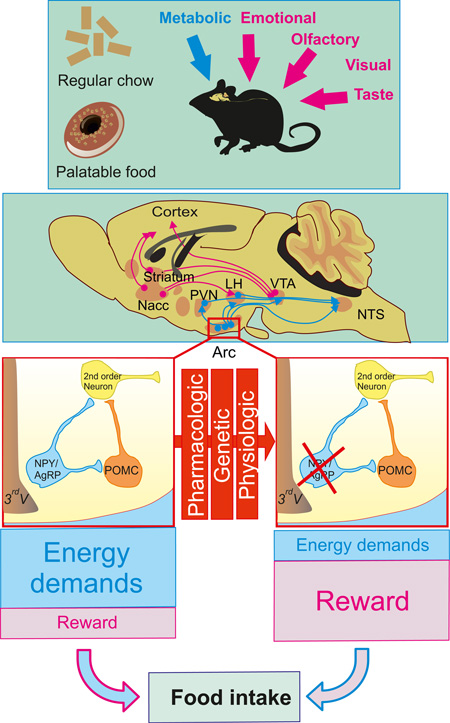
SUMMARY
Feeding behavior is exquisitely regulated by homeostatic and hedonic neural substrates that integrate energy demand as well as the reinforcing and rewarding aspects of food. Understanding the net contribution of homeostatic and reward-driven feeding has become critical due to the ubiquitous source of energy-dense foods and the consequent obesity epidemic. Hypothalamic, agouti-related peptide-secreting neurons (AgRP neurons) provide primary orexigenic drive of homeostatic feeding. Using models of neuronal inhibition or ablation we demonstrate that the feeding response to a fast, ghrelin or serotonin receptor agonist relies on AgRP neurons; however, when palatable food is provided, AgRP neurons are dispensable for an appropriate feeding response. In addition, AgRP-ablated mice present exacerbated stress-induced anorexia and palatable food intake—a hallmark of comfort feeding. These results suggest that when AgRP neuron activity is impaired, neural circuits sensitive to emotion and stress are engaged and modulated by food palatability and dopamine signaling.
Graphical Abstract
INTRODUCTION
The last several decades have witnessed a pandemic expansion of pathologies related to high-fat and high-carbohydrate diets including obesity, diabetes, dyslipidemia and cardiovascular diseases—collectively referred to as metabolic syndrome (Eckel et al., 2005; Reaven, 2006). Globally, there has been both an increased intake of energy-dense foods that are high in fat, sugar and salt, and a concomitant decrease in physical activity (Eckel et al., 2005; Reaven, 2006). Despite the increasing prevalence and burden of metabolic syndrome, effective and safe treatments to reverse the epidemic remain limited.
Appropriate energy homeostasis results from the exquisite balance between energy intake and energy expenditure. Several determinants of feeding behavior have been extensively studied and encompass homeostatic regulation of nutrient intake, generally attributed to a hypothalamic-brainstem circuitry (Saper et al., 2002). Non-homeostatic feeding is, in part, a consequence of the potent reinforcing and motivational properties of food, closely tied to the release of dopamine in limbic brain regions, which is particularly stimulated by high-fat and high-sugar (HFHS) foods (Di Chiara and Imperato, 1988; Dallman et al., 2005; Narayanan et al., 2010). Although the presence of high-fat, nutrient-rich food is essentially ubiquitous in developed countries, not all individuals with access to these foods consume them in excess or become obese. It is thus likely that certain individuals become more vulnerable to the hedonic or reinforcing effects of calorie-rich food, and ultimately form eating habits that become disassociated from the homeostatic mechanisms that normally maintain energy balance (Berthoud, 2004). Understanding the functional connection between reward-driven nutrient intake and homeostatic control of energy balance is a key step toward a clinical breakthrough in the treatment of hyperphagia and obesity.
The arcuate nucleus (ARC) of the hypothalamus is regarded as a primary location for the integration of circulating signals of hunger and satiety. “First order neurons” located in the ARC are the first to respond to the circulating signals of hunger and satiety. Neurons that make pro-opiomelanocortin (POMC) produce melanocortin peptides including α–melanocyte-stimulating hormones (α-MSH). Binding of α-MSH to G protein-coupled melanocortin receptors 3 & 4 (MCR3 & MCR4) leads to decreased food intake and increased energy expenditure. Conversely, energy deprivation triggers increased activity of the neighboring neurons that make neuropeptide Y (NPY), agouti-related peptide (AgRP) and γ-aminobutyric acid (GABA) (AgRP neurons) resulting in increased inhibitory tone onto POMC neurons and competitive inhibition of melanocortin signaling at post-synaptic neurons located in many brain regions (Morton et al., 2006). Serotonin (5-hydroxytryptamine, 5-HT) is known to have anorexic properties and serotonergic drugs have been widely used as anti-obesity treatments (Lam and Heisler, 2007). Serotonergic innervation of ARC neurons mediates the satiety action of serotonin through the concerted activation of POMC neurons and inhibition of AgRP neurons (Heisler et al., 2006). Administration of ghrelin, a stomach-derived 28 amino-acid peptide, in both humans and rodents was shown to increase food intake through the binding to the growth hormone secretagogue receptor 1 (GHS-R) (Kojima et al., 1999). We and others have shown that AgRP neuron integrity is crucial for the ghrelin-induced feeding response (Chen et al., 2004; Luquet et al., 2007).
Receptors for 5-HT, leptin, or ghrelin are also found in extra-arcuate (ARC) targets including pontine, brainstem and midbrain structures. Although the direct access of circulating hormones such as ghrelin or leptin to these structures lying away from blood brain barrier (BBB) entry point is still a matter of debate, injection of these hormones in the ventral tegmental area (VTA) or parabrachial nucleus (PBN) can inhibit feeding (Abizaid et al., 2006; Figlewicz et al., 2003; Fulton et al., 2006; Hommel et al., 2006; Jerlhag et al., 2007; Lee et al., 1998; Naleid et al., 2005 ; Quarta et al., 2009; Zigman et al., 2006). These observations raise the question of the specific contribution of ARC versus extra-ARC neurons in the balance between reward-driven or energy-driven nutrient intake. This question becomes crucial in when sugar- and fat-rich diets are readily available and may contribute to addictive-like consummatory behavior (DiLeone et al., 2012).
Mouse models with transient or chronic loss of AgRP neurons’ activity provide an ideal tool to dissect the role of homeostatic versus non-homeostatic regulation of energy intake. Taking advantage of these models, we demonstrate that feeding behavior on normal chow diet relies on AgRP neurons; however, when a high-palatability diet is substituted, the AgRP neurons are no longer necessary. We also show that AgRP-ablated mice consume excess palatable food and are hypersensitive to stress-induced anorexia. We propose that activation of hedonic circuitry overrides the homeostatic circuitry in the chronic inhibition of AgRP neurons.
RESULTS
AgRP-ablated mice have impaired hunger-associated feeding responses on a standard diet
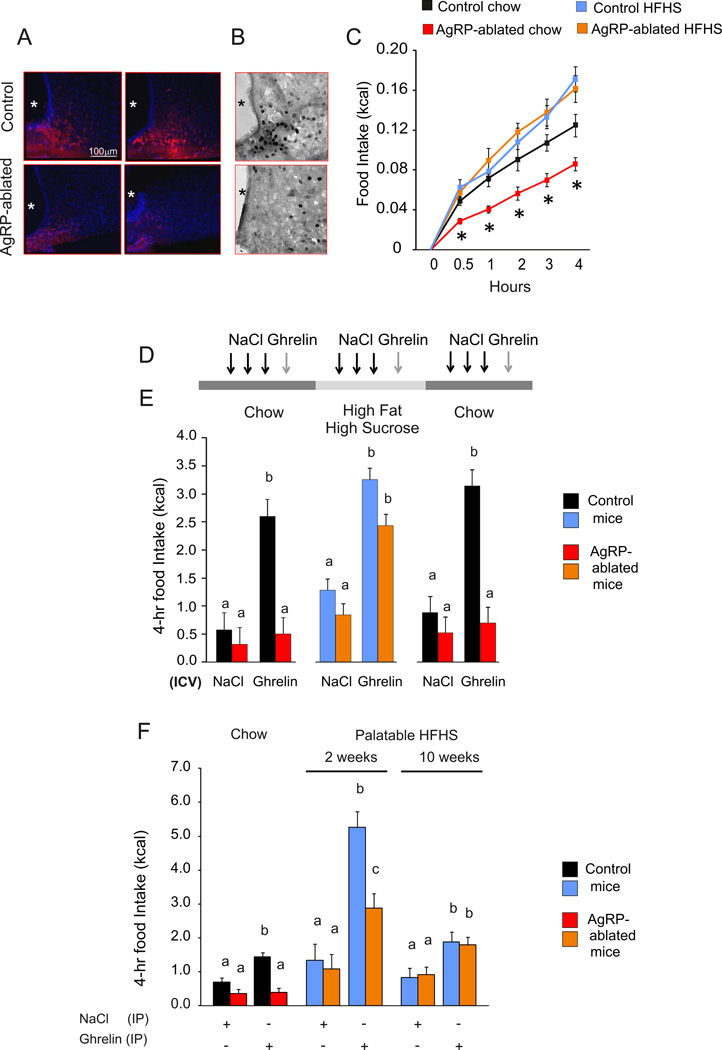
Activation of hypothalamic AgRP neurons promotes robust feeding (Aponte et al., 2011; Krashes et al., 2011). To decipher the contribution of AgRP neurons in hedonic versus non-hedonic regulation of feeding we used a model in which AgRP neurons can be selectively ablated (Joly-Amado et al., 2012; Luquet et al., 2005; Luquet et al., 2007). Briefly, mice with the human diphtheria toxin (DT) receptor under the control of Agrp regulatory elements (AgRPDTR) are injected with DT during the first week after birth (AgRP-ablated mice); control mice of the same genotype are injected with saline (control mice). We reproduced our previous observation that neonatal ablation of AgRP neurons does not affect daily chow intake (see Figure S1A); however, feeding responses to peripherally or centrally injected ghrelin were abolished (See Figure S1B, S1C) (Joly-Amado et al., 2012; Luquet et al., 2005; Luquet et al., 2007). Peripheral injection of bio-active, fluorescently labeled ghrelin (Leyris et al., 2011; Schaeffer et al., 2013) resulted in robust accumulation of fluorescence in the ARC, while this signal was virtually absent in mice lacking AgRP neurons (Figure 1A). In addition, ghrelin induced Fos in ARC neurons following intra-cerebroventricular (ICV) injection, which was lost in AgRP-ablated mice (Figure 1B). These observations establish that AgRP neurons are important for ghrelin binding and action.
Figure 1. Food palatability restores ghrelin-induced feeding in AgRP-ablated mice.
(A and B) Representative confocal images of brain sections after injection (IV) of active fluorescent ghrelin (A) and immunohistochemistry for c-fos after 24 h fast (B) of AgRP-ablated (lower panel) and control (top panel) mice. Blue: Hoechst, red: incorporated fluorescent ghrelin (A), dark; Fos immune-staining (B). *:3rd ventricle. (C) 4-h caloric response to fast in AgRP-ablated (red, orange) or control (black, blue) mice presented with chow (black, red) or HFHS diet (blue, orange). (D) Representative time bar of three (daily) ICV injections of vehicle, and octanoyled ghrelin on the fourth day in control (black, blue) and AgRP-ablated (red, orange) mice presented consecutively with a chow, HFHS diet and then chow diet. Three days of acclimation were allowed with the new diet before the next series of injection. (E) 4-h cumulative caloric intake in 4-month-old control (black, blue) and AgRP-ablated mice (red, orange) after ICV injection of NaCl or octanoyled ghrelin injection. (F) 4-h cumulative caloric intake after IP injection of octanoyled ghrelin in 4-month-old control (black, blue) and AgRP-ablated mice (red, orange) fed with a chow or 2 or 10 weeks of HFHS diet. n=6 minimum in each group. *, indicated P<0.05, control vs AgRP-ablated mice. a, b and c differ significantly (P<0.05). Data are expressed as mean ± SEM. (See also Figure S1).
Food palatability restores feeding response to ghrelin in mice lacking AgRP neurons
We have observed previously that feeding after a fast can be restored according to the degree of palatability of food presented for refeeding (Luquet et al., 2005; Luquet et al., 2007). Provision of a highly palatable, high-fat and high-sucrose (HFHS) diet allowed a normal feeding response after a fast by AgRP-ablated mice (Figure 1C). This observation raises the possibility that the palatability or reward value of food might compensate for defective feeding in AgRP-ablated mice. Feeding response to centrally injected ghrelin was assessed in control or AgRP-ablated mice fed either regular chow or HFHS diet on several consecutive days (Figure 1D). ICV injection of ghrelin produced a significant increase in food intake by both groups of mice on HFHS diet (Figure 1E), supporting the hypothesis that ghrelin-induced feeding in the absence of AgRP neurons is dependent on diet palatability. Body-weight measurements confirmed that food intake rather than spillage had occurred during the 4 h following the injection (see Figure S1D). Ghrelin-induced increase in locomotor activity was identical between control and AgRP-ablated mice indicating that AgRP neurons are not essential for ghrelin-induced locomotor activity (see Figure S1E). We also assessed the feeding response to peripherally administered ghrelin in the same groups of animals with chow diet, versus 2 or 10 weeks on HFHS diet. Whereas ghrelin injected IP did not trigger chow intake by mice lacking AgRP neurons, it significantly increased consumption after being on the HFHS diet for 2 or 10 weeks (Figure 1F). While control animals exhibited a more robust increase in feeding response to ghrelin after 2 weeks of HFHS-diet exposure, the responses of both groups were identical after 10 weeks of HFHS-diet exposure. This result is consistent with the observation that diet-induced obesity leads to ghrelin resistance (Briggs et al., 2010; Briggs et al., 2014) and suggests that diet-induced obesity promotes a phenotype similar to that achieved with loss of AgRP neurons.
Feeding relies on food palatability and dopamine tone when activity of AgRP neurons is compromised
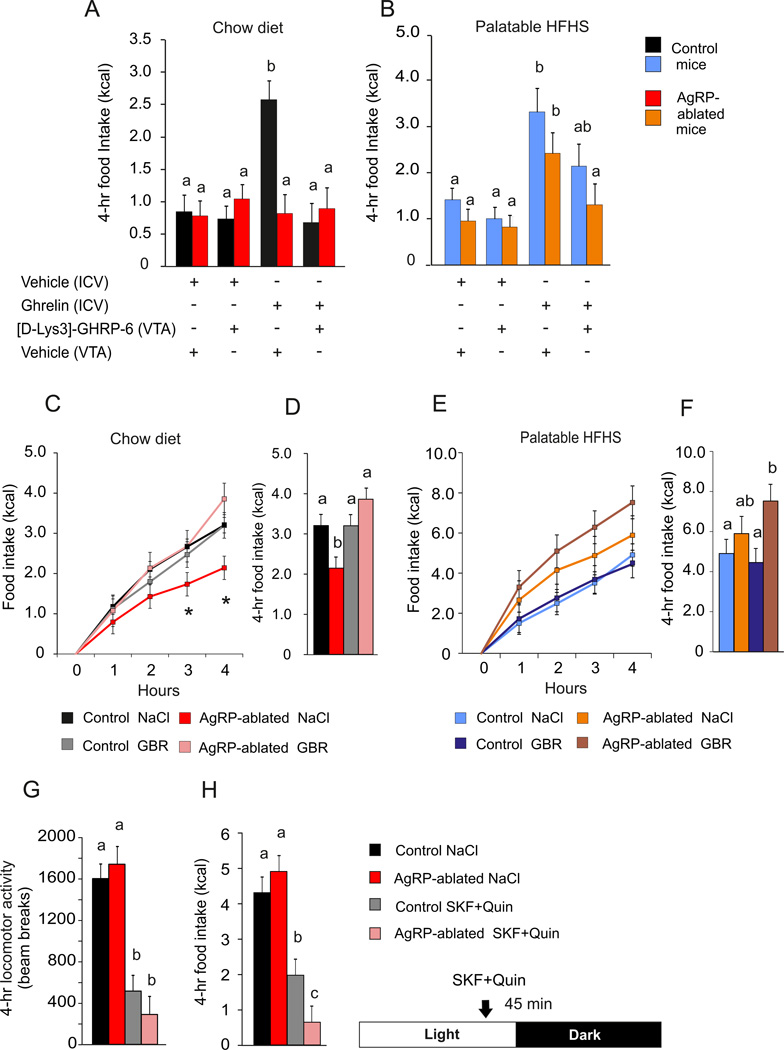
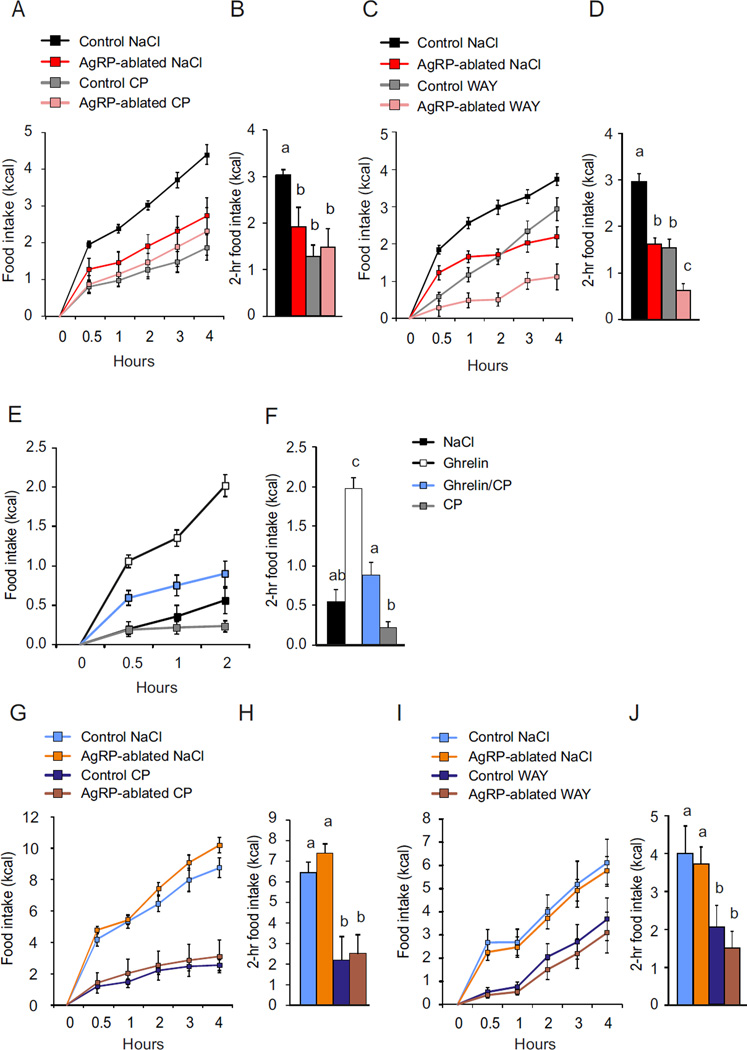
We reasoned that in the absence of AgRP neurons, orexigenic drive might rely on dopamine tone because the ghrelin receptor is expressed in the VTA (Mani et al., 2014) where its activation has been shown to trigger feeding (Abizaid et al., 2006; Egecioglu et al., 2010; Jerlhag et al., 2007; Naleid et al., 2005). Control and AgRP-ablated mice were implanted with ICV and intra-VTA cannulas and feeding responses to ghrelin were monitored on chow or HFHS diet in presence or absence of [D-Lys3]-GHRP-6, a selective antagonist of the ghrelin receptor (Traebert et al., 2002) that is known to reduce food intake (Ishii et al., 2002). Ghrelin (ICV) induced a significant increase in both chow and HFHS-diet intake in control mice, but this effect was restricted to the HFHS diet in AgRP-ablated mice (Figure 2A, 2B). Concomitant administration of the ghrelin receptor antagonist in the VTA reduced the orexigenic action observed in both control and AgRP-ablated mice (Figure 2A, 2B). These data support the hypothesis that VTA ghrelin is required for the orexigenic action of ghrelin in AgRP-ablated mice fed a palatable diet.
Figure 2. Feeding responses rely on dopamine tone in AgRP-ablated mice.
(A and B) 4-h cumulative caloric intake of AgRP-ablated (red, blue) or control (black, orange) mice fed with a chow (A) or HFHS diet (B), after a concomitant ICV and intra-VTA injection of vehicle or octanoyled ghrelin and [D-lys3-GHRP-6] or vehicle, respectively. (C, D, E and F) 4-h caloric response (C and E) and cumulative caloric intake (D and F) after a fast in AgRP-ablated (red, rose, orange and brown) or control (black, grey, blue and navy blue) mice presented with chow (black, red, grey and rose) or HFHS diet (blue, orange, navy blue and brown) injected IP with NaCl or a selective dopamine reuptake inhibitor (GBR). (G and H) Spontaneous locomotor activity (G) and caloric intake (H) of 6-month-old lean control animals (black, grey) and AgRP-ablated (red, rose) at the onset of the dark period after an injection IP (45 min before dark period) of the selective D1R agonist SKF 38393 hydrobromide and selective D2R agonist quinpirole hydrochloride (SKF+ Quin). n=6 minimum in each group. a, b and c differ significantly (P<0.05). Data are expressed as mean ± SEM. (See also Figure S2).
To further implicate the importance of dopamine signaling, we used a dopamine re-uptake inhibitor to determine if that would enhance chow consumption after a fast by AgRP-ablated mice. Fasted mice were tested on chow or HFHS diet in conjunction with peripheral injection of saline or GBR 12935 (Billes and Cowley, 2007). We found that pre-treatment with GBR selectively restored 4-h chow intake in response to a fast in AgRP-ablated mice without affecting control mice (Figure 2C, 2D). Importantly, the same treatment did not change feeding after a fast in control or AgRP-ablated mice when assessed on the HFHS diet (Figure 2E, 2F). GBR injection also increased locomotor activity selectively in AgRP-ablated mice when refed on chow (See Figure S2A).
We also took the reciprocal approach by exploring the anorectic action of dopamine receptor agonists in AgRP-ablated mice. Control and AgRP-ablated mice were injected with a mixture of selective dopamine D1 and D2 receptor agonists (D1/D2 mixture) that produce anorexia (Cooper and Al-Naser, 2006; Kuo, 2002; Romero-Pico et al., 2013). Animals were injected with saline or D1/D2 mixture 45 min before the onset of the dark period. AgRP-ablated mice displayed similar decrease in spontaneous locomotor activity to control mice (Figure 2G), but show enhanced sensitivity to the anorectic action of the agonists (Figure 2H). Monoamine quantification in various brain regions did not reveal any compensatory changes in dopamine content between control and AgRP-ablated mice (see Figure S3). Likewise, the abundance of various dopamine related mRNAs was unaffected (see Figure S4). These results support our hypothesis that feeding by mice lacking AgRP neurons relies on dopaminergic reward circuitry.
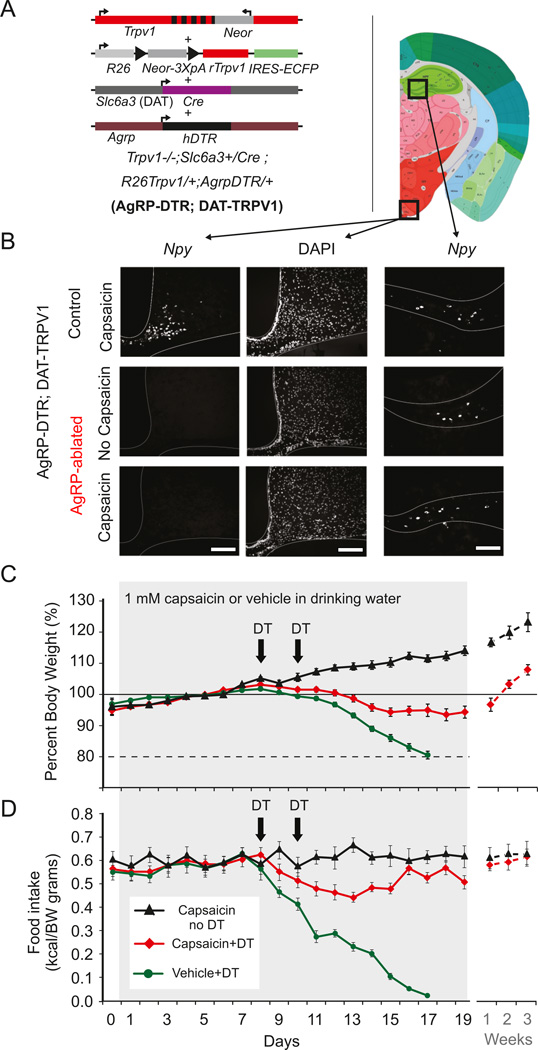
Because compensatory mechanisms in mice lacking AgRP neurons from birth cannot be completely ruled out, we also tested whether enhancing food palatability and dopamine neurons activity could prevent starvation that follows ablation of AgRP neurons in adult animals. AgRPDTR mice were put on a genetic background in which activation of dopamine neurons is mediated through the restricted expression of Trpv1, the capsaicin-receptor, in dopamine neurons; referred to as AgRPDTR; DATTRPV1 mice, see Figure 3A (Guler et al., 2012). Adult AgRPDTR; DATTRPV1 mice were presented with a palatable high-fat diet and the consequence of AgRP neuron ablation was evaluated in the presence or absence of capsaicin in the drinking water (Figure 3C,3D). AgRP-neuron ablation led to rapid body weight loss and starvation (Figure 3B, C, 3D); however, mild activation of dopamine neurons with capsaicin in the water prevented body weight loss and starvation in AgRPDTR; DATTRPV1 mice provided with a palatable diet (Figure 3C, 3D). When these mice were on a chow diet, capsaicin treatment was unable to prevent starvation (data not shown).
Figure 3. Mild activation of dopamine neurons prevents starvation following acute ablation of AgRP neurons in adult mice.
(A) Trpv1−/−; Slc6a3+/Cre; R26Trpv1/+ mice (DATTRPV1; (Guler et al., 2012)) were crossed to AgrpDTR/+ mice (Luquet et al., 2005) on the C57Bl/6 background to generate AgRPDTR. Resulting triple transgenic mice conditionally express rat Trpv1 (rTrpv1) gene from Gt(ROSA)26Sor (R26) locus only in dopamine neuron expressing CRE recombinase under the dopamine transporter (Slc6a3 or DAT) promoter on the Trpv1 knockout background (Trpv1−/−; Neor: neomycin resistance gene; IREs-ECFP: internal ribosome entry site-enhanced cyan fluorescent protein). (B) Fluorescent in situ hybridization for NPY (left and right panel) and DAPI counterstaining (middle panel) in arcuate (left panel) and hippocampus (right panel) of control (upper panel) and AgRPDTR mice (lower panel) injected with diphtheria toxin. Scale bar, 200 µm. (C) Body weight change (%) and food intake (kcal/g BW) of AgRPDTR; DATTRPV1 with capsaicin (1 mM) in drinking water (black triangle; n=6 mice), or injected twice with diphtheria toxin (DT) with capsaicin (red diamond; n=14 mice) or vehicle (green circle; n=15 mice) in drinking water. Following AgRP-neuron ablation, all of the AgRPDTR; DATTRPV1 mice with no access to capsaicin treatment had a concomitant decrease in food intake and body weight within 7 days. Mice at 80% of baseline bodyweight were not rescued and sacrificed.
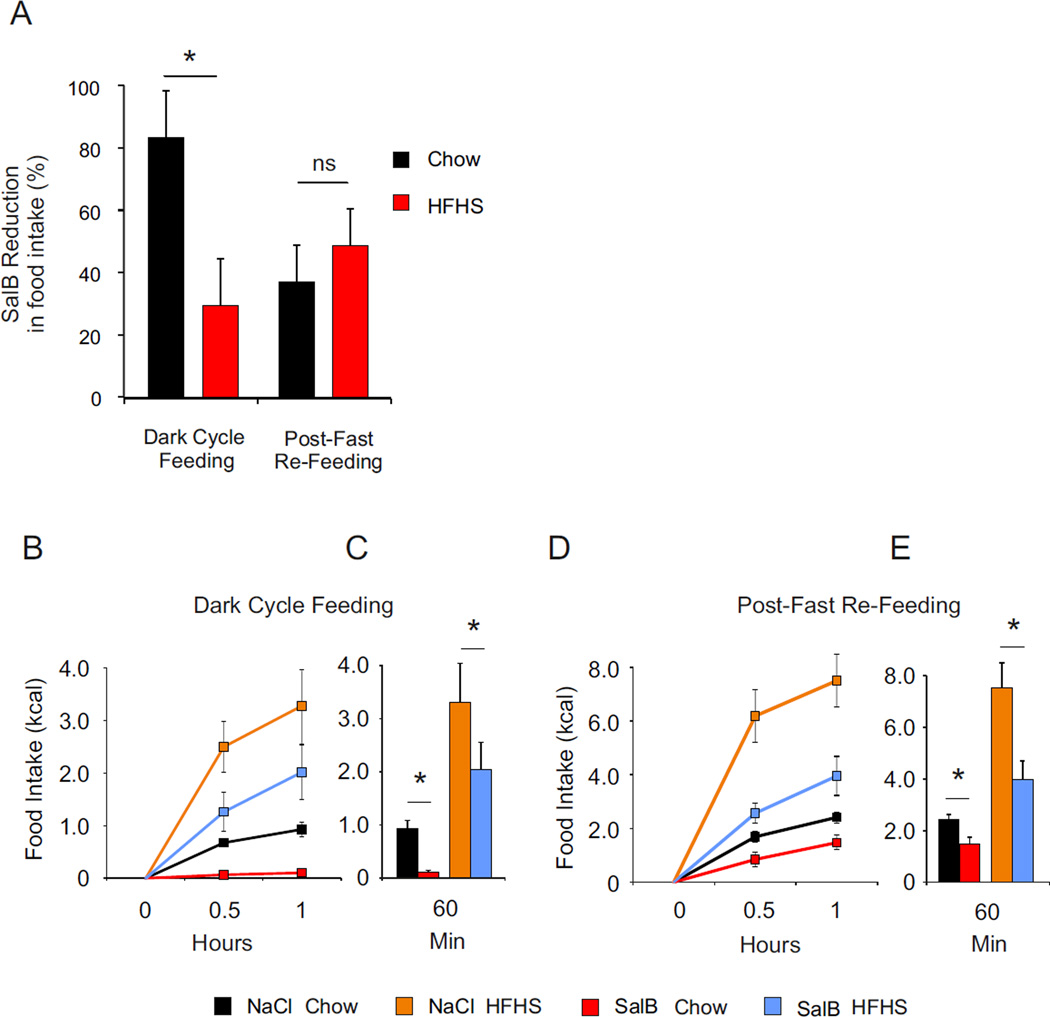
We also tested the effect of transient inhibition of AgRP neurons in adult mice using a designer receptor exclusively activated by designer drug (DREADD) approach. Male mice expressing Cre specifically in AgRP neurons (Agrpires-Cre) received bilateral stereotactic injection of the Cre-dependent AAV9-hSyn-DIO-HA-KOR expressing the newly designed Gi-coupled DREADD using the kappa-opioid receptor (KORD) activated by the salvinorin B (SalB): a pharmacologically inert ligand (Vardy et al., 2015). SalB-mediated AgRP neuron silencing was assessed during spontaneous feeding at the onset of the dark period and in response to a fast on both chow and HFHS diet. Acute silencing of AgRP neurons resulted in a ~80% decrease in spontaneous chow intake compared to saline injection, whereas palatable diet intake was inhibited by only ~30% (Figure 4A, 4B, 4C). Following a fast, SalB-mediated inhibition of AgRP neurons resulted in a ~50% decrease in food intake regardless of the diet palatability. (Figure 4A, 4D, 4E). These results show that depending of metabolic status, the contribution of metabolic demands and reward differentially contribute to overall nutrient intake.
Figure 4. Acute silencing of AgRP neurons differentially impact on chow and palatable food intake.
(A) Salvorin-B (SalB) mediated inhibition of food intake in percentage over saline injection inhibition of food intake initiated for each animal. Comparison between groups was performed using analysis of variance for each paradigm. Values are expressed as mean ± SEM issue of the analysis of variance. * P<0.05 Ns = non-significant. (B, C) 2-h food intake (kcal) after onset of dark period (B, C) or after a fast (D, E). Values are expressed of mean ± SEM of animals treated with Saline or SalB under a standard and a HFHS diet. Food intake was recorded at 0.5 and 1 h. Values (C, E) are expressed as mean ± SEM at 1 h. Comparison was performed between Saline and SalB treatment using a nonparametric Wilcoxon–Mann–Whitney test. * P<0.05.
To determine whether palatability or nutrient content could explain the difference in feeding response observed in mice lacking AgRP neurons, control and AgRP ablated mice were compared for preference of either caloric (sucrose) or non-caloric (saccharin) solution verses water in a 2-bottle paradigm; both groups had similar preferences for sucrose or saccharin with a greater preference for sucrose over saccharin (see Figure S5A) as well as similar shift in preference under conditions of increasing sucrose concentration in both 2 h and overnight-consumption tests (see Figure S5 B–D). These data indicate that control and AgRP-ablated mice have similar responses to sweeteners, suggesting that fat in the HFHS diet is more likely to affect the palatability preference displayed by AgRP-ablated mice.
Ablation of AgRP neurons reveals a functional dichotomy in the anorectic action of serotonin according to food palatability
5-HT innervation of ARC neurons mediates satiety by the concerted activation of both the Gi-coupled 5-HT1B receptors on AgRP neurons and Gq-coupled 5-HT2C receptor activation on POMC neurons (Heisler et al., 2006). This arrangement predicts that AgRP-ablated mice should manifest reduced sensitivity to the anorectic effects of 5HT1BR compared to the 5HT2CR agonist. Feeding response after a fast on a chow diet was monitored following administration of the selective 5-HT1BR agonist CP94253 (CP) and 5-HT2CR agonist WAY161503 (WAY) using doses that produce significant reduction in refeeding (Heisler et al., 2006). While pretreatment with CP 45 min prior to refeeding produced a ~50% decrease in chow intake in control animals, this treatment was ineffective compared to saline in mice lacking AgRP neurons (Figure 5A, 5B). WAY injection produced a greater anorectic response in AgRP-ablated mice than in control mice (Figure 5C, 5D). These results provide evidence that, despite the fact that several other brain regions express 5-HT1B receptors, AgRP neurons are a primary mediator the anorectic action of 5-HT1BR agonist.. Having established that AgRP neurons are a principal mediator of both ghrelin and 5-HT1BR agonist responses, we predicted that transient inhibition of AgRP neurons with one agonist should reduce feeding by the other agonist in wild-type animals. Feeding responses to peripherally injected ghrelin (500 µg/kg IP) alone or in association with a pretreatment with CP (8 mg/kg, IP) were measured for 2 h in wild-type mice; CP inhibited ghrelin-induced feeding as predicted (Figure 5E, 5F). Quantification of serotonin content in various brain areas did not reveal any adaptive change as a consequence of AgRP-neuron ablation (See Figure S3).
Figure 5. Satiety action of 5-HTR agonist requires AgRP neurons integrity on chow diet but not palatable diet.
(A, B, C and D) 4-h caloric response to fast (A and C) and 2-h cumulative food intake (B, D) of control (black, grey) and AgRP-ablated mice (red, rose) fed a chow diet, injected IP, 45 min prior refeeding, with NaCl (black, red) or the 5-HT1BR agonist CP (A and C, grey, rose) or the 5-HT2CR agonist WAY (B and D, grey, rose). (E and F) 2-h caloric intake (E) and cumulative (F) after an injection (IP) of NaCl (black) ghrelin (white box) associated with CP (blue box) and CP alone (grey box). (G, H, I, and J) 4-h response (G and I) and cumulative (H and J) caloric intake to fast in AgRP-ablated (orange, brown) or control (blue, navy blue) mice fed HFHS diet, injected 45 min prior to refeeding with NaCl (blue, orange), CP (G and H navy blue, brown) or WAY (I and J. navy blue, brown). n=6–10 mice in each group. a, b and c, differ significantly (P<0.05). Data are expressed as mean ± SEM.
Based on the radical change in the ghrelin response that we observed by manipulating diet palatability, we also assessed feeding response to 5-HT receptor agonists when mice were provided with a palatable diet using the same experimental design as in Figure 5A–D. With substitution of a HFHS-diet, both saline-treated control and AgRP-ablated mice displayed similar feeding response and had similar anorectic responses to both 5-HT2CR and 5-HT1BR agonists (Figure 5 G–J), suggesting again that AgRP neurons are dispensable for the anorectic action of these serotonin agonists when assessed on a palatable diet.
Feeding behavior in the absence of AgRP neurons: a model of comfort feeding
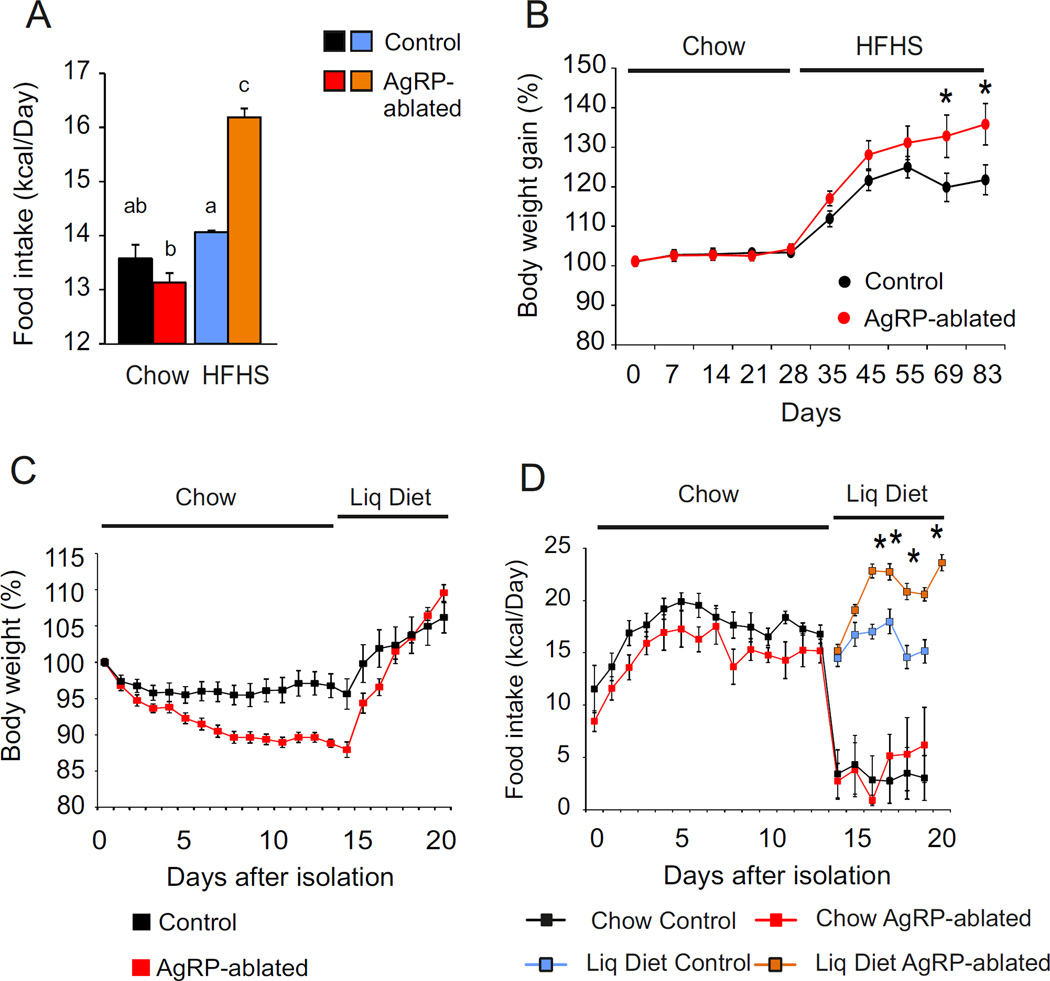
Our data suggest that ingestion of a palatable diet is relatively independent of AgRP neurons. We predict, therefore, that mice lacking AgRP neurons represent a good model of “comfort feeding”, in which feeding in response to energy demands is crippled due to the lack of hypothalamic orexigenic drive and relies essentially on reward. We evaluated daily caloric intake over a period of 4 months during which animals were exposed to chow for 2 months after weaning and then shifted to HFHS diet for another 2-month period. Food intake was measured weekly by group-housed mice. Whereas chow intake was similar between the two groups before and after shifting diets, shifting AgRP-ablated mice onto HFHS diet resulted in sustained overfeeding (Figure 6A) and body weight gain (Figure 6B).
Figure 6. Ablation of AgRP neurons creates a context favorable to comfort feeding.
(A) Weekly averaged caloric intake over 2 months by control (black, blue) and AgRP-ablated (red, orange) mice presented with chow (black, red) and then HFHS diet (blue, orange). (B) Body-weight gain of control (black) and AgRP-ablated (red) mice monitored during the shift to HFHS diet. (C and D) Evaluation of body weight change (C) and caloric intake (D) by AgRP-ablated (red, orange) or control (black, blue) mice over 20 days after social isolation fed with chow diet (black, red) and presented with a liquid diet on the five remaining days (blue, orange). n=6–10 mice in each group. * P<0.05, control vs AgRP-ablated mice. a, b and c differ significantly (P<0.05). Data are expressed as mean ± SEM.
Reciprocally, social isolation achieved by singly housing control or AgRP-ablated mice was used to quantify the action of a mild stress on food intake (Hao et al., 2001). Two-month-old control and AgRP-ablated mice with the same body weight were singly housed and given normal chow. Body weight loss was significantly greater in AgRP-ablated mice during the 2 weeks following social isolation (Figure 6C). When access to palatable liquid diet was provided, the body weight of the AgRP-ablated mice was rapidly regained (Figure 6C); furthermore, while control animals maintained caloric intake, the AgRP-ablated mice consumed more calories (Figure 6D). Our finding that AgRP-ablated mice exhibit exaggerated hyperphagia on a palatable diet and greater anorexia during stress is consistent with the hypothesis that, in the absence of AgRP neurons, feeding is less motivated by energy demand and more sensitive to changes in stress and reward value (Chuang et al., 2011).
AgRP-ablated mice display impaired feeding but abnormal anticipation on food-restricted schedule
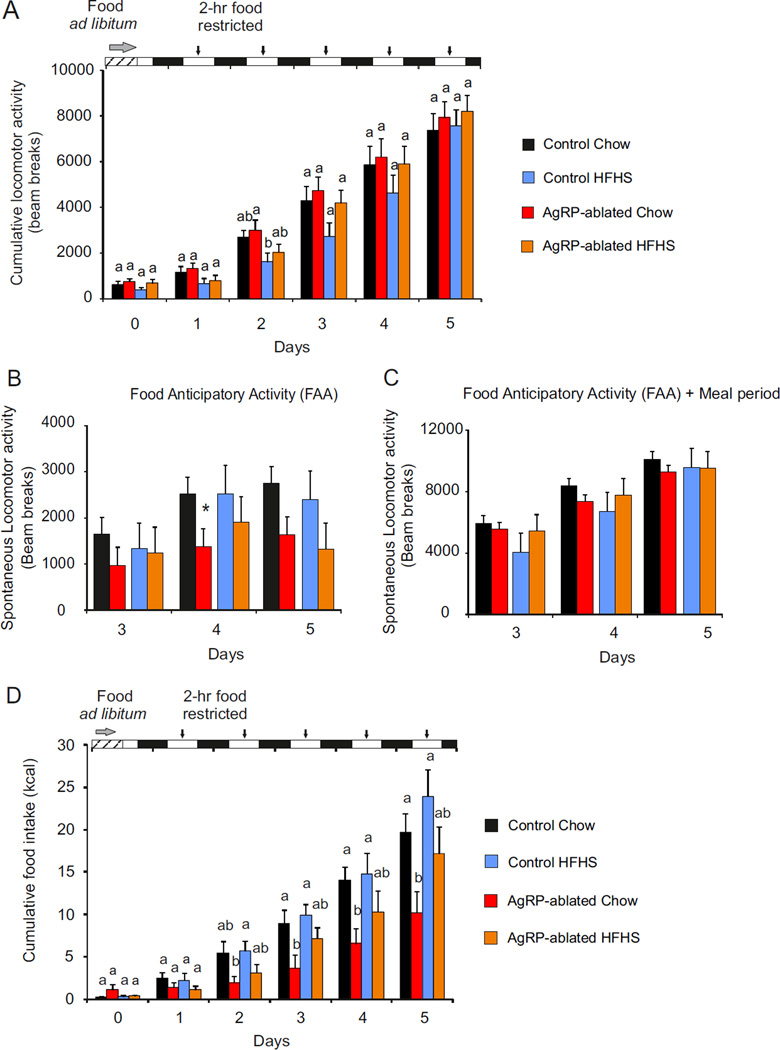
Restricting feeding to a short time window is a paradigm used to assess food-seeking behavior in response to scheduled meals. Animals with limited access to food learn to anticipate food availability by increasing locomotion (food anticipatory activity, FAA) and optimize their consummatory behavior during that period. Ghrelin signaling is critical for FAA and consumption (Blum et al., 2009; Verhagen et al., 2011). When assessed on chow diet, we found that total locomotor activity by AgRP-ablated mice was initially decreased relative to control mice but activity recovered by day 5; when the mice were on HFHS diet, the AgRP-ablated group recovered normal locomotion faster (Figure 7A). AgRP-ablated mice had decreased in FAA on chow diet that reached significance only on day 4 after the beginning of the restriction (Figure 7B); no deficit FAA was observed by AgRP-ablated mice on the HFHS diet.
Figure 7. Food palatability restores feeding deficit of AgRP-ablated mice on feeding restricted schedule.
Five-day cumulative locomotor activity (A) and cumulative caloric intake (D) of control (black, blue) and AgRP-ablated (red, orange) mice with 2-h restricted access to chow (black, red) or HFHS diet (blue, orange); n=6 per group, a, b and c, differ significantly (P<0.05). Time bars: food ad libitum (striped) daylight (white), night (black). Arrows indicate 2-h food access. (B and C) Spontaneous locomotor activity of control (black, blue) and AgRP-ablated (red, orange) mice fed with a chow (black and red) or HFHS diet (blue and orange) at day 3, 4 and 5 of restricted schedule, recorded during the 2 h before food being available (B) and during the 2 h before food access (FAA) and 2 h of food availability (Meal) (C); ANOVA with groups, diet and days and their interactions as factors showed a significance diminished locomotor activity referred as Food Anticipatory Activity (FAA) in mice lacking AgRP neurons only when fed a chow diet (from the ANOVA; 2307 ± 206,3 vs 1327 ± 225.9 under a chow diet P<0,01. 2082 ± 346.1 vs 1489 ± 325.1 under HFHS, ns). N= 6 per group *, P <0.05, control vs AgRP-ablated mice. Data are expressed as mean ± SEM. (See also Figure S6, S7).
Spontaneous activity during the 2 h before the meal and the 2 h of meal availability were the same for all four groups (Figure 7C). Chow consumption was significantly blunted in AgRP-ablated mice when compared to control mice (Figure 7D), but more so by the AgRP ablated mice on chow diet than on HFHS diet (see Figure S6A, S7A). These results agree with a study showing that partial ablation of AgRP neurons leads to defective adaptation of feeding and FAA (Tan et al., 2014). (Figure 7D, 7B and also Figure S7A). The observation that FAA behavior is intact even when ghrelin action on AgRP neurons is not possible suggests that ghrelin-dependent anticipatory behavior is mediated by other neural circuits.
DISCUSSION
The mammalian brain has evolved several very efficient and redundant, interconnected neural circuits that encodes both metabolic needs and reward in the complex sequence of feeding behavior. While the hedonic drive to eat energy-dense food has the evolutionary advantage of promoting a strong motivational response to seek and consume nutrients and insure survival in time of scarcity, it has become a challenge because of the radical change in feeding habits in modern society characterized by an almost ubiquitous source of calorie-dense foods (Saper et al., 2002). Understanding the net contribution of homeostatic-energy-driven and non-homeostatic-reward-driven feeding has become critical and requires a deeper understanding of the neuronal mechanism by which energy needs and reward participate in food intake.
AgRP neurons represent a critical component of the homeostatic circuitry that respond to decreased nutrient availability by engaging the full sequence of feeding behaviors (Aponte et al., 2011; Carter et al., 2013; Cowley et al., 2003; Cowley et al., 1999; Krashes et al., 2011). Furthermore, ablation of AgRP neurons in adult mice results in profound anorexia (Bewick et al., 2005; Gropp et al., 2005; Luquet et al., 2005; Xu et al., 2005). Thus, these neurons represent a critical component of the homeostatic circuitry.
To address the contribution of AgRP neurons in reward and non-reward driven feeding, we used various models in which AgRP neuron activity is compromised. We found that while AgRP neuron integrity is required to mediate normal feeding after a fast, administration of ghrelin or a 5-HT1BR agonist, or during meal-restricted schedule; however, AgRP neurons are dispensable when feeding is assessed on HFHS diet. Ghrelin-induced feeding by AgRP-ablated mice required the integrity of ghrelin-signaling pathway in the VTA, and enhancing dopamine tone or food palatability selectively restored feeding to either post-natal or adult AgRP neuron ablation. Reciprocally, dopamine receptor agonists had a more pronounced satiety action in AgRP-ablated mice compared to controls. Importantly, long-term exposure to HFHS diet which results in ghrelin resistance in AgRP neurons (Briggs et al., 2010) produced a behavioral response to ghrelin similar to that observed in AgRP-ablated mice. Mice lacking AgRP neurons are also more susceptible to both stress-induced anorexia and palatable-diet hyperphagia. The palatability effect is observed after neonatal ablation, after adult ablation or with transient inactivation of AgRP neurons; thus, this phenomenon is not entirely due to developmental effects.
Our results suggest that when AgRP neuron activity is impaired, neural circuits sensitive to emotion and stress are engaged and modulated by food palatability and dopamine signaling.
Our data demonstrate that peripherally injected ghrelin can increase consumption of palatable food in a VTA-dependent manner when AgRP neurons are absent. This result is reminiscent of a recent study showing that ghrelin action on dopaminergic neurons is sufficient to drive food-reward in a context of chronic stress, referred to as ‘comfort feeding’ (Chuang et al., 2011). Our observations are also in line with a study showing that loss of AgRP neuron function promotes greater excitability of VTA dopamine neurons (Dietrich et al., 2012). We show that dopamine neuron activity and food palatability are both important contributors to food intake when AgRP neuron activity is chronically compromised.
From an evolutionary standpoint, animals have evolved mechanisms to cope with ever-present potential for starvation. AgRP neurons are most active during starvation conditions and they send axonal projections to many brain regions (Broberger et al., 1998) presumably to orchestrate adaptive responses that facilitate energy conservation (e.g. by inhibiting metabolism, growth, reproduction and satiation) while promoting foraging and feeding (Betley et al., 2013). Thus, in the absence of AgRP neurons we predict that suppression of energy-demanding activities would be impaired and that feeding would depend on alternative neural circuitry. Chronic desensitization of AgRP neurons due to long-term exposure to an energy rich diet could result in the progressive replacement of metabolic demand by reward seeking as the main driver of food intake. Reciprocally, consuming highly palatable food may provide stronger taste signals as well as increased dopamine release by the VTA and ultimately adaptive change in the reward circuitry (Lockie and Andrews, 2013). During chronic exposure to palatable diet orosensory inputs relaying food reward might gradually prevails on AgRP-mediated inputs relaying metabolic needs. The overall consequence would be to minor the consequence of AgRP neuron activity onto nutrient intake. In these conditions the contribution of AgRP neurons could be largely bypassed by extrinsic hedonic neural circuitry.
In addition any attempt to chronically decrease AgRP neuron activity as a pharmacological intervention might have the adverse consequence of enhancing neural circuits that are more susceptible to emotion (stress or anxiety) and food reward—hallmarks of ‘comfort feeding’ (Dallman et al., 2005).
EXPERIMENTAL PROCEDURES
Animals
All animal experiments were performed with approval of the Animal Care Committee of the University Paris Diderot-Paris 7. Mice (8–9 months old, otherwise stated in the text) were housed individually in stainless steel cages in a room maintained at 22.5±1°C with lights scheduled from 7 am to 7 pm. Animals had access to water and food ad libitum unless otherwise specified. Details are provided in the supplemental experimental procedures.
Drugs and peptides
Drugs or peptide were dissolved according to the manufacturer in vehicle solution; saline for IP injection and artificial cerebrospinal fluid (Alzet, Cupertino, USA) for central injection. Acetylated ghrelin (Polypeptide, Strasbourg, France) was either administrated IP (150 nmol/kg Body Weight; BW) or centrally (1 nmole per mouse at a rate of 0.4 µl/min in a final volume of 0.8 µl). Antagonist (D-Lys3]-GHRP-6 (Tocris Bioscience, Bristol, UK) of the ghrelin receptor (GHS-R1a) was administered centrally (1 nmole per mouse at a rate of 0.4 µl/min in a final volume of 0.8 µl). Selective serotonin receptor agonist (Tocris Bioscience, Bristol, UK) were administrated IP at 10 µl/g of BW: 5-HT1BR agonist CP94253 (CP, 8 mg/kg), 5-HT2CR agonist WAY 161503 (WAY, 7 mg/kg). Dopamine receptor agonists and an inhibitor of dopamine re-uptake (Tocris Bioscience, Bristol, UK) were administrated IP at 10 µl/g of BW; final concentrations were selective dopamine receptor-1 agonist (D1R) SKF 38393 hydrobromide (10 mg/kg), selective Dopamine receptor-2 (D2R) agonist quinpirole hydrochloride (0.2 mg/kg), Inhibitor of dopamine uptake GBR 12935 dihydrochloride (GBR, 7.5 mg/kg). Capsaicin (97% by HPLC, Sigma, MO) containing drinking water was prepared as a 1-M stock solution in ethanol and diluted 1,000-fold in 1% Tween-80 (1mM final concentration). Control solution contained 0.1% ethanol, 1% Tween-80. Diphtheria toxin (DT, 50 µg/kg; List Biological Laboratories, Campbell, CA) or vehicle injection was administered via subcutaneous or intramuscular routes in neonates or adult animal respectively. Salvorin B (SalB) was dissolved in DMSO and injected IP at 10mg/kg as described in (Vardy et al., 2015).
Fluorescent ghrelin synthesis and binding in vivo
Bioactive fluorescent ghrelin was obtained as described (Schaeffer et al., 2013). Briefly, active fluorescent ghrelin (25 nmole in 100 µl) was injected i.v. into the tail vein, in both control and AgRP-ablated mice fed with either chow diet or HFHS diet. Terminally anesthetized mice at 5–10 min post-injection were perfused via the heart with 10 mL of potassium phosphate buffer 0.1 M (PBS). Brains were collected and immersed for 24 h in 4% paraformaldehyde solution (PFA) and subsequently sliced using a Leica vibratome (50-µm coronal slices) and prepared for confocal imaging as described in supplemental methods (Schaeffer et al., 2013).
Spontaneous activity and feeding
Spontaneous feeding and locomotors activity (beam breaks/h) were measured using automated feeding and activity recording in Labmaster (TSE Systems GmbH, Bad Homburg, Germany). Each cage was equipped with a set of highly sensitive feeding and drinking sensors for automated online measurement and embedded in a frame with an infrared light beam-based locomotion monitoring system. The sensors for detection of movement operate efficiently in both light and dark phases, allowing continuous recording. Mice were individually housed and acclimated to the chambers for 48 h before experimental measurements.
Acute feeding after a fast
Feeding after a fast was carried out as follows: cage bedding was changed and food removed at 10 am until the next day at 10 am when carefully weighed food was replaced and weighed again 4 h later. Body weight was measured before and after the fasting period and 4 h after refeeding. When appropriate, 30 min prior to replacing the food, animals received IP injection of vehicle or selected compounds.
Time restricted feeding schedule
Mice were subjected to a time-restricted feeding protocol. Six control and 6 AgRP-ablated mice were housed individually in activity recording system with an automated food and liquid access control (Labmaster, TSE Systems GmbH, Bad Homburg, Germany). Following 72 h of adaptation to the new environment with food and drink ad libitum, mice were temporally restricted access to food from 12 pm to 14 pm every day for 5 consecutive days. Spontaneous locomotor activity, food and water intake were monitored continuously. When appropriate, food spillage was collected and subtracted from the calculated food intake.
Data analysis
The results are expressed as mean ± SEM. Variance equality was analysed by F-test (Microsoft Excel, Issy-Les-Moulineaux, France) and comparisons between groups were carried out using a Student’s t test or by a nonparametric Mann–Whitney–Wilcoxon’s test (Minitab, Paris, France). When appropriate, analyses of variances were performed followed either by a Bonferroni or Tukey’s post hoc test with the appropriate parameters and their interaction as factor (Minitab, Paris, France). Unless otherwise indicated in the text, a P-value of <0.05 was indicated by * and considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by young investigator ATIP grant from the Centre National la Recherche Scientifique (CNRS), a research and equipment grant from the Région Île-de-France, from the University Paris Diderot-Paris 7, from the ‘Agence Nationale de la Recherche’ ANR-09-BLAN-0267-02 and ANR 11 BSV1 021 01. RGP received a post-doctoral grant from the Région Île-de-France. A.J-A. received a National Merit Scholarship from the French Department of National Education and Research, a research grant from the Société Francophone du Diabète and a research grant from the SFNEP-ANTADIR. R.D received a fellowship from the Région Ile de France and merit grant from the Société Francophone de Nutrition (SFN-LU). C.C. received a PhD fellowship from the CNRS and a research grant from the Société Francophone du Diabète-Roche. We acknowledge the technical platform metabolism of the Unit ‘Biologie Fonctionnelle et Adaptative’, (University Paris Diderot, Sorbonne Paris Cité, BFA, UMR 8251 CNRS, F-75205 Paris, France) for metabolic and molecular analysis, and the animal core facility ‘Buffon’ of the University Paris Diderot Paris 7/Institut Jacques Monod, Paris for animal husbandry and breeding. We thank Olja Kacanski for administrative support, Gaëlle Charlon, Le Parco Isabelle, Sandrine Olivré and Ludovic Maingault for care of animals. We also thank Colin T. Phillips and Hicham Lamrini for providing essential help in collecting stress-induced responses and feeding data in AgRP-ablated animals. We acknowledge Bryan Roth for kindly providing the KOR viruses. Finally, we would like to thank Michael Cowley and Scott Sternson for valuable scientific input and Diane Durnam for manuscript editing.
Footnotes
AUTHOR CONTRIBUTIONS
A.J-A., R. G.P., designed and performed most of the experiments. R. G. P developed the ghrelin aspect while A. J-A collected data on serotonin. J. C, C. C, A-S. D, A. L participated in in vivo experiments and indirect calorimetry, C.R performed HPLC measurement and N.K and S M performed molecular analysis. M. S, F.L and B. D contributed for fluorescent ghrelin analysis and A.L performed c-fos studies. J-A. F, J.M and P. V designed and provided fluorescent ghrelin. R. P, S. P and A. G contributed data with TRPV-dependent activation of dopamine neurons. E, W and M. K contributed DREADD inhibition of AgRP neurons with T. H and C. M contributed to data analysis and interpretation and also help with the manuscript. S.L acquired funding, conceptualized and interpreted the studies and wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental information includes 7 figures and supplemental experimental procedures.
The authors declare that they have no conflicts of interest.
REFERENCES
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiology & behavior. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32:822–834. doi: 10.1038/sj.npp.1301155. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Lockie SH, Benzler J, Wu Q, Stark R, Reichenbach A, Hoy AJ, Lemus MB, Coleman HA, Parkington HC, et al. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology. 2014 doi: 10.1210/en.2013-1861. en20131861. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. The Journal of clinical investigation. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain research. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Guler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, Palmiter RD. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat Commun. 2012;3:746. doi: 10.1038/ncomms1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Avraham Y, Bonne O, Berry EM. Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: effects of tyrosine. Pharmacology, biochemistry, and behavior. 2001;68:273–281. doi: 10.1016/s0091-3057(00)00448-2. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Role of ghrelin in streptozotocin-induced diabetic hyperphagia. Endocrinology. 2002;143:4934–4937. doi: 10.1210/en.2002-220612. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. Embo J. 2012;31:4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DY. Co-administration of dopamine D1 and D2 agonists additively decreases daily food intake, body weight and hypothalamic neuropeptide Y level in rats. J Biomed Sci. 2002;9:126–132. doi: 10.1007/BF02256023. [DOI] [PubMed] [Google Scholar]
- Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000245. [DOI] [PubMed] [Google Scholar]
- Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology. 1998;136:304–307. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- Leyris JP, Roux T, Trinquet E, Verdie P, Fehrentz JA, Oueslati N, Douzon S, Bourrier E, Lamarque L, Gagne D, et al. Homogeneous time-resolved fluorescence-based assay to screen for ligands targeting the growth hormone secretagogue receptor type 1a. Anal Biochem. 2011;408:253–262. doi: 10.1016/j.ab.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Lockie SH, Andrews ZB. The hormonal signature of energy deficit: Increasing the value of food reward. Mol Metab. 2013;2:329–336. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–225. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perello M, Andrews ZB, Zigman JM. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522:3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009;54:89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–1247. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]
- Romero-Pico A, Novelle MG, Folgueira C, Lopez M, Nogueiras R, Dieguez C. Central manipulation of dopamine receptors attenuates the orexigenic action of ghrelin. Psychopharmacology. 2013;229:275–283. doi: 10.1007/s00213-013-3096-7. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdie P, Bourrier E, Dehouck B, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab. 2014;3:694–704. doi: 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. Journal of neuroendocrinology. 2002;14:580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron. 2015;86:936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol. 2011;21:384–392. doi: 10.1016/j.euroneuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.