Abstract
Background
Marijuana is seeing increased therapeutic use, and is the world’s third most-popular recreational drug following alcohol and tobacco. This widening use poses increased exposure to potentially toxic combustion by-products from marijuana smoke and the potential for public health concerns.
Objectives
To compare urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) among self-reported recent marijuana users and nonusers, while accounting for tobacco smoke exposure.
Methods
Measurements of PAH and VOC metabolites in urine samples were combined with questionnaire data collected from participants in the National Health and Nutrition Examination Surveys (NHANES) from 2005 to 2012 in order to categorize participants (≥18 years) into exclusive recent marijuana users and nonusers. Adjusted geometric means (GMs) of urinary concentrations were computed for these groups using multiple regression analyses to adjust for potential confounders.
Results
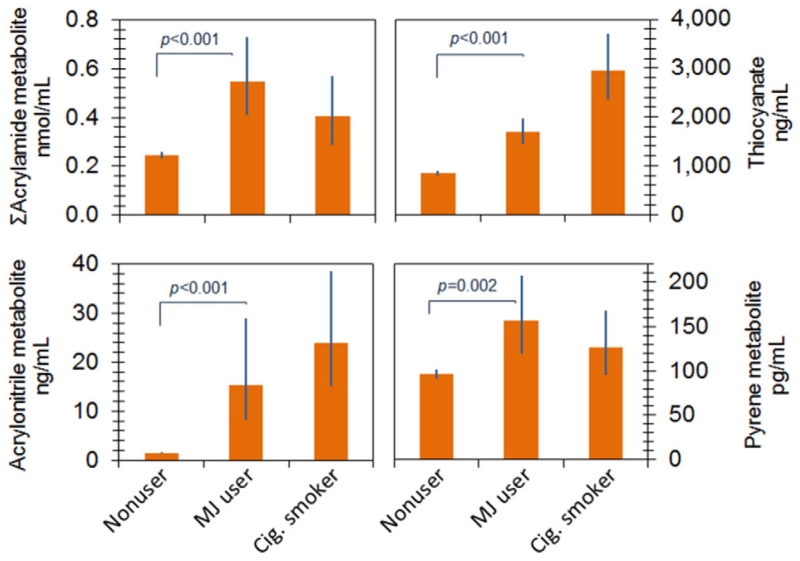
Adjusted GMs of many individual monohydroxy PAHs (OH-PAHs) were significantly higher in recent marijuana users than in nonusers (p < 0.05). Urinary thiocyanate (p < 0.001) and urinary concentrations of many VOC metabolites, including metabolites of acrylonitrile (p < 0.001) and acrylamide (p < 0.001), were significantly higher in recent marijuana users than in nonusers.
Conclusions
We found elevated levels of biomarkers for potentially harmful chemicals among self-identified, recent marijuana users compared with nonusers. These findings suggest that further studies are needed to evaluate the potential health risks to humans from the exposure to these agents when smoking marijuana.
Keywords: Cannabis smoke, Biomonitoring, PAHs, VOCs, Secondhand smoke, Environmental tobacco smoke (ETS)
1. Introduction
Marijuana, prepared from Cannabis sativa, has been increasingly used as a therapeutic agent, and ranks as the world’s third most-popular recreational drug following alcohol and tobacco (Murray et al., 2007; UNODC, 2014). The United Nations Office on Drugs and Crime (UNODC) estimated that worldwide 177 million people aged 15–64 years used cannabis at least once in 2012 (UNODC, 2014). Marijuana use in the USA was allowed for medical purpose under Federal law in 1937 when the Marijuana Tax Act was passed. In 1942, marijuana was removed from the US pharmacopeia, and in 1970, it was classified as a drug with ‘no accepted medical use’ (Murray et al., 2007). Since California became the first state to legalize medical marijuana use in 1996, 23 states and the District of Columbia in US have legalized medical marijuana use. In contrast with past-month tobacco use among persons ≥ 12 years old in US, which decreased from 30.4% in 2002 to 25.5% in 2013, the proportion using marijuana in the past month increased from 6.2% in 2002 to 7.5% in 2013 (SAMHSA, 2014).
Most people use marijuana for medical and recreational activities because it contains psychoactive constituents such as the primary cannabinoid — Δ9-tetrahydrocannabinol (THC) (Murray et al., 2007). Medicinally, marijuana is used to relieve symptoms, such as nausea and vomiting caused by cancer chemotherapy, appetite loss in patients with AIDS, muscle spasticity and chronic pain in patients with neurological disorders, and glaucoma (Hall and Degenhardt, 2003). Recreationally, the use of marijuana can provide temporary experiences, including euphoria, relaxation, heightened mood (Hall and Degenhardt, 2009). Use of marijuana can also cause many adverse health consequences, including anxiety and panic in naive users (Hall and Degenhardt, 2009), impaired respiratory function (Taylor et al., 2002), chronic bronchitis (Tetrault et al., 2007), elevated risks of increased heart rate (Jones, 2002) and myocardial infarction (Mittleman et al., 2001), and possibly lung cancer (Aldington et al., 2008; Berthiller et al., 2008). Long-term marijuana use is also associated with impaired cognitive abilities (Solowij et al., 2002), changes in brain function (Block et al., 2000), and use during pregnancy has been associated with reduced birth weight in the offspring, but not all studies (English et al., 1997; Fergusson et al., 2002; Gray et al., 2010; Brown and Graves, 2013; Huizink, 2014; Mark et al., 2015).
Similar to tobacco users, smoking is the main route desired by many marijuana users as this is the most efficient way to achieve the desired psychoactive effects within a short time (Huestis, 2002; Clark et al., 2004; Ware et al., 2005; Hall and Degenhardt, 2009; Schauer et al., 2015). Unlike cigarette smoke (i.e., main stream, side stream, secondhand and even third-hand smoke) which has been extensively investigated, information on chemical constituents produced through smoking marijuana is limited (Fehr and Kalant, 1972; Lee et al., 1976; Rickert et al., 1982; Chait and Pierri, 1989; Moir et al., 2008; Maertens et al., 2009). Collectively, these studies have indicated that marijuana smoke contains similar toxic chemical constituents as tobacco smoke, including polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs). Nevertheless, existing literature data is insufficient to evaluate actual body burdens of these harmful chemicals in marijuana users.
In this study, we use self-reported questionnaire data along with serum cotinine (sCOT) concentrations measured in adult participants (≥18 years old) of the National Health and Nutrition Examination Surveys (NHANES) during the period 2005 to 2012 to classify the participants as exclusive marijuana users and nonusers of marijuana or tobacco. We subsequently evaluated and compared the urinary concentrations of PAH and VOC metabolites among them.
2. Materials and methods
2.1. Study design and participants
The National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) conducts NHANES, a cross-sectional health examination survey conducted in two-year cycles; its sample is representative of the US civilian non-institutionalized population. Survey participants are randomly selected using a complex, stratified, multistage probability design. A sample weight is assigned to each participant to account for the complex survey design (including oversampling), survey non-response, and post-stratification. Details on sample design and weight calculation were described online by NCHS (US-CDC, 2013). The NCHS Research Ethics Review Board (ERB) protected the rights and welfare of NHANES participants. In accordance with Federal regulations, the NCHS ERB reviewed and approved NHANES protocols and any changes made to them. Signed informed consent was obtained from each participant or their parent/guardian prior to collecting any data.
Participants included in this study were ≥18 years old. Monohydroxy PAH metabolites (OH-PAHs) were measured in a subsample of participants from four survey cycles: 2005–2006, 2007–2008, 2009–2010 and 2011–2012. Totally 9064 participants had all valid measurements of OH-PAHs, urinary creatinine (UCre) and sCOT. VOC metabolites were measured in a subsample of participants from two survey cycles: 2005–2006 and 2011–2012. Totally, 5407 participants had all valid measurements of VOC metabolites, UCre and sCOT. Urinary thiocyanate (SCN) was measured in participants from four survey cycles: 2005–2006, 2007–2008, 2009–2010 and 2011–2012. Totally, 19,065 participants had all valid measurements of SCN, UCre and sCOT. Urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (tNNAL) was measured in participants from three survey cycles: 2007–2008, 2009–2010 and 2011–2012. Totally, 20,679 participants had all valid measurements of tNNAL, UCre and sCOT.
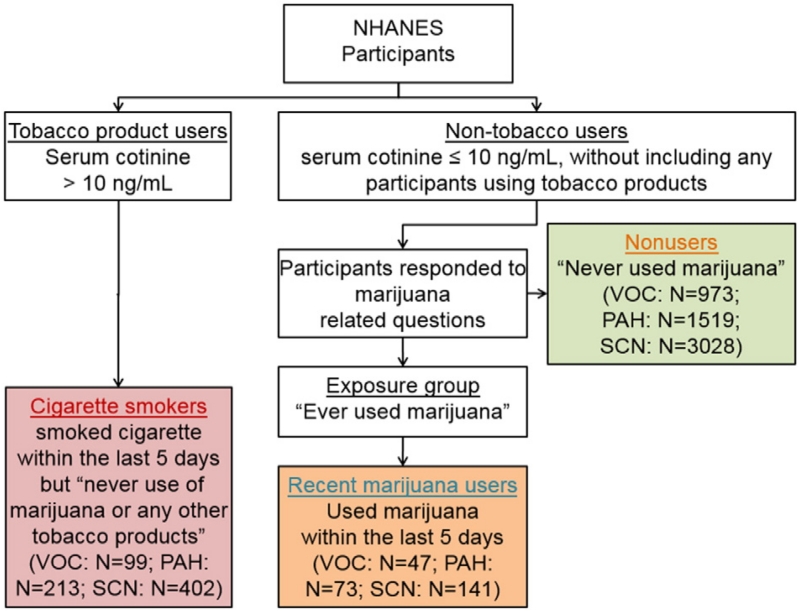
Study participants were classified as non-tobacco users if their sCOT levels were ≤10 ng/mL (Pirkle et al., 1996, 2006), and they reported not using any tobacco or nicotine products (i.e., cigarette, cigar, pipe, snuff, chewing tobacco, nicotine patch) within the five days prior to their NHANES physical examination (Wei et al., under review, 2015b, 2015c). Among non-tobacco users, those who reported use of marijuana within the five days prior to examination were classified as recent marijuana users based on their responses to the question “last time used marijuana or hashish”, and those who reported never using marijuana were classified as nonusers if they responded “no” to the question “ever used marijuana or hashish”. Those participants who used marijuana at least once prior to examination but not within the last five days were not included in either of those two groups. Exclusive cigarette smokers were identified if their sCOT was >10 ng/mL, and they self-reported cigarette smoking within the five days prior to examination, but neither used marijuana nor any other tobacco products. Owing to the lack of the information regarding to how marijuana products were consumed by the participants, we were unable to conduct a ‘fine’ classification among marijuana users into groups such as “marijuana smokers or users of edibles”. Fig. 1 shows the classification tree for nonusers of marijuana or tobacco, recent marijuana users and cigarette users, and their sample size characteristics are given in Table 1.
Fig. 1.
Category chart for identifying nonusers, recent marijuana users and cigarette smokers. Urinary PAH metabolite concentrations were available in four NHANES cycles: 2005–2006, 2007–2008, 2009–2010 and 2011–2012. Urinary VOC metabolite concentrations were available in two NHANES cycles: 2005–2006 and 2011–2012. Urinary thiocyanate concentration was available in four NHANES cycles: 2005–2006, 2007–2008, 2009–2010 and 2011–2012. Active exclusive cigarette smokers were selected if their sCOT was >10 ng/mL, and they self-reported cigarette smoking within the five days prior to examination, but neither used marijuana nor any other tobacco products.
Table 1.
Sample size characteristics of nonusers, recent marijuana users and cigarette smokers.
| Nonuser |
Marijuana usera |
Cigarette smoker |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VOC | PAH | SCN | VOC | PAH | SCN | VOC | PAH | SCN | |
| All | 973 | 1519 | 3028 | 47 | 73 | 141 | 99 | 213 | 402 |
| Gender | |||||||||
| Male | 377 | 636 | 1150 | 35 | 48 | 91 | 65 | 122 | 222 |
| Female | 596 | 883 | 1878 | 12 | 25 | 50 | 34 | 91 | 180 |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 281 | 424 | 931 | 30 | 40 | 82 | 40 | 88 | 167 |
| Non-Hispanic Black | 207 | 310 | 573 | 8 | 15 | 26 | 22 | 34 | 64 |
| Mexican American | 241 | 382 | 886 | 8 | 10 | 22 | 28 | 54 | 114 |
| Others | 244 | 403 | 638 | 1 | 8 | 11 | 9 | 37 | 57 |
| Age (year) | |||||||||
| 18–45 | 702 | 1045 | 2073 | 33 | 56 | 104 | 52 | 118 | 218 |
| ≥46 | 271 | 474 | 955 | 14 | 17 | 37 | 47 | 95 | 184 |
Participants used marijuana within 5 days prior to their NHANES physical examination.
2.2. Laboratory measurements
We measured urinary VOC metabolites using ultra-high performance liquid chromatography (UPLC) coupled with electrospray ionization (ESI) tandem mass spectrometry (UPLC-ESI/MSMS) with limits of detection (LOD) ranging from 0.5 to 20 nanograms per milliliter (ng/mL) (Alwis et al., 2012). We measured urinary SCN using ion chromatography coupled with ESI-MS/MS with a LOD of 20 ng/mL (Blount and Valentin-Blasini, 2006; Valentín-Blasini et al., 2007). We measured urinary OH-PAHs using isotope-dilution gas chromatography (GC) coupled with MS or MS/MS with LODs in the range of 1.0–19 picograms per milliliter (pg/mL) (Li et al., 2006, 2014). We measured sCOT using HPLC coupled with atmospheric ionization (API)–MS/MS with a LOD of 0.015 ng/mL for all NHANES participants (Bernert et al., 2000). We measured urinary tNNAL, a major metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), using HPLC-ESI-MS/MS method with a LOD of 0.6 pg/mL (Xia et al., 2005). Urinary creatinine concentration was measured using a colorimetric method based on Jaffé rate reaction.
Biological samples were collected, shipped and stored according to strict quality assurance and quality control (QA/QC) rules that involved both internal and external surveillance. For example, all collection materials, vacuum sample vials, and storage containers used were prescreened for background contamination levels (US-CDC, 2014a, 2014b). Laboratory blanks and QC samples were also simultaneously processed and analyzed to assure the quality of the analytical results to meet the accuracy and precision specification of the QC/QA program of Division of Laboratory Sciences at the US CDC (Caudill et al., 2008).
2.3. Statistical analysis
Statistical analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA) and SUDAAN (version 11.0.0; RTI International, Research Triangle Park, NC, USA). Because we combined multiple survey cycles, we first merged the data regarding biomarker concentrations and those tobacco/marijuana associated questionnaire data, and then calculated new sample weights for each participant according to the recommendations of the NCHS (US-CDC, 2014a, 2014b). Briefly, new sample weights equal to 1/n of the sample weights provided in the NHANES, where n refers to the total number of release cycles in which the biomarker concentrations were available. In all of our analyses, statistics were adjusted for the new sampling weights and the nonrandom sampling design.
Both VOC and PAH metabolites were measured in participants from a one-third sample of all participants while serum cotinine was measured in all participants. Owing to the generally low response rates to marijuana related questions, we were able to identify 47 and 73 recent marijuana users (use of marijuana within the last 5 days prior to their NHANES physical examination) who had valid measurements of sCOT, urinary creatinine (UCre), VOC and PAH metabolites, respectively. This limited number of samples did not allow us to systematically examine the associations between urinary measurements and demographic (i.e. race and gender) or socioeconomic (i.e. income and education) variables, but rather to evaluate whether the overall differences between marijuana user and nonuser categories were significant on the basis of expected large differences in biomarker levels between these two groups. Nevertheless, in order to compare biomarker levels among marijuana users and nonusers, sample weighted linear regression models were used to estimate least-squares geometric means (lsGMs) and 95% confidence intervals (95% CI) from log10 transformed urinary biomarker concentrations, with log10-sCOT and log10-UCre as covariates. Log10-sCOT was included to account for potential confounding from exposure to secondhand tobacco smoke, and log10-UCre was included to account for variation arising from urine dilutions among spot samples (Barr et al., 2005). The term “adjusted” GMs was used to refer to lsGMs throughout the manuscript. In addition, concentrations below the LOD were substituted with the LOD divided by the square root of two. Statistical analysis was confined to measurements with a frequency of detection greater than 60% to avoid undue influence on the estimates caused by imputed values. In all cases, using Satterwaite-adjusted F statistics, difference in least-squares means among different groups with a null hypothesis probability level of <0.05 was considered as statistical significance.
3. Results
OH-PAHs were detected in nearly all urine samples nonusers, recent marijuana users and cigarette smokers from NHANES 2005–2012 (Table 2). Most of the VOC metabolites were detected in ≥62% urine samples. Benzene metabolites, trans, trans-muconic acid (MU) and N-acetyl-S-(phenyl)-l-cysteine (PMA), were only detected in 51.2% and 29.5% respectively in urine samples from recent marijuana users. Cigarette smokers had higher detection rates for MU (69%) and PMA (43%).
Table 2.
Urinary metabolites of PAHs and VOCs, abbreviations and detection percentages. For the categories, refer to Fig. 1.
| Parent compound | Urine metabolite | Detection percentage (%) |
|||
|---|---|---|---|---|---|
| Abbreviation | Nonuser | Marijuana usera | Cigarette smoker | ||
| Fluorene | 2-Hydroxyfluorene | 2-OH-FLU | 100 | 100 | 100 |
| 3-Hydroxyfluorene | 3-OH-FLU | 100 | 100 | 100 | |
| 9-Hydroxyfluorene | 9-OH-FLU | 100 | 100 | 100 | |
| Naphthalene | 1-Hydroxynaphthalene | 1-OH-NAP | 99.9 | 100 | 100 |
| 2-Hydroxynaphthalene | 2-OH-NAP | 99.9 | 100 | 100 | |
| Phenanthrene | 1-Hydroxyphenanthrene | 1-OH-PHE | 100 | 100 | 100 |
| 2-Hydroxyphenanthrene | 2-OH-PHE | 100 | 100 | 100 | |
| 3-Hydroxyphenanthrene | 3-OH-PHE | 100 | 100 | 100 | |
| Pyrene | 1-Hydroxypyrene | 1-OH-PYR | 100 | 100 | 100 |
| Acrolein | N-acetyl-S-(3-hydroxypropyl)-l-cysteine | 3HPMA | 100 | 100 | 100 |
| N-acetyl-S-(2-carboxyethyl)-l-cysteine | CEMA | 97.4 | 97.8 | 100 | |
| Acrylamide | N-acetyl-S-(2-carbamoylethyl)-l-cysteine | AAMA | 98.4 | 100 | 100 |
| N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine | GAMA | 62.3 | 80.4 | 83.3 | |
| Acrylonitrile | N-acetyl-S-(2-cyanoethyl)-l-cysteine | CYMA | 85.8 | 100 | 100 |
| Benzene | trans, trans-Muconic acid | MU | 51.2 | 50.0 | 68.8 |
| N-acetyl-S-(phenyl)-l-cysteine | PMA | 29.5 | 28.3 | 42.7 | |
| 1,3-Butadiene | N-acetyl-S-(3,4-dihydroxybutyl)-l-cysteine | DHBMA | 99.6 | 100 | 100 |
| N-acetyl-S-(4-hydroxy-2-buten-1-yl)-l-cysteine | MHBMA3 | 95.5 | 95.7 | 100 | |
| Carbon-disulfide | 2-Thioxothiazolidine-4-carboxylic acid | TTCA | 66.7 | 80.4 | 74.0 |
| Crotonaldehyde | N-acetyl-S-(3-hydroxypropyl-1-methyl)-l-cysteine | HPMMA | 99.9 | 100 | 100 |
| Cyanide | Thiocyanate | SCN | 100 | 100 | 100 |
| N,N-dimethylformamide | N-acetyl-S-(N-methylcarbamoyl)-l-cysteine | AMCC | 98.3 | 100 | 100 |
| Ethylbenzene, styrene | Phenylglyoxylic acid | PGA | 89.9 | 95.7 | 89.6 |
| Propylene oxide | N-acetyl-S-(2-hydroxypropyl)-l-cysteine | 2HPMA | 97.1 | 100 | 100 |
| Styrene | Mandelic acid | MA | 99.3 | 95.7 | 97.9 |
| Toluene | N-acetyl-S-(benzyl)-l-cysteine | BMA | 99.2 | 100 | 100 |
| Xylene | 2-Methylhippuric acid | 2MHA | 93.9 | 91.3 | 100 |
| 3-Methylhippuric acid + 4-Methylhippuric acid | 3MHA + 4MHA | 99.2 | 100 | 100 | |
Participants used marijuana within 5 days prior to their NHANES physical examination.
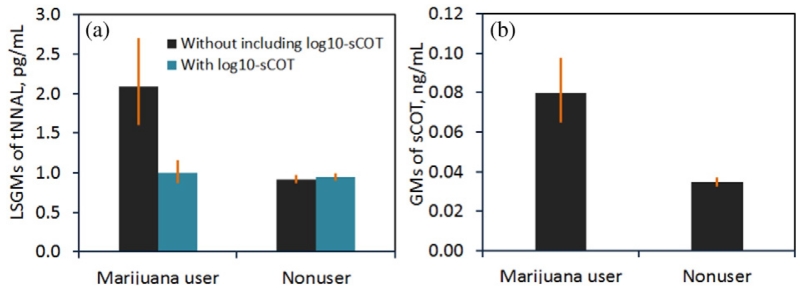
Fig. 2 shows GMs of tNNAL concentrations among different marijuana use status groups. Without including log10-sCOT in the regression analysis, we found recent marijuana users had a significantly higher adjusted GM of tNNAL (2.13 pg/mL) than nonusers (0.96 pg/mL). After adding log-sCOT in the regression analysis as a covariate, no significant statistical differences in adjusted GMs were observed between recent marijuana users and nonuser groups.
Fig. 2.
Tobacco specific biomarker measurements among recent marijuana users and nonusers. (a) Least square geometric means of urinary total NNAL (pg/mL) calculated with and without log10-transformed serum cotinine as one covariate in the multiple regression analysis. (b) Geometric means of serum cotinine among marijuana users and nonusers. Error bars indicate 95% confidence intervals. Urinary NNAL data was available in NHANES 2007–2012.
Table 3 presents the adjusted GMs for 9 OH-PAHs metabolized from naphthalene (NAP), fluorene (FLU), phenanthrene (PHE) and pyrene (PYR), using sample weighted linear regression models with log10-sCOT and log10-UCre as covariates. Compared to nonusers, recent marijuana users had significantly higher concentrations for 1-OH-NAP (p-value = 0.016), 1-OH-PYR (p = 0.002), 2-OH-FLU (p < 0.001), 3-OH-FLU (p < 0.001) and 3-OH-PHE (p = 0.011). Adjusted GMs of 2-OH-NAP, 1-OH-PHE and 2-OH-PHE were elevated more than 11% among recent marijuana users compared to nonusers, but their concentration levels were not statistically significantly different.
Table 3.
Adjusted geometric means (95% confidence interval) of urinary concentrations (pg/mL) of PAH metabolites in nonusers, marijuana users, and cigarette smokers. For the categories, refer to Fig. 1. Estimates were computed using sample weighted linear regression models with log10-sCOT and log10-UCre as covariates.
| PAH metabolite | Adjusted geometric means (95% CI), pg/mLa |
p-Valued | R 2 | ||
|---|---|---|---|---|---|
| Nonuser | Marijuana userb | Cigarette smokerc | |||
| 1-OH-NAP | 1498 (1351, 1661) | 2995 (1787, 5019) | 5926 (3550, 9892) | 0.016 | 0.29 |
| 2-OH-NAP | 3214 (3015, 3428) | 3661 (3037, 4413) | 9471 (6730, 13,328) | 0.233 | 0.44 |
| 2-OH-FLU | 199 (189, 209) | 322 (252, 411) | 581 (463, 729) | < 0.001 | 0.64 |
| 3-OH-FLU | 71.4 (67.7, 75.4) | 138 (111, 172) | 255 (198, 330) | < 0.001 | 0.65 |
| 9-OH-FLU | 254 (239, 270) | 246 (203, 298) | 300 (216, 417) | 0.732 | 0.45 |
| 1-OH-PHE | 125 (119, 132) | 162 (128, 205) | 162 (123, 212) | 0.057 | 0.44 |
| 2-OH-PHE | 61 (58.3, 63.9) | 68 (55.2, 83.8) | 71 (55.5, 90.9) | 0.349 | 0.46 |
| 3-OH-PHE | 68.3 (65, 71.9) | 91.8 (74.8, 113) | 97.1 (75.6, 125) | 0.011 | 0.50 |
| 1-OH-PYR | 95.8 (90.9, 101) | 157 (119, 206) | 127 (95, 168) | 0.002 | 0.45 |
Abbreviations: CI — confidence interval; R2 — coefficient of determination.
Sample sizes for nonusers, recent marijuana users, cigarette users were 1519, 73, and 213, respectively.
Participants used marijuana within 5 days prior to their NHANES physical examination.
Exclusive cigarette smokers were selected if their sCOT was >10 ng/mL, and they self-reported cigarette smoking within the five days prior to examination, but neither used marijuana nor any other tobacco products.
p-Values between recent marijuana user and nonusers using Satterwaite-adjusted F test.
Table 4 presents the adjusted GMs for those VOC metabolites with detection rates above 60%. Recent marijuana users had significantly higher urinary metabolite concentrations of acrylamide (p < 0.001), acrylonitrile (p < 0.001), 1,3-butadiene (MHBMA3, p = 0.037), and cyanide (SCN, p < 0.001), than did nonusers. Urinary metabolites of crotonaldehyde (4%), propylene oxide (1%), styrene (3%), and xylene (1–15%) were elevated among recent marijuana users compared with nonusers, but these increases were not statistically significant (p-values from 0.07–0.73). Compared with nonusers, the highest increase (approximately 13-fold) was observed for N-acetyl-S-(2-cyanoethyl)-l-cysteine (CYMA, a urinary metabolite of acrylonitrile) in recent marijuana users.
Table 4.
Adjusted geometric means of VOC metabolites among nonusers, marijuana users, and cigarette users. For the categories, refer to Fig. 1. Estimates were computed using sample weighted linear regression models with log10-sCOT and log10-UCre as covariates.
| VOC metabolite | Adjust geometric means (95% CI), ng/mLa |
p-Valuee | R 2 | ||
|---|---|---|---|---|---|
| Non-user | Marijuana userb | Cigarette smokerd | |||
| CEMA | 77.5 (73.4, 81.8) | 82.7 (65.5, 105) | 118 (78.7, 176) | 0.629 | 0.52 |
| 3HPMA | 241 (228, 255) | 274 (229, 328) | 642 (429, 960) | 0.213 | 0.50 |
| AAMA | 40.2 (37.6, 42.9) | 97.1 (72.7, 130) | 67.2 (46.8, 96.7) | <0.001 | 0.52 |
| GAMA | 15.1 (14.1, 16.2) | 29.2 (20.7, 41.1) | 25.1 (18.4, 34.3) | 0.002 | 0.43 |
| CYMA | 1.44 (1.34, 1.56) | 15.4 (8.15, 29) | 24 (15.0, 38.5) | <0.001 | 0.77 |
| DHBMA | 221 (207, 235) | 252 (221, 287) | 257 (195, 339) | 0.070 | 0.66 |
| MHBMA3 | 4.85 (4.44, 5.30) | 7.06 (5.08, 9.81) | 32.4 (18.8, 56) | 0.037 | 0.52 |
| HPMMA | 303 (287, 320) | 316 (261, 383) | 1180 (789, 1766) | 0.689 | 0.56 |
| SCNc | 850 (799, 904) | 1682 (1437, 1969) | 2957 (2364, 3698) | <0.001 | 0.44 |
| AMCC | 84.4 (76.2, 93.4) | 107 (81.4, 141) | 340 (215, 539) | 0.138 | 0.46 |
| PGA | 116 (106, 128) | 139 (105, 183) | 152 (90, 256) | 0.180 | 0.37 |
| 2HPMA | 46.4 (42.6, 50.6) | 49 (39.8, 60.3) | 116 (74.5, 180) | 0.633 | 0.28 |
| MA | 135 (127, 144) | 143 (116, 176) | 221 (143, 343) | 0.645 | 0.55 |
| BMA | 6.60 (6.10, 7.14) | 6.15 (4.53, 8.36) | 6.49 (4.07, 10.4) | 0.678 | 0.35 |
| 2MHA | 27.3 (24.6, 30.4) | 30.3 (21.5, 42.8) | 85.8 (50.7, 145) | 0.612 | 0.29 |
| 3MHA + 4MHA | 174 (157, 194) | 221 (172, 284) | 631 (406, 980) | 0.152 | 0.41 |
| TTCA | 8.64 (7.54, 9.89) | 9.32 (6.21, 14.0) | 4.93 (2.47, 9.87) | 0.730 | 0.13 |
Abbreviations: CI — confidence interval; R2 — coefficient of determination.
Sample sizes for nonusers, recent marijuana users, and cigarette users except thiocyanate were 973,47, and 99, respectively.
Participants used marijuana within 5 days prior to their NHANES physical examination.
Sample sizes of thiocyanate for nonuser, marijuana user, and cigarette user were 3028, 141, and 402, respectively.
Exclusive cigarette smokers were selected if their sCOT was >10 ng/mL, and they self-reported cigarette smoking within the five days prior to examination, but neither used marijuana nor any other tobacco products.
p-Values between recent marijuana user and nonusers using Satterwaite-adjusted F test.
4. Discussion
We observed higher levels of many potentially toxic by-products of combustion (PAHs and VOCs) in recent marijuana users compared to nonusers in the present study. To our knowledge, this is the first examination to date of body burdens of harmful organic compounds in self-reported exclusive marijuana users who participated in NHANES. These findings suggest that further studies are needed to evaluate the potential health risks to humans from the exposure to these agents when smoking marijuana.
In order to obtain exclusive samples to evaluate the exposure to marijuana, we excluded the participants if they had either sCOT >10 ng/mL (Pirkle et al., 1996, 2006) or self-reported using any tobacco products (i.e., cigarette, cigar, pipe, snuff, chewing tobacco, nicotine patch) at the time of the survey. However, we still observed higher GMs of tNNAL and sCOT in recent marijuana users compared with nonusers. Since both NNAL and COT are tobacco-specific biomarkers (Hecht et al., 2008; Wei et al., 2014), this finding suggests that recent marijuana users were likely co-exposed to secondhand tobacco smoke at the time when the surveys were conducted. It was also plausible that some marijuana users might add tobacco to marijuana to assist burning when marijuana is smoked (Hall and Degenhardt, 2009). SCOT has been measured in every survey cycle while tNNAL was not available before 2007 (Bernert et al., 2010; Wei et al., under review, 2015b, 2015c), we thus included log10-sCOT as a covariate in the regression analysis to account for the confounding from tobacco smoke. Equal adjusted GMs of tNNAL between recent marijuana users and nonusers (Fig. 2) indicates the effectiveness of this approach.
Previous studies have documented PAHs from tobacco combustion, but few studies have characterized PAH levels in marijuana smoke. Under controlled laboratory conditions, Moir et al. (2008) measured PAH concentrations in both mainstream and sidestream marijuana smoke, and they found the pattern of chemicals in tobacco smoke was similar to that in marijuana smoke. Using the biomonitoring data, we found that recent marijuana users had significantly higher adjusted GMs of 1-OH-NAP, 2- and 3-OH-FLU, 3-OH-PHE and 1-OH-PYR than did nonusers (Fig. 3 and Table 3). These results confirm that marijuana smoke is an important source of exposure to PAHs.
Fig. 3.
Adjusted geometric means (GMs) of urinary concentrations of thiocyanate, metabolites of acrylamide, acrylonitrile and pyrene among nonusers, recent marijuana users (MJ user), and cigarette smokers (Cig. Smoker). Categories refer to Fig. 1. Estimates were computed using sample weighted linear regression models with log10-sCOT and log10-Cre as covariates. Error bars indicate 95% confidence intervals.
OH-PAH levels in marijuana users were generally lower compared with cigarette smokers, which was consist with the earlier report of lower PAH concentrations in mainstream marijuana smoke compared with mainstream tobacco smoke (Moir et al., 2008), except for 1-OH-Pyre. We observed elevated adjusted GM level of 1-OH-PYR in exclusive marijuana users than in cigarette users although the difference was not statistically significant. This difference could be attributed to the variations resulted from different sampling duration since last substance (cigarette or marijuana) use. Small sample size for marijuana users could also introduce large variation. Future studies are needed to better characterize biomarker patterns among substance user groups, while accounting for such factors as use frequency and amount of product consumed.
Significantly higher adjusted GMs for many urinary VOC metabolites in recent marijuana users than in nonusers suggest that marijuana smoke is also an important source of exposure to a number of toxic VOCs. Recent marijuana users had slightly elevated adjusted GMs of urinary TTCA (metabolite of CS2). A previous study reported that each commercial cigarette and marijuana cigarette could deliver approximately 2 μg CS2 in mainstream smoke (Horton and Guerin, 1974), but caution should be used when reviewing these data as Pankow et al. (2004) reported higher CS2 concentration in mainstream tobacco smoke. Furthermore, no recent data is available for the concentrations of CS2 in marijuana smoke. Other factors that could plausibly explain these concentration differences include planting environment/soil characteristics, fertilizers used, product storage, smoking conditions and other exposure sources such as diet.
Adjusted GMs of urinary CYMA (metabolite of acrylonitrile), MHBMA3 (metabolite of 1,3 butadiene) in recent marijuana users were nearly 13 times and 1.5 times of those in nonusers, respectively, but significantly lower than in cigarette users. In contrast, Moir et al. (2008) found higher concentrations of acrylonitrile and 1,3-butadiene in both mainstream and sidestream marijuana smoke compared with those in tobacco smoke. The best plausible explanation for this observation, similar to that of the OH-PAHs, could be the lower frequency of marijuana smoking and the smaller amount of product consumed by marijuana users (Jamal et al., 2014; SAMHSA, 2014).
Most PAHs and VOCs are ubiquitous in the environment and none of them are marijuana-specific compounds. Other sources, such as vehicle exhaust, coal combustion and diet, could be important exposure sources (Li et al., 2008; Alwis et al., 2012). These sources could ‘dilute’ the contributions to the body burdens from marijuana smoke especially when marijuana was infrequently used and the amount consumed was small. In addition, some VOC metabolites, i.e. BMA, can be formed from multiple sources (Lovreglio et al., 2010). For example, benzyl alcohol, a widespread constituent in cosmetic products can be metabolized into BMA. Other factors that cannot be ruled out include differences in the absorption, distribution, metabolism and excretion mechanisms of the chemicals in human body. Collectively, these factors might explain the inconsistent statistical results for the multiple metabolites of fluorene and phenanthrene.
Our findings should be interpreted in the context of several limitations. The sparsity of marijuana users precluded demographical analysis and comprehensive exposure characterization but it was still possible to evaluate differences in biomarker levels between marijuana users and nonuser categories. Although screened from a representative sample of the general US population, owing to small sample sizes, the results presented in this study could not reflect overall marijuana exposure characteristics in the US population, but rather be informative to plan future systematic assessment of exposure to marijuana use. Due to the ubiquity of PAHs and VOCs in environment, this study assumed that contributions from background sources (i.e. vehicle exhaust, gas/oil/coal, wood smoke, dietary, etc.) occurred at comparable levels across different categories. Comparison of biomarker levels between cigarette smokers and marijuana users was also limited and requires further exploration by including detailed product use information (i.e. use frequency and amount) in the regression analysis. Characterizing exposure patterns among persons using multiple combustion products are also needed. Higher concentrations for many PAHs and VOCs in sidestream marijuana smoke compared to sidestream tobacco smoke (Moir et al., 2008) suggest higher potential risks of persons being exposed to harmful constituents in secondhand marijuana smoke (SHMS) than secondhand tobacco smoke under comparable smoking and environmental conditions. Unfortunately, neither laboratory measurements nor the questionnaire data from NHANES surveys are available to identify the respondents exposed to SHMS. Furthermore, a highly accurate and sensitive laboratory method (Wei et al., 2015c), measuring marijuana-specific psychoactive constituents, such as Δ9-THC, and their metabolites in biological matrices, would be helpful to assess actual exposure to both firsthand and secondhand marijuana smoke in future studies.
5. Conclusions
This study reported for the first time identified higher levels of many potentially toxic by-products of combustion (PAHs and VOCs) among recent marijuana users than nonusers. These findings suggest that further studies are needed to evaluate potential health risks to humans from the exposure to these agents when smoking marijuana. Meanwhile, future work, such as assessing exposure characteristics and health risks in large-scale marijuana users using a sensitive biomonitoring method for measuring marijuana-specific cannabinoids and their metabolites are recommended for a better evaluation of marijuana smoke exposure.
Acknowledgment
We thank Rey Decastro at the US CDC for his thoughtful input into the statistical analysis in this article.
Footnotes
Competing financial interests
The authors declare no actual or potential competing financial interests.
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
References
- Aldington S, Harwood M, Cox B, Weatherall M, Beckert L, Hansell A, Pritchard A, Robinson G, Beasley R. Cannabis use and risk of lung cancer: a case–control study. Eur. Respir. J. 2008;31(2):280–286. doi: 10.1183/09031936.00065707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary voc metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS) Anal. Chim. Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113(2):192. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J. Anal. Toxicol. 2000;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the US population from secondhand smoke exposure. Cancer Epidemiol. Biomark. 2010;19(11):2969–2977. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- Berthiller J, Straif K, Boniol M, Voirin N, Benhaïm-Luzon V, Ayoub WB, Dari I, Laouamri S, Hamdi-Cherif M, Bartal M. Cannabis smoking and risk of lung cancer in men: a pooled analysis of three studies in Maghreb. J. Thorac. Oncol. 2008;3(12):1398–1403. doi: 10.1097/JTO.0b013e31818ddcde. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LLB, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL. Cerebellar hypoactivity in frequent marijuana users. Neuroreport. 2000;11(4):749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Blount BC, Valentin-Blasini L. Analysis of perchlorate, thiocyanate, nitrate and iodide in human amniotic fluid using ion chromatography and electrospray tandem mass spectrometry. Anal. Chim. Acta. 2006;567(1):87–93. doi: 10.1016/j.aca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Brown HL, Graves CR. Smoking and marijuana use in pregnancy. Clin. Obstet. Gynecol. 2013;56(1):107–113. doi: 10.1097/GRF.0b013e318282377d. [DOI] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat. Med. 2008;27(20):4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- Chait L, Pierri J. Some physical characteristics of Nida marijuana cigarettes. Addict. Behav. 1989;14(1):61–67. doi: 10.1016/0306-4603(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Clark A, Ware M, Yazer E, Murray T, Lynch M. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62(11):2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- English D, Hulse G, Milne E, Holman C, Bower C. Maternal cannabis use and birth weight: a meta-analysis. Addiction. 1997;92(11):1553–1560. [PubMed] [Google Scholar]
- Fehr KOB, Kalant H. Analysis of cannabis smoke obtained under different combustion conditions. Can. J. Physiol. Pharmacol. 1972;50(8):761–767. doi: 10.1139/y72-111. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109(1):21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin. Chem. 2010;56(9):1442–1450. doi: 10.1373/clinchem.2010.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Medical marijuana initiatives. CNS Drugs. 2003;17(10):689–697. doi: 10.2165/00023210-200317100-00001. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Stepanov I, Jensen J, Anderson A, Hatsukami DK. Metabolism of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to its biomarker total nnal in smokeless tobacco users. Cancer Epidemiol. Biomark. 2008;17(3):732–735. doi: 10.1158/1055-9965.EPI-07-2843. [DOI] [PubMed] [Google Scholar]
- Horton A, Guerin M. Quantitative determination of sulfur compounds in the gas phase of cigarette smoke. J. Chromatogr. A. 1974;90(1):63–70. doi: 10.1016/s0021-9673(01)94774-0. [DOI] [PubMed] [Google Scholar]
- Huestis MA. Cannabis(marijuana) — effects on human behavior and performance. Forensic Sci. Rev. 2002;14(1):15–60. [PubMed] [Google Scholar]
- Huizink A. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;52:45–52. doi: 10.1016/j.pnpbp.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. [accessed on June 30, 2015];Current cigarette smoking among adults — United States, 2005–2013. MMWR. 2014 63(47):1108–1112. Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a4.htm. [PMC free article] [PubMed] [Google Scholar]
- Jones RT. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002;42(S1):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Novotny M, Bartle K. Gas chromatography/mass spectrometric and nuclear magnetic resonance spectrometric studies of carcinogenic polynuclear aromatic hydrocarbons in tobacco and marijuana smoke condensates. Anal. Chem. 1976;48(2):405–416. doi: 10.1021/ac60366a048. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, Patterson DG, Sjödin A. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid–liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal. Chem. 2006;78(16):5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Pittman EN, Hilton D, Hubbard K, Carmichael H, Parker J, Calafat AM, Sjödin A. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal. Bioanal. Chem. 2014;406:3119–3129. doi: 10.1007/s00216-014-7676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008;107(3):320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Lovreglio P, Barbieri A, Carrieri M, Sabatini L, Fracasso ME, Doria D, Drago I, Basso A, D’Errico MN, Bartolucci GB. Validity of new biomarkers of internal dose for use in the biological monitoring of occupational and environmental exposure to low concentrations of benzene and toluene. Int. Arch. Occup. Environ. Health. 2010;83(3):341–356. doi: 10.1007/s00420-009-0469-7. [DOI] [PubMed] [Google Scholar]
- Maertens RM, White PA, Rickert W, Levasseur G, Douglas GR, Bellier PV, McNamee JP, Thuppal V, Walker M, Desjardins S. The genotoxicity of mainstream and sidestream marijuana and tobacco smoke condensates. Chem. Res. Toxicol. 2009;22(8):1406–1414. doi: 10.1021/tx9000286. [DOI] [PubMed] [Google Scholar]
- Mark K, Desai A, Terplan M. Marijuana use and pregnancy: prevalence, associated characteristics, and birth outcomes. Arch. Womens Ment. Health. 2015:1–7. doi: 10.1007/s00737-015-0529-9. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103(23):2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat. Rev. Neurosci. 2007;8(11):885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Pankow JF, Luo W, Tavakoli AD, Chen C, Isabelle LM. Delivery levels and behavior of 1,3-butadiene, acrylonitrile, benzene, and other toxic volatile organic compounds in mainstream tobacco smoke from two brands of commercial cigarettes. Chem. Res. Toxicol. 2004;17(6):805–813. doi: 10.1021/tx0342316. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Bernert JT, Caudill SA, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke: 1988–2002. Environ. Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke — the third national health and nutrition examination survey, 1988 to 1991. JAMA J. Am. Med. Assoc. 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- Rickert WS, Robinson J, Rogers B. A comparison of tar, carbon monoxide and ph levels in smoke from marihuana and tobacco cigarettes. Can. J. Public Health. 1982;73(6):386. [PubMed] [Google Scholar]
- SAMHSA [Accessed on: June 16, 2015];Results from the 2013 national survey on drug use and health: summary of national findings. 2014 Available from http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf.
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, US, 2014. Am. J. Prev. Med. 2015 doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Fergusson DM, Milne BJ, Horwood LJ, Moffitt TE, Sears MR, Poulton R. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97(8):1055–1061. doi: 10.1046/j.1360-0443.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch. Intern. Med. 2007;167(3):221–228. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC . World Drug Report. United Nations Office on Drugs and Crime; [accessed on June 14, 2015]. 2014. Available from http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. [Google Scholar]
- US-CDC [Accesed on November 12, 2015];Survey design factors—sample design, weighting and variance estimation. 2013 Available from http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/intro.htm.
- US-CDC [Accessed on November 12, 2014];Biological specimens collection. 2014a Available from http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/labdoc_g.htm.
- US-CDC [Accessed on July 24th, 2015];The National Health and Nutrition Examination Survey (NHANES) analytic and reporting guidelines. 2014b Available from http://www.cdc.gov/nchs/nhanes/survey_methods.htm.
- Valentín-Blasini L, Blount BC, Delinsky A. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J. Chromatogr. A. 2007;1155(1):40–46. doi: 10.1016/j.chroma.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Ware M, Adams H, Guy G. The medicinal use of cannabis in the UK: results of a nationwide survey. Int. J. Clin. Pract. 2005;59(3):291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- Wei B, Bernert JT, Blount BC, Sosnoff CS, Wang L, Richter P, Pirkle JL. Temporal Trends of Secondhand Smoke Exposure: Nonsmoking Workers in the United States (NHANES 2001–2010) 2015a doi: 10.1289/EHP165. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Blount BC, Xia B, Wang L. Assessing exposure to tobacco-specific carcinogen NNK using its urinary metabolite NNAL measured in US population: 2011–2012. J. Expo. Sci. Environ. Epidemiol. 2015b doi: 10.1038/jes.2014.88. http://dx.doi.org/10.1038/jes.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, Wang L. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta. 2014;436:290–297. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Wang L, Blount BC. Analysis of cannabinoids and their metabolites in human urine. Anal. Chem. 2015c;87(20):10183–10187. doi: 10.1021/acs.analchem.5b02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, McGuffey JE, Bhattacharyya S, Sellergren B, Yilmaz E, Wang L, Bernert JT. Analysis of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal. Chem. 2005;77(23):7639–7645. doi: 10.1021/ac058027u. [DOI] [PubMed] [Google Scholar]