Abstract
Purpose
The biology of HIV-associated cancers may differ depending on immunologic and virologic context during development. Therefore, an understanding of the burden of Kaposi's sarcoma (KS) and non-Hodgkin lymphoma (NHL) relative to antiretroviral therapy (ART), virologic suppression, and CD4 count is important.
Patients and Methods
KS and NHL diagnoses during 1996 to 2011 were identified among patients with HIV infection in eight clinical cohorts in the United States. Among patients in routine HIV clinical care, the proportion of cases in categories of ART use, HIV RNA, and CD4 count at diagnosis were described across calendar time. Person-time and incidence rates were calculated for each category.
Results
We identified 466 patients with KS and 258 with NHL. In recent years, KS was more frequently diagnosed after ART initiation (55% in 1996 to 2001 v 76% in 2007 to 2011; P-trend = .02). The proportion of patients with NHL who received ART was higher but stable over time (83% overall; P-trend = .81). An increasing proportion of KS and NHL occurred at higher CD4 counts (P < .05 for KS and NHL) and with undetectable HIV RNA (P < .05 for KS and NHL). In recent years, more person-time was contributed by patients who received ART, had high CD4 counts and had undetectable HIV RNA, whereas incidence rates in these same categories remained stable or declined.
Conclusion
Over time, KS and NHL occurred at higher CD4 counts and lower HIV RNA values, and KS occurred more frequently after ART initiation. These changes were driven by an increasing proportion of patients with HIV who received effective ART, had higher CD4 counts, and had suppressed HIV RNA and not by increases in cancer risk within these subgroups. An improved understanding of HIV-associated cancer pathogenesis and outcomes in the context of successful ART is therefore important.
INTRODUCTION
People with HIV infection have a highly elevated risk of Kaposi's sarcoma (KS) and non-Hodgkin lymphoma (NHL)1 related to loss of immunologic control of human herpesvirus-8 and Epstein-Barr virus, respectively.2,3 With widespread use of effective antiretroviral therapy (ART), risk has declined dramatically in the HIV population.4,5 However, KS and NHL incidence remains much higher than in the general population,6 and elevations may persist after patients with HIV infection have achieved virologic suppression and immune recovery.7 These HIV-associated cancers also contribute substantially to the total US KS and NHL burden.8
Trends in earlier ART initiation and increased emphasis on linkage and retention into care have changed the characteristics of the HIV population. Among US patients with HIV in clinical care in 2008, 83% had been prescribed ART and 72% had suppressed levels of HIV RNA compared with 74% prescribed ART and 46% with suppressed HIV RNA in 2000.9 Given this shift, the fraction of KS and NHL cases occurring among patients with HIV administered effective ART may be increasing despite the benefits of ART.
Because viral suppression and immune recovery combat KS and NHL development, the biology of cancers that arise despite effective ART likely differ from those that develop in patients with HIV and uncontrolled viremia and severe immunosuppression. An understanding of the KS and NHL burden among people with HIV infection who have received effective ART is important because these cancers may require different therapeutic approaches. In this study, we characterize KS and NHL diagnoses relative to ART use, virologic suppression, and CD4 count in a large clinical HIV population.
PATIENTS AND METHODS
The study population included adults with HIV infection from eight US HIV clinical cohorts that comprise the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) and followed from 1996 to 2011.10 This calendar period spans from the beginning of widespread combination ART availability to the last year of systematic identification of cancer diagnoses in CNICS. CNICS collects data through electronic medical records, including information on demographics, ART use, diagnoses, CD4 count, and HIV RNA measurements. Cancer diagnoses have been verified through a standardized process.11 Each CNICS site obtained local institutional review board approval.
Demographic characteristics were described for patients with KS and NHL who received a diagnosis on or after a first visit at a CNICS site over three calendar periods (1996 to 2001, 2002 to 2006, and 2007 to 2011). Diagnoses were categorized as having occurred in or out of routine clinical care. Start of routine clinical care was defined as the date when the second of two consecutive HIV outpatient visits occurred ≥ 1 month apart and within 6 months. Routine clinical care was considered to continue as long as clinical visits were not > 12 months apart. If a patient went > 12 months without a visit, the case was recategorized as out of routine clinical care until the original two-visit criterion was met again. Trends in the proportion of KS and NHL cases diagnosed in routine care across calendar periods were evaluated by Cochran-Armitage test.
We restricted further analyses to time in routine clinical care because we could obtain reliable information on ART and laboratory measures during this time only. KS and NHL cases diagnosed during routine care were characterized based on ART use, immune status, and virologic status at the time of cancer diagnosis. We also specifically characterized cases of diffuse large B-cell lymphoma (DLBCL), the most common NHL subtype. ART initiation was defined as the first concurrent use of three or more different antiretroviral medications. ART status was categorized as no ART, ART initiation < 6 months prior (when immune reconstitution inflammatory syndrome is most likely),12 or ART initiation ≥ 6 months prior. Immune status at cancer diagnosis was based on the latest CD4 count within 6 months before cancer diagnosis. Virologic status was based on the latest HIV RNA value 3 to 9 months before cancer diagnosis. We assessed HIV RNA measurements 3 months earlier than CD4 counts because HIV RNA is highly variable over short periods, and values closer to cancer diagnosis may be affected by management changes during diagnostic evaluation for cancer. If CD4 count or HIV RNA values were unavailable in these windows, the cases were excluded from analyses of those characteristics (< 15% of cases were excluded for this reason).
We described the proportion of KS and NHL cases in each ART category as well as in CD4 count and HIV RNA categories across calendar periods. We evaluated changes in the proportion of cases in each category across periods by using χ2 as a global test for differences and the Cochran-Armitage test to evaluate trends for particular categories over time. Because CD4 count and HIV RNA can be evaluated as continuous variables, changes in the mean CD4 count and HIV RNA (log10-transformed) were also described nonparametrically by using LOWESS curves,13 and trends were evaluated with linear regression.
To evaluate whether changes in the characteristics of cancer cases resulted from changes in the underlying HIV population or changes in cancer risk, we divided person-time in routine clinical care into the aforementioned ART, CD4 count, and HIV RNA categories. Person-time was used to calculate KS and NHL incidence rates, and Poisson regression was used to evaluate time trends in incidence.
Standardized incidence ratios were calculated within categories that compared observed cancer counts with expected counts. Expected counts were calculated within strata of age, sex, and race by multiplying follow-up time observed in each stratum by stratified incidence rates in the general population obtained from SEER cancer registries.14 KS incidence rates were based on 1973 to 1979 SEER data because subsequent rates are heavily influenced by the HIV epidemic.8
RESULTS
Among 24,901 patients in CNICS during 1996 to 2011, there were 466 KS diagnoses and 258 NHL diagnoses, including 135 DLBCL diagnoses. Most cases were among white men (Table 1). The median age at cancer diagnosis increased across time from 37 years for KS and 40 years for NHL during 1996 to 2001 to 42 years for KS and 46 years for NHL during 2007 to 2011 (Table 1).
Table 1.
Patients With Kaposi's Sarcoma and Non-Hodgkin Lymphoma Diagnosed in the Centers for AIDS Research Network of Integrated Clinical Systems From 1996 to 2011
| Patient Characteristics at Cancer Diagnosis | Kaposi's Sarcoma, No. (%) | Non-Hodgkin Lymphoma, No. (%) | ||||
|---|---|---|---|---|---|---|
| 1996-2001 | 2002-2006 | 2007-2011 | 1996-2001 | 2002-2006 | 2007-2011 | |
| Total | 127 | 197 | 142 | 75 | 109 | 74 |
| Sex | ||||||
| Female | 4 (3) | 8 (4) | 4 (3) | 11 (15) | 12 (11) | 9 (12) |
| Male | 123 (97) | 189 (96) | 138 (97) | 64 (85) | 97 (89) | 65 (88) |
| Median age at diagnosis (IQR) | 37 (33-43) | 40 (36-44) | 42 (35-47) | 40 (37-46) | 42 (37-47) | 46 (38-50) |
| Race* | ||||||
| White | 89 (75) | 122 (65) | 95 (67) | 46 (67) | 56 (52) | 46 (62) |
| Black | 22 (19) | 50 (27) | 23 (16) | 21 (30) | 36 (34) | 18 (24) |
| Other | 8 (7) | 16 (9) | 24 (17) | 2 (3) | 15 (14) | 10 (14) |
| Diagnosis during routine clinical care† | ||||||
| Yes | 41 (32) | 85 (43) | 70 (49) | 42 (56) | 61 (56) | 47 (64) |
| No | 86 (68) | 112 (57) | 72 (51) | 33 (44) | 48 (44) | 27 (36) |
Abbreviation: IQR, interquartile range.
Seventeen patient cases of Kaposi's sarcoma and eight patient cases of non-Hodgkin lymphoma are missing information on race.
Routine clinical care was defined as starting when two HIV outpatient visits occurred that were ≥ 1 month and < 6 months apart and continuing for as long as HIV outpatient visits occurred at least every 12 months.
Among KS cases, 42% (n = 196) were diagnosed during routine clinical care, with the proportion increasing over time from 32% in 1996 to 2001 to 49% in 2007 to 2011 (P-trend = .005; Table 1). Among NHL cases, 58% (n = 150) were diagnosed during routine clinical care. This proportion did not change significantly across time (P-trend = .354). In the most recent calendar period, 64% of cases were diagnosed during routine care.
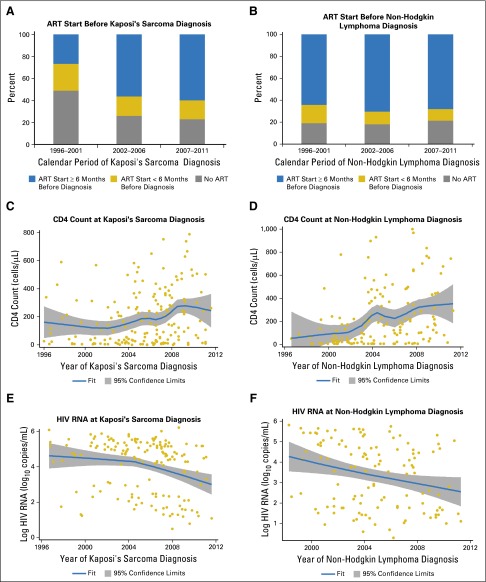
Among cases diagnosed during routine care, ART status at diagnosis changed over time for KS (P = .019) but not for NHL (P = .809). Although only 26% of KS diagnoses occurred ≥ 6 months after ART initiation during 1996 to 2001, most KS cases were diagnosed ≥ 6 months after ART initiation in the later periods, reaching 60% in 2007 to 2011 (P-trend = .008; Fig 1A). For NHL, 68% of all diagnoses occurred ≥ 6 months after ART initiation, and this proportion did not change noticeably over time (Fig 1B).
Fig 1.
Antiretroviral therapy (ART), immune, and virologic status of patients with HIV in routine clinical care who were diagnosed with Kaposi's sarcoma and non-Hodgkin lymphoma. (A) and (B) The proportion of Kaposi's sarcoma and non-Hodgkin lymphoma patient cases that occurred at various times relative to ART initiation over three calendar periods. (C) and (D) The distribution of CD4 counts at diagnosis of Kaposi's sarcoma and non-Hodgkin lymphoma across time, including a LOWESS curve to illustrate the smoothed trend over time. (E) and (F) The distribution of HIV RNA values on the log10 scale at diagnosis of Kaposi's sarcoma and non-Hodgkin lymphoma across time, including a LOWESS curve. ART start was defined as the first date at which there was concurrent use of at least three different antiretroviral medications. CD4 counts at cancer diagnosis were defined as the most recent values within 6 months before cancer diagnosis. HIV RNA measurements at cancer diagnosis were defined as the most recent values 3 to 9 months before cancer diagnosis. HIV RNA values below the detection limit were randomly assigned a value between the detection limit and zero. Although cancers were ascertained starting in 1996, the first patient with non-Hodgkin lymphoma in the Centers for AIDS Research Network of Integrated Clinical Systems with an HIV RNA measurement 3 to 9 months before diagnosis was observed in 1998.
The mean CD4 count at diagnosis increased with calendar time for both KS (P = .001) and NHL (P < .001). For KS, this increase was most notable during 2003 to 2008, whereas a general increasing trend was observed over the entire period for NHL (Figs 1C and 1D). During 2007 to 2011, 15% of KS cases and 24% of NHL cases were diagnosed at CD4 counts ≥ 500 cells/μL, whereas fewer than one half were diagnosed at CD4 counts < 200 cells/μL.
The mean HIV RNA value (3 to 9 months before cancer diagnosis) decreased over time for both KS and NHL (Figs 1E and 1F), but the decrease was significant only for NHL (P = .014 for NHL; P = .087 for KS). During 2007 to 2011, 29% of KS cases and 51% of NHL cases were diagnosed when HIV RNA was suppressed (< 500 copies/mL).
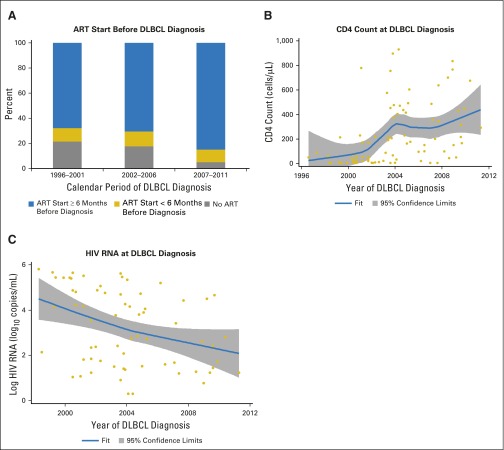
For the 82 DLBCL cases diagnosed during care, patterns were similar to those for NHL overall (Appendix Fig A1, online only). The proportion of diagnoses occurring ≥ 6 months after ART initiation did not change significantly over time (P = .616), but DBLCL diagnoses were made at higher CD4 counts and lower HIV RNA levels in more recent calendar years (P-trend < .001 for CD4 count; P-trend = .018 for HIV RNA; Appendix Fig A1). During 2007 to 2011, 85% of DLBCL diagnoses were made ≥ 6 months after ART initiation, 26% were diagnosed at CD4 counts ≥ 500 cells/μL, and 67% were diagnosed when HIV RNA was suppressed.
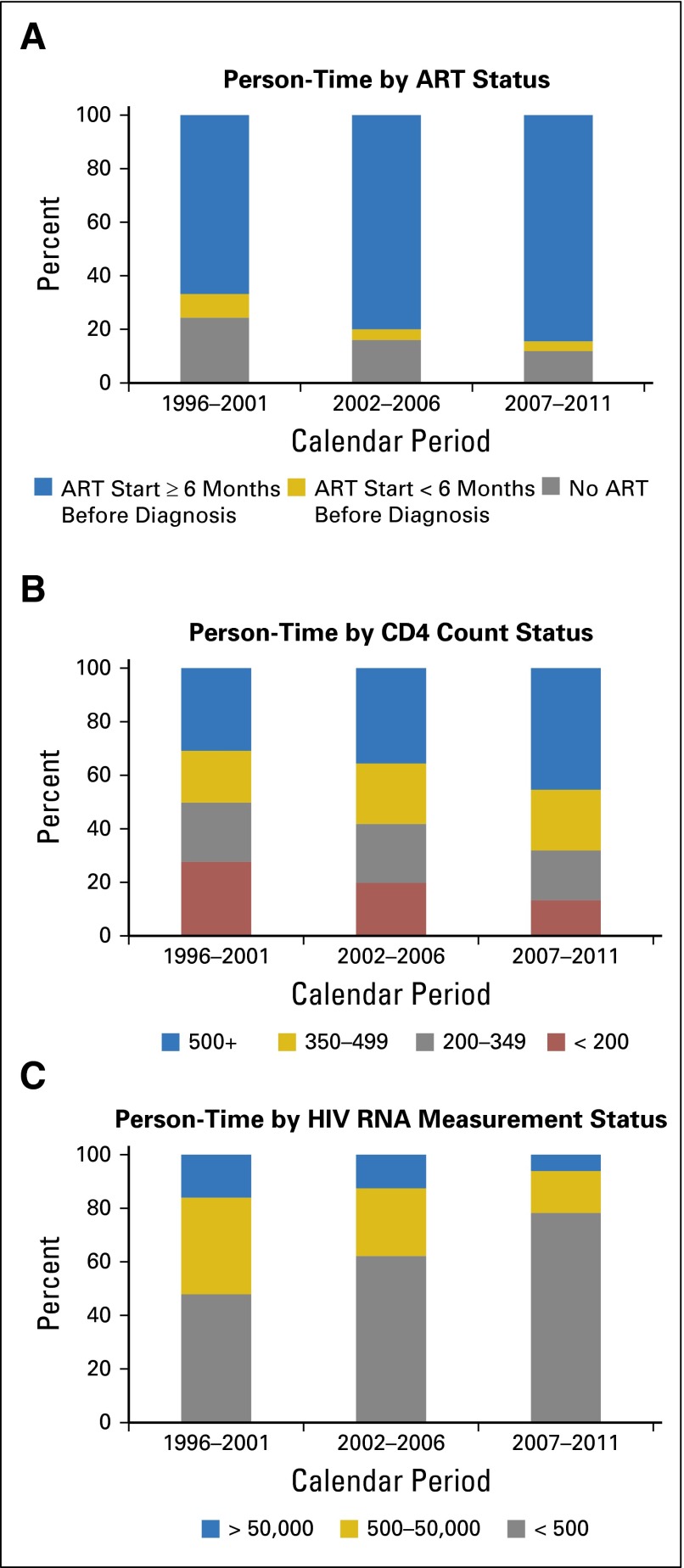
We also observed calendar trends when examining the underlying person-time of patients followed during routine care. Across time, more person-time was contributed when patients had received ART for ≥ 6 months and less by patients who had not initiated ART (Fig 2A). In more recent calendar years, more person-time was contributed by patients with higher CD4 counts and lower HIV RNA values (Figs 2B and 2C). During 2007 to 2011, 45% of person-time was contributed by patients with CD4 counts > 500 cell/μL compared with 30% in 1996 to 2001. Similarly, 76% of person-time was contributed by patients with suppressed HIV RNA in 2007 to 2011 compared with 45% in 1996 to 2001.
Fig 2.
Proportion of person-time contributed to each antiretroviral therapy (ART), CD4 count, and HIV RNA category by calendar period among patients with HIV in routine clinical care. Person-time categories are based on follow-up of the entire Centers for AIDS Research Network of Integrated Clinical Systems HIV population in routine clinical care and were time updated to account for changes in (A) ART status, (B) CD4 count, and (C) HIV RNA values.
Overall, KS and NHL incidence rates decreased 5% and 8% per year, respectively, during 1996 to 2011 (incidence rate ratios, 0.95 and 0.92 per year, respectively; Table 2). KS incidence decreased across time among patients who had not received ART and increased among those with HIV RNA values between 500 and 50,000 copies/mL. KS incidence did not significantly change for any other ART, CD4 count, or HIV RNA category, although a borderline decrease was observed among patients with HIV RNA < 500 copies/mL (Table 2). NHL incidence decreased among patients who received ART for > 6 months, among those with CD4 counts < 200 cells/μL, and among those with HIV RNA values < 500 copies/mL (Table 2). No changes in NHL incidence over time were observed for other ART, CD4 count, or HIV RNA categories.
Table 2.
Incidence Rates of Kaposi's Sarcoma and Non-Hodgkin Lymphoma Among Patients With HIV in Routine Clinical Care
| Time-Updated Patient Characteristics | Incidence Rate (per 100,000 person-years) by Calendar Period | IRR (per increasing calendar year) | P-Trend | ||
|---|---|---|---|---|---|
| 1996-2001 | 2002-2006 | 2007-2011 | |||
| Kaposi's sarcoma | |||||
| Total | 243.7 | 232.2 | 168.1 | 0.95 | .003 |
| ART status | |||||
| No ART | 501.5 | 373.8 | 323.4 | 0.94 | .043 |
| ART < 6 months | 733.8 | 1,040.1 | 795.6 | 1.00 | .999 |
| ART ≥ 6 months | 88.3 | 164.0 | 119.4 | 1.01 | .988 |
| CD4 count (cells/μL) | |||||
| < 200 | 674.2 | 816.7 | 609.7 | 0.98 | .540 |
| 200-349 | 129.2 | 234.2 | 255.6 | 1.03 | .565 |
| 350-499 | 36.7 | 114.0 | 136.7 | 1.05 | .461 |
| ≥ 500 | 46.1 | 36.2 | 61.5 | 1.05 | .528 |
| HIV RNA (copies/mL) | |||||
| > 50,000 | 729.0 | 1,088.9 | 967.8 | 1.01 | .660 |
| 500-50,000 | 141.5 | 166.6 | 356.6 | 1.10 | .037 |
| < 500 | 152.3 | 104.3 | 85.7 | 0.93 | .051 |
| Non-Hodgkin lymphoma | |||||
| Total | 249.6 | 166.6 | 112.9 | 0.92 | < .001 |
| ART status | |||||
| No ART | 200.6 | 186.9 | 202.1 | 0.99 | .902 |
| ART < 6 months | 467.0 | 485.4 | 331.5 | 0.95 | .385 |
| ART ≥ 6 months | 238.4 | 146.9 | 91.0 | 0.90 | < .001 |
| CD4 count (cells/μL) | |||||
| < 200 | 855.7 | 539.0 | 336.4 | 0.93 | .012 |
| 200-349 | 96.9 | 87.8 | 180.4 | 1.04 | .537 |
| 350-499 | 36.7 | 156.7 | 85.9 | 0.98 | .757 |
| ≥ 500 | 23.1 | 63.4 | 67.7 | 1.03 | .644 |
| HIV RNA (copies/mL) | |||||
| > 50,000 | 729.0 | 518.5 | 414.8 | 0.94 | .165 |
| 500-50,000 | 101.1 | 230.6 | 231.8 | 1.05 | .335 |
| < 500 | 228.4 | 83.4 | 78.5 | 0.90 | .004 |
Abbreviations: ART, antiretroviral therapy; IRR, incidence rate ratio.
Across all calendar periods, KS incidence in every ART, CD4 count, and HIV RNA category was higher than general population rates (Table 3). Compared with the general US population, KS incidence in 2007 to 2011 was 710 times higher among those who received ART for ≥ 6 months, 430 times higher among those with CD4 counts ≥ 500 cells/μL, and 430 times higher among those with HIV RNA < 500 copies/mL. NHL incidence was also higher than general population rates in almost every category across all periods. Compared with the general population, NHL incidence in 2007 to 2011 was 3.4 times higher among those who received ART for ≥ 6 months, 3.1 times higher among those with CD4 counts ≥ 500 cells/μL, and 2.9 times higher among those with HIV RNA < 500 copies/mL.
Table 3.
Standardized Incidence Ratios for Kaposi's Sarcoma and Non-Hodgkin Lymphoma Among White and Black Patients With HIV in Routine Clinical Care
| Time-Updated Patient Characteristics | Standardized Incidence Ratio (95% CI) | ||
|---|---|---|---|
| 1996-2001 | 2002-2006 | 2007-2011 | |
| Kaposi's sarcoma | |||
| ART status | |||
| No ART | 5,000 (3,100 to 7,600) | 3,200 (2,000 to 4,700) | 3,100 (1,900 to 4,700) |
| ART < 6 months | 9,200 (5,200 to 15,000) | 12,000 (7,200 to 18,000) | 6,200 (3,200 to 11,000) |
| ART ≥ 6 months | 740 (370 to 1,300) | 1,200 (900 to 1,500) | 710 (530 to 930) |
| CD4 count (cells/μL) | |||
| < 200 | 5,900 (4,000 to 8,600) | 6,400 (4,900 to 8,200) | 3,900 (2,700 to 5,400) |
| 200-349 | 1,300 (450 to 3,000) | 1,600 (970 to 2,500) | 1,600 (980 to 2,400) |
| 350-499 | 400 (21 to 1,900) | 950 (470 to 1,700) | 660 (330 to 1,200) |
| ≥ 500 | 550 (97 to 1,700) | 310 (110 to 710) | 430 (230 to 720) |
| HIV RNA (copies/mL) | |||
| > 50,000 | 7,500 (4,400 to 12,000) | 10,000 (8,100 to 14,000) | 9,200 (6,200 to 13,000) |
| 500-50,000 | 1,300 (490 to 2,600) | 1,500 (820 to 2,400) | 3,000 (1,900 to 4,400) |
| < 500 | 1,500 (800 to 2,500) | 700 (450 to 1,000) | 430 (280 to 630) |
| Non-Hodgkin lymphoma | |||
| ART status | |||
| No ART | 6.7 (2.3 to 15) | 11 (5.9 to 18) | 8.8 (4.1 to 17) |
| ART < 6 months | 22 (8.5 to 45) | 18 (6.1 to 41) | 17 (5.8 to 39) |
| ART ≥ 6 months | 12 (8.0 to 17) | 7.0 (5.3 to 9.0) | 3.4 (2.4 to 4.6) |
| CD4 count (cells/μL) | |||
| < 200 | 39 (27 to 54) | 24 (17 to 33) | 10 (5.5 to 17) |
| 200-349 | 5.5 (1.5 to 14) | 4.3 (1.9 to 8.5) | 6.8 (3.7 to 12) |
| 350-499 | 2.3 (0.12 to 11) | 8.1 (4.5 to 13) | 3.4 (1.5 to 6.7) |
| ≥ 500 | 0 (0 to 4.3) | 3.2 (1.5 to 6.1) | 3.1 (1.7 to 5.1) |
| HIV RNA (copies/mL) | |||
| > 50,000 | 35 (20 to 56) | 27 (18 to 40) | 19 (9.0 to 36) |
| 500-50,000 | 6.5 (2.6 to 14) | 12 (7.5 to 18) | 10 (5.6 to 17) |
| < 500 | 5.8 (3.3 to 9.7) | 3.7 (2.3 to 5.6) | 2.9 (1.9 to 4.3) |
Abbreviation: ART, antiretroviral therapy.
DISCUSSION
Among US patients with HIV infection, the clinical context in which KS and NHL occur has changed considerably during the effective ART era. Since 1996, more cases have been diagnosed after entry into routine clinical care, with 64% of NHL cases and 49% of KS cases diagnosed during routine care in 2007 to 2011. As anticipated, the overall incidence of KS and NHL decreased over calendar time, likely due to increasing use and effectiveness of ART. However, more cases were diagnosed among patients who either received ART, had high CD4 counts, or had suppressed HIV RNA. In fact, in 2007 to 2011, most cases occurred ≥ 6 months after ART initiation and in patients with CD4 counts ≥ 200 cell/μL. For these cancers, new clinical approaches may be needed because the clinical presentation, treatment response, and prognosis likely differ from KS and NHL diagnosed in the setting of severe immunosuppression.
The growing fraction of cancers diagnosed during routine clinical care likely reflects the larger proportion of the HIV population engaged in care in more recent times. For example, among US individuals with HIV infection entering care, an estimated 82% were retained in routine care in 2008 compared with 61% in 2000.15 Engagement in routine care could reduce cancer risk by encouraging ART initiation and adherence, and one might anticipate that engagement in care would facilitate detection of cancers at an early stage, which would lead to a facilitation of treatment. Because our population was defined by attendance at an HIV clinic, we could not obtain information on cancers among people who never visit an HIV clinic. Thus, we likely underestimated the total proportion of cancers in patients outside routine care and could not calculate accurate incidence rates in these patients.
For KS and NHL diagnosed during routine care, several patient characteristics changed over calendar time that reflect changes in treatment of the underlying HIV population. The proportion of KS cases diagnosed among patients who received ART increased not because KS incidence increased in patients who received ART but because of the growing fraction of the HIV population administered ART. Similar patterns were seen for KS and NHL with respect to HIV RNA and CD4 count (ie, a larger proportion of cases among patients with suppressed HIV RNA and high CD4 counts), even as KS and NHL incidence declined or remained constant in these strata. Again, these trends resulted from the consistently increasing fraction of the HIV population who live with virologic control and immune competence. Although we evaluated trends in ART use, HIV RNA, and CD4 count separately, these clinical characteristics and the changes in the underlying HIV population are inherently related. Improvements in ART access and earlier initiation lead to earlier suppression of HIV RNA and, ultimately, higher CD4 counts.
Other changes in the HIV population have influenced the clinical characteristics of cancer cases. For instance, cancers in the HIV population are typically diagnosed at younger ages than in the general population, which reflects the younger age distribution of the HIV population.16 Nonetheless, both KS and NHL arose at older ages in more recent calendar years in the current study. As the HIV population continues to age, the age distribution of cancer cases will likely continue to shift to older ages. Furthermore, one would expect to see more cancers etiologically related to aging.
Changing clinical characteristics of these cancers may also reflect changing biology. For example, cancers that develop in patients with HIV infection after immune recovery may manifest genetic or epigenetic changes that facilitate evasion from the immune system, whereas cancers that arise in more immunosuppressed populations may not face the same degree of immune selection and may not manifest these changes.17 Given that human herpesvirus-8 and Epstein-Barr virus are genetically heterogeneous,18,19 another possibility is that patients in whom KS or NHL develop after immune reconstitution may be infected with more pathogenic strains. For NHL, the distribution of subtypes has changed over time, with DLBCL and lymphoma of the CNS making up a smaller proportion of diagnoses in more recent years, whereas Burkitt lymphoma and other lymphoma subtypes now make up a larger proportion of diagnoses.20 However, when we limited analyses to one subtype, DLBCL, we still observed changes in the immunologic and virologic context of the diagnoses over time. As the HIV population ages, KS and NHL cases may resemble cases seen in elderly populations.21 Among patients administered ART, the presence of circulating T cells with immunosenescent phenotypes is associated with a KS diagnosis, which indicates that cancer-promoting mechanisms related to aging may be important.22
In this study, even patients with HIV who received ART, had high CD4 counts, or had suppressed HIV RNA had higher KS and NHL incidence than the general population. This could be due to lingering weaknesses in the immune system. For instance, although ART initiation corresponds with gradual increases in immune responses to human herpesvirus-8,23-25 these responses might not be as robust as in individuals with no history of immunosuppression. HIV could also have persistent direct effects on KS and NHL risk because the HIV latent reservoir is not eliminated with ART use. HIV viremia after ART initiation predicts NHL risk independently of CD4 count,26 and recent studies provide evidence that HIV proteins may directly encourage B-cell clonogenicity.27
Changes in the clinical context of these cancers may have important implications for treatment and prognosis. For KS and NHL cases diagnosed in the setting of uncontrolled HIV, initiation of ART is a major component of cancer treatment.2 For KS, ART alone can lead to significant regression, presumably due to improved immune function and control of human herpesvirus-8.2 However, as the current study shows, KS and NHL develop in many patients despite ART, and intensification or other ART changes may not be beneficial.28,29 For NHL, how the immunologic and virologic context at diagnosis influences tumor biology and disease aggressiveness currently is unclear. A previous CNICS study found that patients with NHL diagnosed while receiving ART had worse survival than those with NHL diagnosed before ART initiation.20 However, for HIV-associated NHL, and specifically DLBCL, patients with higher CD4 counts have been observed to have better responses and improved survival.30,31 Further study could help to determine whether associations reflect differences in patterns of care or whether consistent predictors can be identified that correspond to underlying biologic differences.
This study has limitations. Limited information was available for cancers diagnosed outside routine clinical care; thus, the study focused mostly on cancers diagnosed during routine care. In addition, although the study population was large, we lacked enough cases to cross-classify them according to ART, HIV RNA measurement, and CD4 count. Furthermore, the CNICS sites are geographically diverse but are all based in large US academic medical centers, which may differ from other HIV care settings.32
This study also has important strengths. We assessed cancer diagnoses across a large multisite cohort that has systematically yielded important clinical characteristics, including ART use, CD4 counts, and HIV RNA measurements. Cancer diagnoses in CNICS were verified through a standardized and comprehensive process. Furthermore, because of the long follow-up in CNICS, we could characterize cancer diagnoses over an extended span of calendar time.
In conclusion, KS and NHL presented a continued burden in the HIV population in the modern ART era, but presenting characteristics for these malignancies are changing. In the United States, more cases are diagnosed while patients are in routine care, receive ART, have a relatively intact immune system, and have suppressed HIV replication. These changes largely reflect the dramatic changes over time in treatment of the underlying HIV population. In the future, more studies are needed to understand whether KS and NHL cases that arise during ART use differ biologically from cases that arise during severe immunosuppression and uncontrolled HIV replication. If biologic differences are demonstrated, they may suggest different approaches to effectively prevent and treat these cancers in the modern ART era.
Acknowledgment
We thank the patients, principal investigators, co-investigators, and research staff at participating Centers for AIDS Research Network of Integrated Clinical Systems sites at the following institutions: Case Western Reserve University; University of Alabama at Birmingham; University of California, San Francisco; University of Washington; University of California, San Diego; Fenway Community Health Center of Harvard University; University of North Carolina at Chapel Hill; and Johns Hopkins University. In particular, we acknowledge Benigno Rodriguez at Case Western Reserve University and Elvin Geng at the University of California, San Francisco, for assistance.
Appendix
Fig A1.
Antiretroviral therapy (ART), immune, and virologic status of patients with HIV in routine clinical care who were diagnosed with diffuse large B-cell lymphoma (DLBCL). (A) The proportion of DLBCL patient cases that occurred at various times relative to ART initiation over three calendar periods. (B) The distribution of CD4 counts at diagnosis of DLBCL across time, including a LOWESS curve to illustrate the smoothed trend over time. (C) The distribution of HIV RNA values on the log10 scale at diagnosis of DLBCL across time, including a LOWESS curve. ART start was defined as the first date at which there was concurrent use of at least three different antiretroviral medications. CD4 counts at cancer diagnosis were defined as the most recent values within 6 months before cancer diagnosis. HIV RNA measurements at cancer diagnosis were defined as the most recent values 3 to 9 months before cancer diagnosis. HIV RNA values below the detection limit were randomly assigned a value between the detection limit and zero. Although cancers were ascertained starting in 1996, the first patient with DLBCL in the Centers for AIDS Research Network of Integrated Clinical System with an HIV RNA measurement 3 to 9 months before diagnosis was observed in 1998.
Footnotes
Listen to the podcast by Dr Little at www.jco.org/podcasts
Supported by the Intramural Research Program of the National Cancer Institute (E.L.Y., A.E.C., and E.A.E.), an Intramural AIDS Research Fellowship Award from the Office of AIDS Research at the National Institutes of Health (E.L.Y.), and National Institutes of Health Grant No. R24 AI067039 to the Centers for AIDS Research Network of Integrated Clinical Systems.
Presented at the International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, MD, October 26 and 27, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth L. Yanik, Chad J. Achenbach, Satish Gopal, Stephen R. Cole, Eric A. Engels
Collection and assembly of data: Elizabeth L. Yanik, Chad J. Achenbach, Joseph J. Eron, Richard D. Moore, W. Christopher Mathews
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Changes in Clinical Context for Kaposi's Sarcoma and Non-Hodgkin Lymphoma Among People With HIV Infection in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Elizabeth L. Yanik
No relationship to disclose
Chad J. Achenbach
Consulting or Advisory Role: ABIVAX
Research Funding: Gilead Sciences
Satish Gopal
No relationship to disclose
Anna E. Coghill
No relationship to disclose
Stephen R. Cole
No relationship to disclose
Joseph J. Eron
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Gilead Sciences, Janssen, AbbVie, ViiV Healthcare
Research Funding: Bristol-Myers Squibb, Gilead Sciences, AbbVie, ViiV Healthcare
Richard D. Moore
No relationship to disclose
W. Christopher Mathews
No relationship to disclose
Daniel R. Drozd
No relationship to disclose
Ayad Hamdan
Speakers’ Bureau: Mallinckrodt
Mary E. Ballestas
No relationship to disclose
Eric A. Engels
No relationship to disclose
REFERENCES
- 1.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 2.Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma. Cancer Lett. 2011;305:150–162. doi: 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: A review of the epidemiological evidence. J Intern Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 4.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 2011;305:1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–335. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: The Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleveland WS, Devlin SJ. Locally-weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 14. National Cancer Institute: SEER-Medicare Linked Database, 2014. http://appliedresearch.cancer.gov/seermedicare.
- 15.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62:356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153:452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 18.Olp LN, Jeanniard A, Marimo C, et al. Whole-genome sequencing of Kaposi’s sarcoma-associated herpesvirus from Zambian Kaposi’s sarcoma biopsy specimens reveals unique viral diversity. J Virol. 2015;89:12299–12308. doi: 10.1128/JVI.01712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingma DW, Weiss WB, Jaffe ES, et al. Epstein-Barr virus latent membrane protein-1 oncogene deletions: Correlations with malignancy in Epstein-Barr virus-associated lymphoproliferative disorders and malignant lymphomas. Blood. 1996;88:242–251. [PubMed] [Google Scholar]
- 20.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105:1221–1229. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yanik EL, Katki HA, Engels EA: Cancer risk among the HIV-infected elderly in the United States. AIDS 30:1663-1668, 2016. [DOI] [PMC free article] [PubMed]
- 22.Unemori P, Leslie KS, Hunt PW, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013;27:1735–1742. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan SG, Hirsch HH, Franceschi S, et al. Kaposi sarcoma herpes virus antibody response and viremia following highly active antiretroviral therapy in the Swiss HIV Cohort study. AIDS. 2010;24:2245–2252. doi: 10.1097/QAD.0b013e32833b7830. [DOI] [PubMed] [Google Scholar]
- 24.Bihl F, Mosam A, Henry LN, et al. Kaposi’s sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi’s sarcoma. AIDS. 2007;21:1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- 25.Bourboulia D, Aldam D, Lagos D, et al. Short- and long-term effects of highly active antiretroviral therapy on Kaposi sarcoma-associated herpesvirus immune responses and viraemia. AIDS. 2004;18:485–493. doi: 10.1097/00002030-200402200-00015. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach CJ, Buchanan AL, Cole SR, et al. HIV viremia and incidence of non-Hodgkin lymphoma in patients successfully treated with antiretroviral therapy. Clin Infect Dis. 2014;58:1599–1606. doi: 10.1093/cid/ciu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolcetti R, Gloghini A, Caruso A, et al. A lymphomagenic role for HIV beyond immune suppression. Blood. 2016;127:1403–1409. doi: 10.1182/blood-2015-11-681411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedland GH, Naidoo P, Abdool-Gafoor B, et al. Case records of the Massachusetts General Hospital. Case 29-2013. A 32-year-old HIV-positive African man with dyspnea and skin lesions. N Engl J Med. 2013;369:1152–1161. doi: 10.1056/NEJMcpc1305985. [DOI] [PubMed] [Google Scholar]
- 29.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357:1352–1353. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 30.Barta SK, Lee JY, Kaplan LD, et al. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2012;118:3977–3983. doi: 10.1002/cncr.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesko CR, Cole SR, Hall HI, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11. Int J Epidemiol. 2016;45:140–150. doi: 10.1093/ije/dyv352. [DOI] [PMC free article] [PubMed] [Google Scholar]