of new therapeutics to selectively deplete or modify their behavior. The need for this has been highlighted in recent clinical trials using CSF1R inhibitors in patients with giant-cell tumors. While these treatments resulted in the preferential depletion of TAMs, and a marked clinical response, it also triggered periorbital edema (Ries et al., 2014; Cassier et al., 2015; Tap et al., 2015), possibly via an effect on lymphatic-associated macrophages which control lymphatic vessel diameter and thus fluid removal (Gordon et al., 2010). Edema may also have resulted from perturbations in vascular homeostasis due to depletion of normal PV macrophages, resulting in vessel leakiness. If this is the case, then PV TAMs in such normal tissues limit rather than promote vascular leakage. These findings indicate that new ways to target only PV macrophages in tumors need to be developed, so that macrophages elsewhere are not adversely affected.
It should also be noted that recent studies have shown that PV TAMs are not the only innate immune cells influencing tumor progression. Neutrophils can also be proangiogenic (Sionov et al., 2015; Tazzyman et al., 2011) and promote metastatic seeding (Coffelt et al., 2015; Wculek and Malanchi, 2015) in some tumor models. However, this may depend on the experimental setting, as another recent study showed that depletion of neutrophils had no effect on metastatic seeding by breast cancer cells in mouse lungs (Kitamura et al., 2015). It remains to be seen whether the selective targeting of PV TAMs per se elicits a compensatory influx and/or activation of tumor-promoting neutrophils. Proangiogenic, MMP9+ neutrophils accumulated in mouse cervical tumors after the pan depletion of CCR2+ TAMs (Pahler et al., 2008). Furthermore, a study showed that two distinct anti-angiogenic agents, initially effective in mouse tumor models, also triggered an influx of Gr1+CD11b+ neutrophils that promoted resistance to further treatment. Targeting Gr1+ cells was not sufficient to lift this blockade, as TAMs were able to compensate for their loss and sustain tumor resistance to anti-angiogenic therapy (Rivera et al., 2015). Studies are now warranted to see whether combining agents that selectively target PV macrophages and tumor-promoting neutrophils yield more sustained anti-tumoral effects, either alone or when administered with frontline therapies.
Acknowledgments
The authors gratefully acknowledge the support of the following funding agencies for their work in this area: Cancer Research UK (C11712/A13028) and Yorkshire Cancer Research (S382) (both to C.E.L.) and NIH (CA172451) (J.W.P.) and NIH CA100324 (A.S.H. and J.W.P.) and the Einstein Integrated Imaging Program (A.S.H.). J.W.P. is also supported by the Wellcome Trust (101067/Z/13/Z) and the Medical Research Council (G1002033). C.E.L. and J.W.P. have no financial interest related to this work. A.S.H. recently moved to be an employee of Flagship Biosciences, Inc. However, she was a postdoctoral scientist at the Albert Einstein College of Medicine in New York when working on this article.
Footnotes
AUTHOR CONTRIBUTIONS
C.E.L. conceived the article and handled correspondence with the journal. C.E.L., A.S.H., and J.W.P. wrote the article, and C.E.L and J.W.P dealt jointly with revisions.
References
- Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res. 2013;319:1626–1634. doi: 10.1016/j.yexcr.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal in presence of nucleic acids. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Müller C, Jegg AM, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, Plate KH, Reiss Y, Murdoch C, De Palma M, Lewis CE. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstege NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, de Visser KE. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Santiago N, Hossain MB, Gabrusiewicz K, Fan X, Gumin J, Marini FC, Alonso MM, Lang F, Yung WK, Fueyo J, Gomez-Manzano C. Soluble Tie2 overrides the heightened invasion induced by anti-angiogenesis therapies in gliomas. Oncotarget. 2016;7:16146–16157. doi: 10.18632/oncotarget.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D, Rodriguez-Tirado C, Kato Y, Kitamura T, Pollard JW, Condeelis J. In vivo subcellular resolution optical imaging in the lung reveals early metastatic proliferation and motility. Intravital. 2015;4 doi: 10.1080/21659087.2015.1086613. http://dx.doi.org/10.1080/21659087.2015.1086613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB. M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J Immunol. 2003;171:2637–2643. doi: 10.4049/jimmunol.171.5.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Szulzewsky F, Yerevanian A, Chen Z, Heinzmann D, Rasmussen RD, Alvarez-Garcia V, Kim Y, Wang B, Tamagno I, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–15094. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget MA, Voorhees JL, Cole SL, Dakhlallah D, Patterson IL, Gross AC, Moldovan L, Mo X, Evans R, Marsh CB, Eubank TD. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLoS One. 2014;9:e98623. doi: 10.1371/journal.pone.0098623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Franklin RA, Li MO. The ontogeny of tumor-associated macrophages: a new understanding of cancer-elicited inflammation. Oncoimmunology. 2014;3:e955346. doi: 10.4161/21624011.2014.955346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Högnäs G, Annala M, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015;5:932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Xu J, Warren CM, Duan D, Li X, Wu L, Iruela-Arispe ML. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120:3152–3162. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MJ, Forster M, Mitchell D, Kaur J, De Caigny C, Kubes P. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J Immunol. 2000;165:7164–7170. doi: 10.4049/jimmunol.165.12.7164. [DOI] [PubMed] [Google Scholar]
- Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75:3479–3491. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Zhang G, Sun B, Yuan H, Huang Y, Zhang J, Wei X, Zhang X, Hou J. The frequency of tumor-infiltrating Tie-2-expressing monocytes in renal cell carcinoma: its relationship to angiogenesis and progression. Urology. 2013;82:974.e9–974.e13. doi: 10.1016/j.urology.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, Maeda K, Shinchi H, Mataki Y, Maemura K, Matsuyama T, Natsugoe S. Clinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancer. Ann Surg Oncol. 2012;19:2264–2271. doi: 10.1245/s10434-012-2263-0. [DOI] [PubMed] [Google Scholar]
- León B, Ardavín C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood. 2008;111:3126–3130. doi: 10.1182/blood-2007-07-100610. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Bricard G, Wang W, Deng Y, Sellers R, Porcelli SA, Pollard JW. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol. 2007;1:288–302. doi: 10.1016/j.molonc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, Miyazaki M, Sakakibara M, Hiramatsu N, Kasahara A, et al. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology. 2013;57:1416–1425. doi: 10.1002/hep.25965. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, Lin C, Iruela-Arispe ML, Dorigo O, Wu L. Macrophage blockade using csf1r inhibitors reverses the vascular leakage underlying malignant ascites in late-stage epithelial ovarian cancer. Cancer Res. 2015;75:4742–4752. doi: 10.1158/0008-5472.CAN-14-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt S, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2015;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S, Leboeuf M, Merad M. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat Immunol. 2015;16:1060–1068. doi: 10.1038/ni.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di Serio C, Naldini L, De Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, Carragher NO, Munro A, Chang A, Bresnick AR, et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212:1433–1448. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11:577–591. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan TE, Xue X, Lin HM, D’Alfonso TM, Ginter PS, Oktay MH, Robinson BD, Ginsberg M, Gertler FB, Glass AG, Sparano JA, Condeelis JS, Jones JG. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. http://dx.doi.org/10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega MÁ, Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–158. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, Jugold M, Besemfelder E, Thomas M, Pasparakis M, Augustin HG. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell. 2014;26:880–895. doi: 10.1016/j.ccell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomäki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129:847–858. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- Tymoszuk P, Evens H, Marzola V, Wachowicz K, Wasmer MH, Datta S, Müller-Holzner E, Fiegl H, Böck G, van Rooijen N, et al. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur J Immunol. 2014;44:2247–2262. doi: 10.1002/eji.201344304. [DOI] [PubMed] [Google Scholar]
- Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, Lewis CE. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]


