Abstract
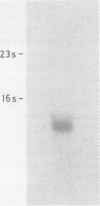
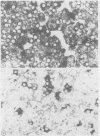
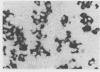
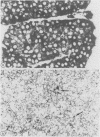
A synthetic oligodeoxynucleotide sequence complementary to the mRNA for the adrenocorticotrophin (ACTH) precursor pro-opiomelanocortin (POMC) was end labelled using digoxigenin. The probe was used to detect POMC mRNA both on nitrocellulose filters and by non-isotopic in situ hybridisation (NISH) in tissue sections. Digoxigenin was identified using anti-digoxigenin alkaline phosphatase. The model system examined was the rat pituitary gland. Removal of both adrenal glands and dexamethasone administration were used to change the concentrations of POMC mRNA in the rat anterior lobe. The labelled probe reacted with a single band of appropriate molecular weight in Northern blot analysis. The distribution of signal in tissue sections and the changes induced by experimental manipulation were as predicted. The results indicate that this method of NISH will prove useful in the detection of specific messenger RNAs in tissue sections of buffered, formalin fixed, paraffin wax embedded material.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch B., Popovici T., Levin M. J., Tuil D., Kahn A. Transferrin gene expression visualized in oligodendrocytes of the rat brain by using in situ hybridization and immunohistochemistry. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6706–6710. doi: 10.1073/pnas.82.19.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Burns J., Graham A. K., Frank C., Fleming K. A., Evans M. F., McGee J. O. Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol. 1987 Aug;40(8):858–864. doi: 10.1136/jcp.40.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissues from different species. J Biol Chem. 1982 Jun 25;257(12):6783–6787. [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Dooley S., Radtke J., Blin N., Unteregger G. Rapid detection of DNA-binding factors using protein-blotting and digoxigenin-dUTP marked probes. Nucleic Acids Res. 1988 Dec 23;16(24):11839–11839. doi: 10.1093/nar/16.24.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwine J. H., Roberts J. L. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem. 1984 Feb 25;259(4):2166–2170. [PubMed] [Google Scholar]
- Enders G., Knotek F. Comparison of the performance and reproducibility of various serological methods and diagnostic kits for the detection of rubella antibodies. J Virol Methods. 1985 May;11(1):1–14. doi: 10.1016/0166-0934(85)90119-3. [DOI] [PubMed] [Google Scholar]
- Guitteny A. F., Fouque B., Mougin C., Teoule R., Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem. 1988 Jun;36(6):563–571. doi: 10.1177/36.6.3259249. [DOI] [PubMed] [Google Scholar]
- Herrington C. S., Burns J., Graham A. K., Bhatt B., McGee J. O. Interphase cytogenetics using biotin and digoxigenin labelled probes II: Simultaneous differential detection of human and papilloma virus nucleic acids in individual nuclei. J Clin Pathol. 1989 Jun;42(6):601–606. doi: 10.1136/jcp.42.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Christensen T., Dalbøge H. Detection of proopiomelanocortin mRNA by in situ hybridization, using a biotinylated oligodeoxynucleotide probe and avidin-alkaline phosphatase histochemistry. Histochemistry. 1988;89(2):109–116. doi: 10.1007/BF00489913. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Sherman T. G., Burke S., Akil H., Davis L. G., Arentzen R., Watson S. J. Detection of proopiomelanocortin mRNA by in situ hybridization with an oligonucleotide probe. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5419–5423. doi: 10.1073/pnas.83.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Landefeld T. D. Detection of prolactin messenger RNA in rat anterior pituitary by in situ hybridization. Am J Pathol. 1986 Oct;125(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- Pringle J. H., Homer C. E., Warford A., Kendall C. H., Lauder I. In situ hybridization: alkaline phosphatase visualization of biotinylated probes in cryostat and paraffin sections. Histochem J. 1987 Sep;19(9):488–496. doi: 10.1007/BF01675419. [DOI] [PubMed] [Google Scholar]
- Tilders F. J., Smelik P. G. Direct neural control of MSH secretion in mammals: the involvement of dopaminergic tubero-hypophysial neurones. Front Horm Res. 1977;4:80–93. doi: 10.1159/000400353. [DOI] [PubMed] [Google Scholar]