Significance
Pathological inflammation resulting from autoimmune, inflammatory, and infectious diseases remains the major cause of morbidity and mortality around the world. We explored the utility of nucleic acid-binding polymers to limit pathological activation of the innate immune response by scavenging nucleic acid-containing debris. We evaluated such polymers following local and systemic delivery for treatment of cutaneous and systemic lupus erythematosus in mice and observed dramatic therapeutic benefit. Remarkably, such treatment does not limit the ability of lupus-prone mice to combat influenza infection. Thus, by selectively targeting activators of the pathogenic immune response rather than the effectors of the innate immune system, nucleic acid scavengers are able to limit pathological inflammation without compromising one’s ability to fight a viral infection.
Keywords: nucleic acid, scavenger, autoimmunity, influenza, inflammation
Abstract
Nucleic acid-containing debris released from dead and dying cells can be recognized as damage-associated molecular patterns (DAMPs) or pattern-associated molecular patterns (PAMPs) by the innate immune system. Inappropriate activation of the innate immune response can engender pathological inflammation and autoimmune disease. To combat such diseases, major efforts have been made to therapeutically target the pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs) that recognize such DAMPs and PAMPs, or the downstream effector molecules they engender, to limit inflammation. Unfortunately, such strategies can limit the ability of the immune system to combat infection. Previously, we demonstrated that nucleic acid-binding polymers can act as molecular scavengers and limit the ability of artificial nucleic acid ligands to activate PRRs. Herein, we demonstrate that nucleic acid scavengers (NASs) can limit pathological inflammation and nucleic acid-associated autoimmunity in lupus-prone mice. Moreover, we observe that such NASs do not limit an animal’s ability to combat viral infection, but rather their administration improves survival when animals are challenged with lethal doses of influenza. These results indicate that molecules that scavenge extracellular nucleic acid debris represent potentially safer agents to control pathological inflammation associated with a wide range of autoimmune and infectious diseases.
Autoimmune disorders such as systemic lupus erythematosus (SLE) are characterized by increased production of antibodies (Abs) against self-nucleic acids and their associated proteins (1–4). A number of Toll-like receptors (TLRs) that assist in recognition of these nucleic acids have been individually targeted to slow and reverse disease progression (5, 6). These therapeutic strategies have shown an essential role for TLR targeting in combating inflammatory disorders; however, their effects, to date, have been modest, and new drugs remain to be tested (7–10).
Endosomal TLRs act as sensors of foreign RNA and DNA, and elicit an innate immune response to pathogens (11, 12). When tolerance is broken, these TLRs are aberrantly activated by self-nucleic acids, often associated with autoantibodies and immune complexes (13, 14). This, in turn, leads to increased downstream activation of signaling cascades and dysregulated expression of proinflammatory cytokines and autoantibodies (15). Despite our increased understanding of TLR biology and attempts to target these particular pathways, we have been unable to develop effective ways to address the source of antigen.
Inappropriate clearance of dying cells and elevated levels of serum RNA and DNA correlates with increased autoimmune disease pathology (16, 17). Studies have shown that targeting the free-circulating RNA and DNA using nucleases can be beneficial in dampening inappropriate TLR activation and improving autoimmune disease outcome (18, 19).
We have previously shown that nucleic acid-binding polymers can act as molecular scavengers and block the ability of artificial nucleic acids to activate TLRs (20–22). In this study, we directly tested the hypothesis that nucleic acid scavengers (NASs) can limit pathological inflammation during the course of autoimmunity, by absorbing free-circulating RNA and DNA or nucleic acid–protein complexes. We used two mouse models of SLE, NZBW F1 and MRLlpr, to assess immune activation and resolution of inflammation in the presence of NASs. Both mouse strains develop spontaneous SLE, which closely mimics clinical human SLE. In addition, we further explored the hypothesis that NASs deliver a therapeutic benefit during autoimmunity without compromising the organism’s ability to fight infections. To test this hypothesis, we used a PR8 influenza infection in the presence of NASs.
Here, we show that NASs are capable of controlling aberrant inflammation in two separate disease models. NAS treatments resulted in improved skin inflammation and also delayed systemic lupus progression. Additionally, we demonstrated that mice treated with NASs were capable of responding to pathogenic infections such as PR8 influenza. Moreover, NAS treatment of mice during a lethal PR8 influenza infection resulted in increased survival rates. It is important to note that our studies employed a widely used NAS: generation-3 PAMAM-G3, [NH2(CH2)2NH2]:(G=3);dendri PAMAM(NH2)32, a cationic polymer (MW6909) with a core of 1,4-diaminobutane. This molecule contains 32 surface amine groups, which allows for high-affinity binding of nucleic acids (23, 24), an important property that results in better NAS efficacy.
Taken together, these results suggest that targeting nucleic acids with NASs could present a therapeutic strategy not only for autoimmune disorders but also for treatment of dangerous acute inflammation.
Results
PAMAM-G3 Treatment of Lupus-Prone Mice (NZBW F1) Exhibiting Chronic Skin Inflammation Significantly Ameliorates Cutaneous Lupus Erythematosus-Like Phenotype.
We hypothesized that nucleic acid scavengers (NASs) might represent a useful treatment strategy for SLE because SLE is associated with anti-nucleic acid autoantibodies (25, 26). Therefore, we evaluated the therapeutic potential of the NAS PAMAM-G3 in NZBW F1 mice. These animals develop an autoimmune disease resembling human SLE and are characterized by high levels of circulating autoantibodies and pro inflammatory cytokines (27). First, to evaluate the potential utility of a NAS in a localized model of lupus erythematosus, we used a well-established murine tape-stripping–induced dermal injury model (28), which mimics cutaneous lupus erythematosus (CLE) in humans. To determine whether NASs can bind extracellular nucleic acids and thereby limit pathology following skin injury in CLE-prone mice, NZBW F1 animals were subjected to tape stripping and subsequently treated with generation 3 PAMAM (PAMAM-G3) subcutaneously (s.c.)
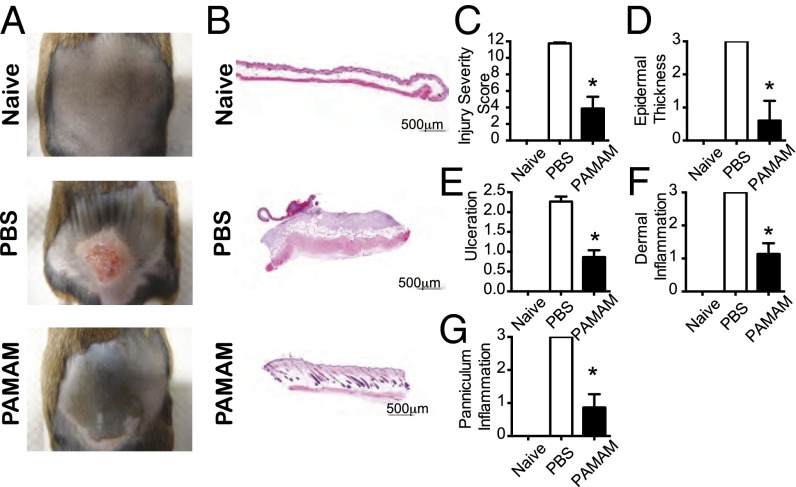
We chose to use PAMAM-G3 in these studies because it binds RNA and DNA with high affinity and blocks TLR activation by artificial nucleic acid agonists in vitro and in vivo (20–22). By contrast, lower-generation PAMAM molecules, with fewer than 32 surface amine groups, were not as effective as PAMAM-G3 at inhibiting CpG DNA and poly(I:C) RNA-mediated activation of TLRs. Higher-generation PAMAM molecules (e.g., PAMAM-G5), are as efficacious as PAMAM-G3 at inhibiting nucleic acid-mediated TLR activation but they are associated with increased toxicity, making it challenging to perform studies with them in lupus-prone mice (21). To choose a therapeutic dosing regimen, we first determined the maximum tolerated dose (MTD) of PAMAM-G3 in NZBW F1 mice (100–200 mg/kg) (21) and then administered this NAS 5- to 10-fold below the MTD. The CLE-prone mice were injured and then treated with PAMAM-G3 (20 mg/kg) twice per week for 14–21 d postinjury. Strikingly, s.c. administration of PAMAM-G3 allowed CLE-prone mice to recover from skin damage much more effectively than control animals (Fig. 1 A and B). Moreover, blinded histopathological analyses of skin samples demonstrated that CLE-prone mice treated with PAMAM-G3 displayed significantly lower disease grades in all pathological categories compared with PBS-treated counterparts (Fig. 1 C–G).
Fig. 1.
PAMAM-G3 treatment of lupus-prone mice (NZBW F1) exhibiting chronic skin inflammation significantly ameliorates CLE-like phenotype. (A) Gross appearance of skin lesions isolated 14 d after tape stripping. NZBW F1 mice treated with PBS (Middle) or PAMAM-G3 (20 mg/kg; Bottom) twice a week. Naïve mice (Top) were not subject to tape stripping. (B) Representative H&E histology of skin lesions 14 d after tape stripping. Data show representative sections from 30 mice. (Scale bars: 500 µm.) (C–G) Pathologic evaluation of skin lesions after tape stripping and PBS or PAMAM-G3 treatment. Data are representative of four independent experiments. n = 12. *P < 0.05.
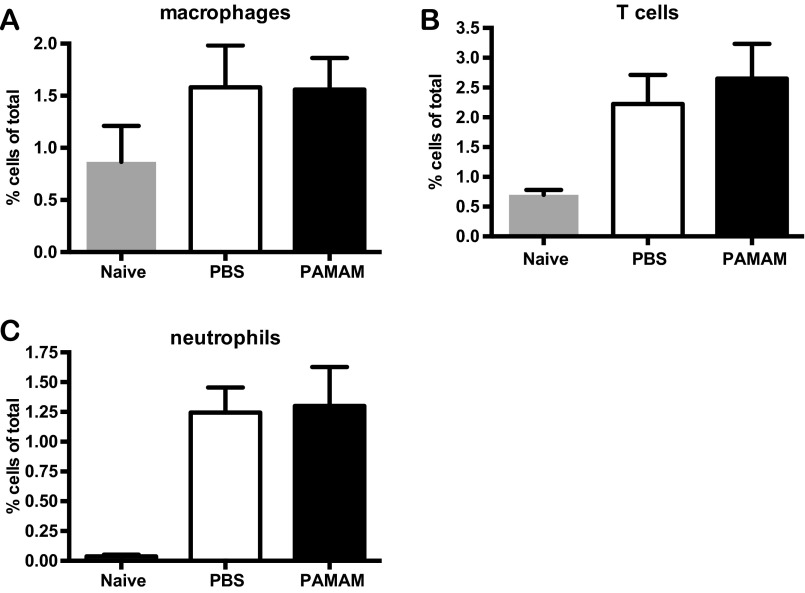
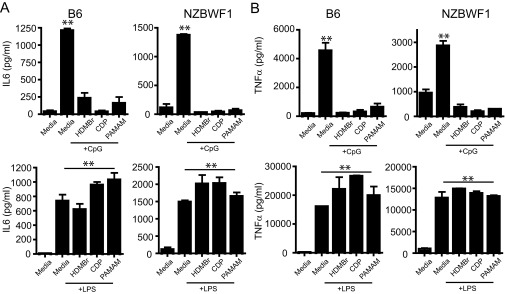
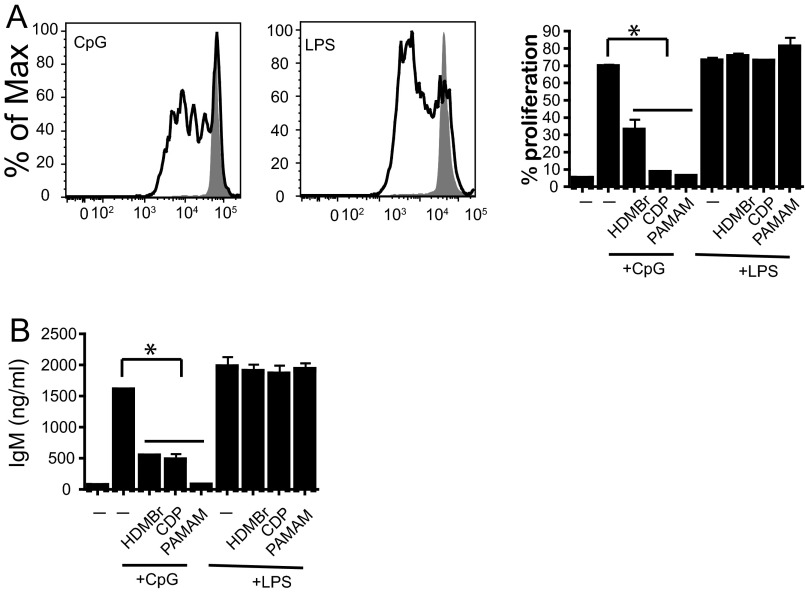
Previous studies in this CLE-prone mouse model have shown that skin damage leads to pronounced immune cell recruitment to the site of injury within 24 h and that the resulting skin inflammation is dependent on signaling through nucleic acid-sensing TLRs (TLR7 and TLR9) (28). Therefore, we evaluated whether immune cell recruitment is affected by NAS treatment. NZBW F1 skin samples were obtained 24 h after tape stripping and treatment with the NAS, and the presence of immune cell infiltrates was analyzed. As shown in Fig. S1 A–C, PAMAM-G3 treatment does not affect immune cell [macrophages, neutrophils, and T cells] infiltration into damaged skin. Therefore, because the inflammatory cells are present in the damaged skin, we next evaluated whether PAMAM-G3 could inhibit activation of the nucleic acid-sensing TLRs on inflammatory cells taken from the NZBW F1 CLE-prone mice compared with wild-type C57/BL6 animals. As shown in Fig. S2, NASs block TLR activation by nucleic acid agonists but not LPS in DCs isolated from NZBW F1 animals similarly to wild-type animals. Thus, NASs can block nucleic acid-driven immune cell activation and inflammatory cytokine production even in a genetic background that is predisposed to rapidly respond to inflammatory triggers (Fig. S2). However, TLR-independent NZBW F1 B-cell activation is unaltered by PAMAM-G3 (Fig. S3). Collectively, these skin damage studies indicate that local administration of NASs facilitates wound healing in a CLE-prone animal following injury by scavenging extracellular nucleic acids and reducing TLR7 and TLR9 activation (28, 29), and this inhibition in turn limits sustained aberrant inflammation.
Fig. S1.
PAMAM-G3 treatment of lupus-prone mice (NZBW F1) exhibiting chronic skin inflammation does not affect initial immune cell infiltration. Immune cell infiltrate in the skin of NZBW F1 mice was characterized before and 24 h after tape-stripping–induced inflammation in the absence and presence of PAMAM-G3, by flow cytometry. Cell percentages are compiled and graphed as macrophages (A), T cells (B), and neutrophils (C). Data are representative of 12 mice per group processed from three independent experiments.
Fig. S2.
NASs block TLR activation by nucleic acid agonists but not LPS in DCs isolated from NZBW F1 animals similarly to wild-type mice. (A and B) NASs block IL-6 and TNFα cytokine production during CpG but not LPS stimulation in both wild-type and lupus-prone (NZBW F1) mice. Bone marrow-derived dendritic cells (DCs) were cultured as previously described. DCs were then cultured in the presence of LPS and CpG as well as 20 µg/mL of each NAS (HDMBr, CDP, and PAMAM-G3). IL-6 and TNFα cytokine production was assessed 18 h later using ELISA. Data are representative of three independent experiments. n = 9 mice per group. **P < 0.01.
Fig. S3.
NASs regulate NZBW F1 B-cell proliferation and Ab production after nucleic acid stimulation. (A) NASs block B-cell proliferation induced by CpG but not LPS stimulation. NZBW F1 splenic B cells were isolated and carboxyfluorescein succinimidyl ester (CFSE) labeled. They were then cultured in the presence of LPS and CpG as well as 20 µg/mL HDMBr, CDP, or PAMAM-G3. Proliferation was assessed using CFSE dilution by flow cytometry. Flow plots show cell proliferation in the presence or absence of stimulation. Graph (Right) compiles proliferation data for stimulations in the presence or absence of NAS. Data are representative of three independent experiments. n = 9 mice per group. *P < 0.05. (B) Polymers block IgM Ab production post CpG but not LPS stimulation. B cells were cultured in the presence of CpG and LPS as well as 20 µg/mL HDMBr, CDP, or PAMAM polymer. Supernatants were collect 72 h poststimulation, and IgM levels were assessed via ELISA. Data are representative of three independent experiments. *P < 0.05.
Systemic PAMAM-G3 Treatment Reduces Glomerular Immune Complex Pathology and C3c Deposits in MRLlpr Mice.
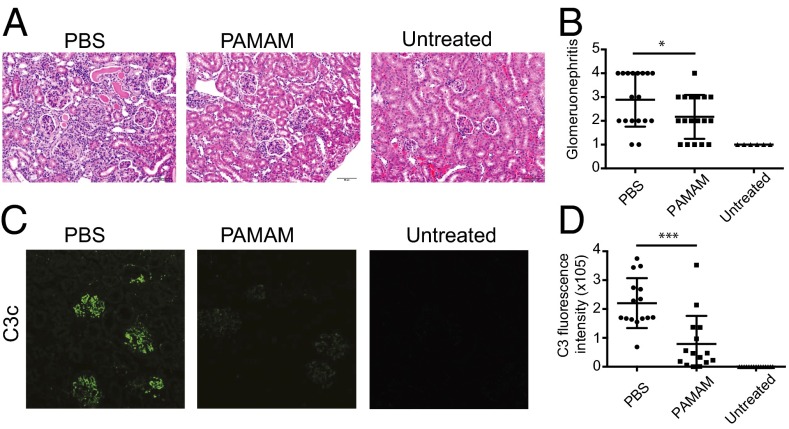
Next, we sought to determine whether NASs can be useful for treatment of chronic inflammatory disease in a murine model of SLE. We evaluated the ability of PAMAM-G3 to reduce glomerulonephritis and circulating autoantibody levels, hallmarks of SLE, in MRLlpr mice (30, 31). As MRLlpr mice spontaneously develop SLE over a few months, we started treating 10- to 12-wk-old male MRLlpr mice twice a week with an i.p. injection of PAMAM-G3 (20 mg/kg). After 10 wks of NAS treatment, SLE-prone mice were analyzed for kidney damage, immune complex deposition in the kidney, and levels of serum autoantibodies. As shown in Fig. 2, histological evaluation of kidney samples demonstrated that PAMAM-G3 treatment significantly reduces glomerulonephritis and kidney damage in MRLlpr mice at 5 mo of age (Fig. 2 A and B).
Fig. 2.
Systemic PAMAM-G3 treatment reduces glomerular immune complex pathology and C3c deposits in MRLlpr mice. (A) Representative H&E histology of paraffin-embedded renal sections from PBS and PAMAM-G3 MRLlpr treated mice as well as wild-type control (untreated) mice. Data show representative sections from 18 mice per group. (Scale bars: 50 µm.) (B) Glomerulonephritis evaluation of kidneys after PBS/PAMAM-G3 treatment. Data are from three independent experiments. Each circle represents one mouse. n = 18. *P < 0.05. (C) Frozen renal sections from PBS and PAMAM-G3 MRLlpr treated mice as well as wild-type control (untreated) mice were stained for complement factor C3c. Data show representative sections from 15 mice per group. Magnification, 20×. (D) Fluorescence intensity of the C3c staining was measured using ImageJ. Data are composites of three independent experiments. Each circle represents one mouse. n = 15. ***P < 0.001.
Because NAS treatment reduced kidney damage and glomerulonephritis, we next assessed how NAS treatment impacted complement deposition in the kidneys of MRLlpr mice. By 5 mo of age, immunofluorescent staining for complement (C3c) deposits revealed that all lupus-prone mice not treated with a NAS had developed C3c deposits throughout the glomerulus (Fig. 2C). By contrast, NAS treatment dramatically reduced deposition of complement C3c in the lupus-prone mice (Fig. 2 C and D). Collectively, these data show that NAS treatment can slow SLE development and glomerulonephritis in MRLlpr animals. These results highlight the potential utility of the NAS approach to treat chronic inflammatory diseases by limiting extracellular nucleic acid debris-induced chronic activation of proinflammatory signaling cascades and by breaking up nucleic acid-containing immune complexes to limit their deposition in the kidney or other organs (32, 33).
Systemic PAMAM-G3 Treatment Decreases Anti-Nuclear Abs and Anti-dsDNA Abs in MRLlpr Mice.
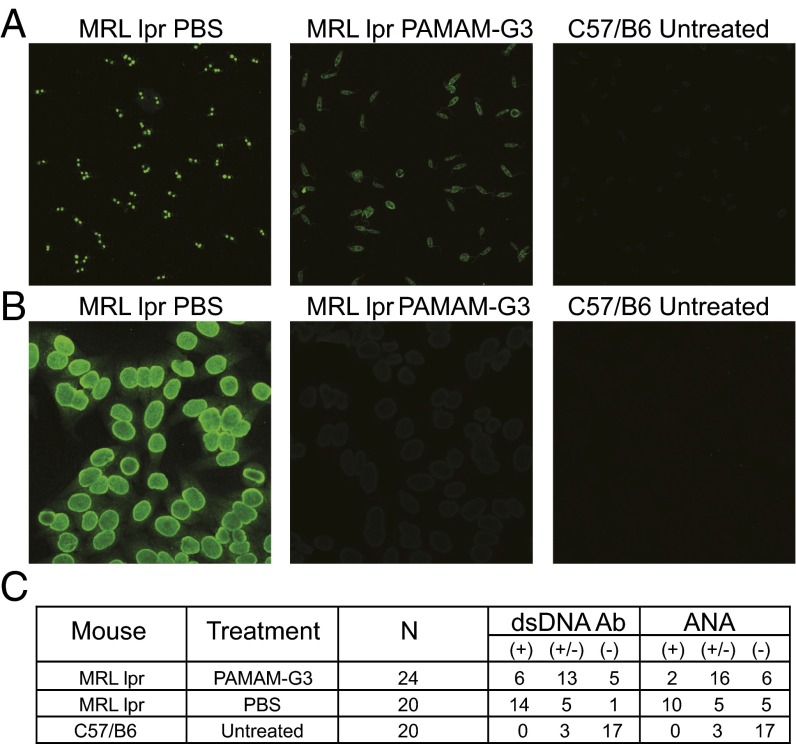
In addition to renal dysfunction, progression of SLE in the MRLlpr animals is characterized by increasing levels of circulating anti-nuclear and anti-DNA Abs, which further exacerbates autoimmunity in the lupus-prone animals (34). To determine whether long-term treatment with a NAS can impact the levels of anti-nuclear and anti-DNA Abs in aging animals prone to SLE, we treated MRLlpr mice with PAMAM-G3 (20 mg/kg twice a week) for 2.5 mo and performed ELISA and immunofluorescent assays on serum collected from the treated animals to determine autoantibody levels. First, we evaluated the effect of NAS treatment on anti-dsDNA Ab levels in lupus-prone mice using an indirect immunofluorescence assay on Crithidia luciliae substrates, a clinically relevant assay used for detection of dsDNA autoantibodies in patients with SLE. We observed high levels of anti-DNA Abs and intense staining when examining samples from PBS-treated mice but much less intense or even absent staining when evaluating serum from PAMAM-G3–treated lupus-prone mice (Fig. 3A). Next we performed a fluorescent anti-nuclear Ab (ANA) assay which is clinically relevant and the most sensitive approach to detect serum Abs against a variety of endogenous nuclear components in their native antigenic form (25). As shown in Fig. 3B, ANA levels are significantly reduced in NAS-treated lupus-prone mice as the staining intensity was significantly decreased, compared with PBS treated. We scored our slides based on an intensity scale (−, absent; +/−, low/intermediate; +/+, intense) (Fig. 3C).
Fig. 3.
Systemic PAMAM-G3 treatment decreases ANAs and anti-dsDNA Abs in MRLlpr mice. (A) Representative Crithidia luciliae kinetoplast DNA slides (anti-dsDNA Abs) from serum of 20-wk-old MRLlpr mice treated with PBS or PAMAM-G3 and 20-wk-old wild-type control (untreated) mice. (B) Representative HEp-2 ANA staining from serum of 20-wk-old MRLlpr mice treated with PBS or PAMAM-G3 and 20-wk-old wild-type control (untreated) mice. (C) Anti-dsDNA and HEp-2 Ab slides were scored for intensity of staining. Data are compiled in the table.
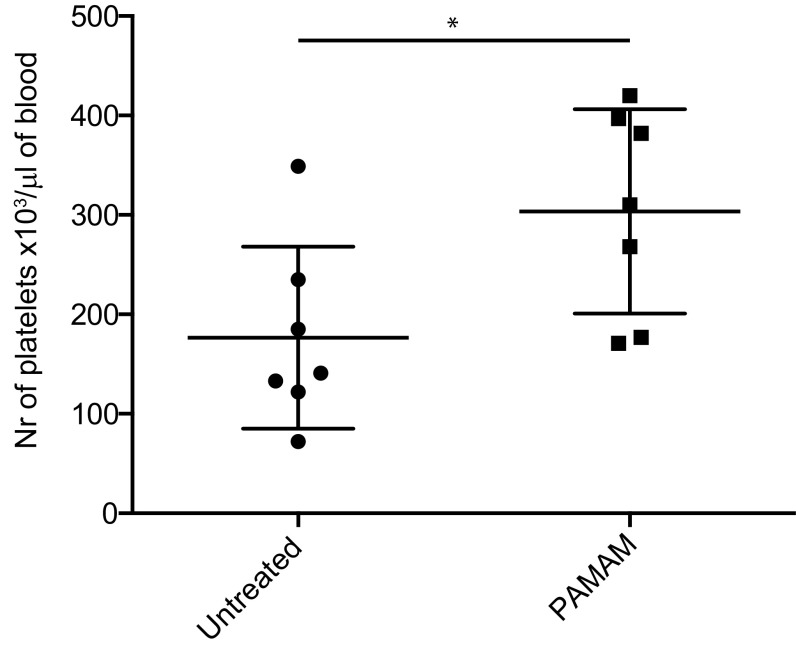
Autoimmune disorders, including SLE, are associated with decreased levels of circulating platelets (35, 36). We assessed circulating platelet counts in SLE animals after NAS treatment. As shown in Fig. S4, NAS treatment maintained platelet counts in the aging lupus-prone mice, whereas platelet counts significantly dropped in animals not treated with a NAS. This result indicates that, when NASs limit circulating levels of nucleic acid-containing debris, chronic platelet activation is limited and platelet counts remain normal.
Fig. S4.
PAMAM-G3 treatment inhibits loss of platelets from the blood of MRLlpr mice. Peripheral blood from 20-wk-old MRLlpr mice treated with PBS or PAMAM-G3 was assessed for platelet counts. Data are representative of three different experiments. n = 7. *P < 0.05.
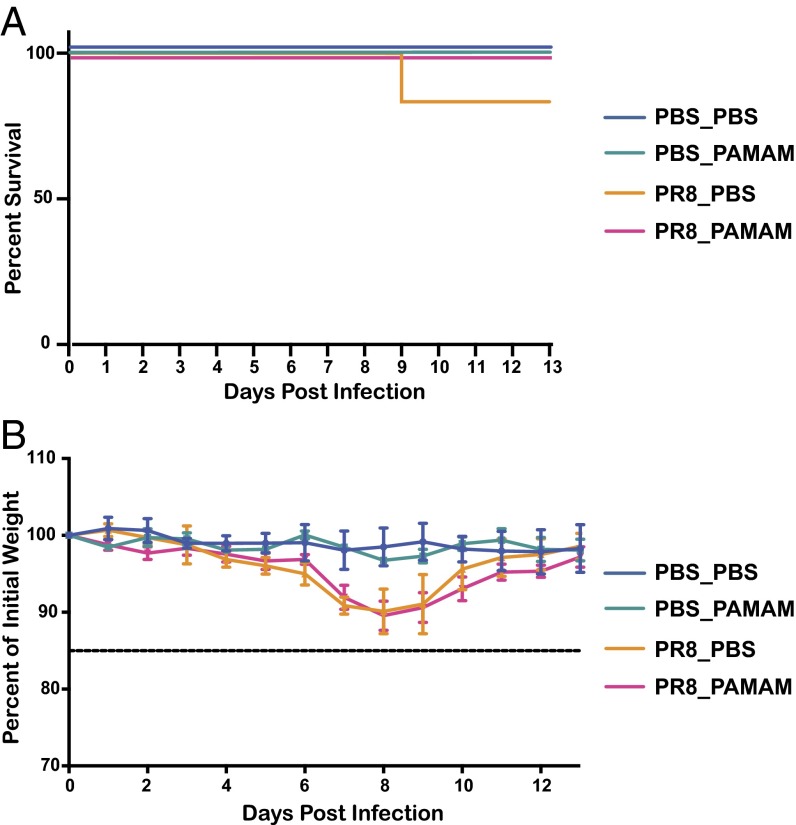
PAMAM-G3 Treatment Does Not Suppress the Immune System of NZBW F1 Animals During PR8 Influenza Infection but Protects C57BL6/J Mice from Lethal PR8 Influenza Infection.
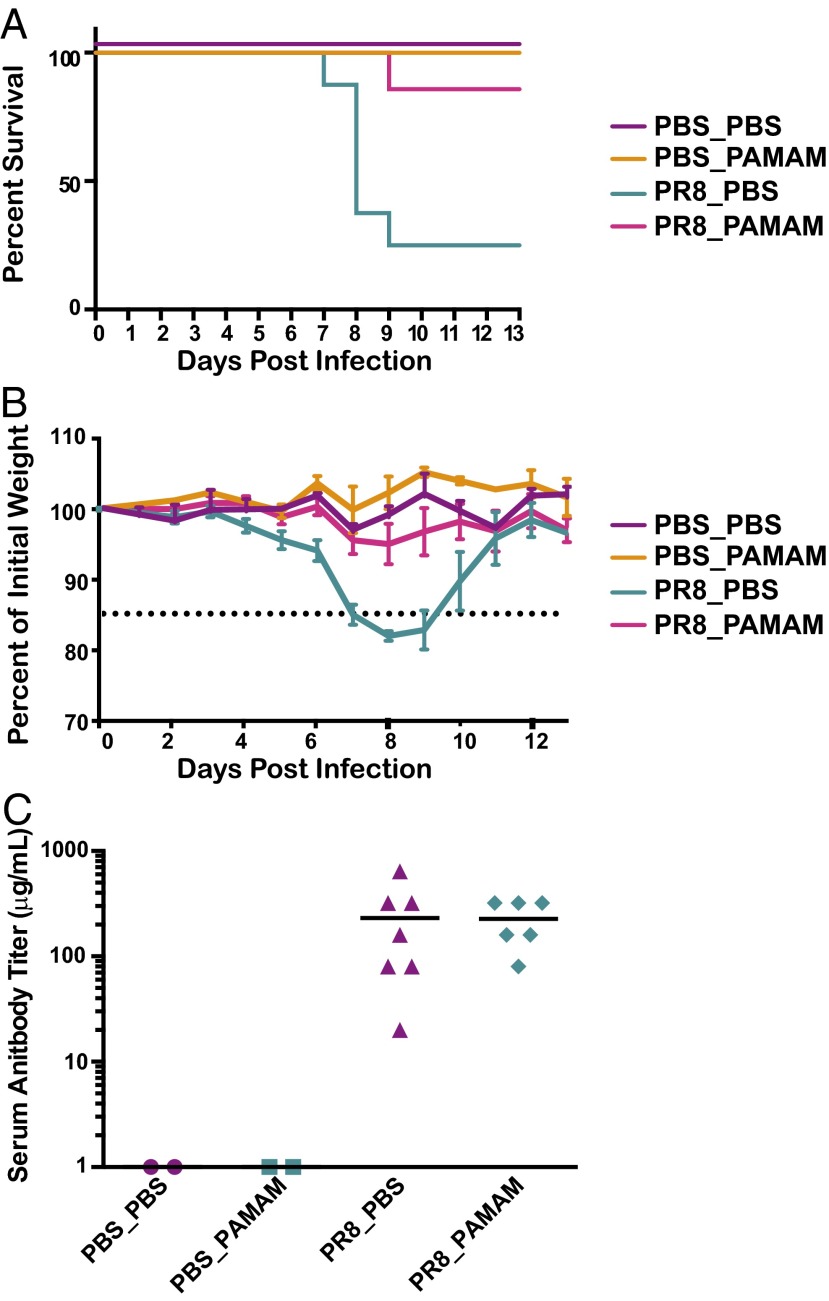
Currently marketed autoimmune disease-combatting drugs and specifically lupus treatments result in severe immune suppression and an array of side effects (16). To determine whether NAS treatment results in immune suppression as well, we evaluated the effects of PAMAM-G3 treatment in a viral infection model in vivo using the same dosing strategy that proved effective in the lupus-prone mice (PAMAM-G3, 20 mg/kg, twice per week). Lupus-prone animals were challenged intranasally with the mouse-adapted influenza A H1N1 strain, PR8 at a mouse lethal dose of 10% (mLD10) to determine whether NAS treatment would result in increased morbidity and mortality. Mice were monitored daily and sacrificed if they lost >15% of their body weight. NAS treatment did not affect animal mortality following influenza infection at an mLD10 (Fig. 4A). Moreover this treatment did not increase morbidity, as monitored by weight loss (Fig. 4B). At this relatively low dose, however, we observed that influenza challenge resulted in 16% fatality in lupus-prone animals not treated with the NAS, whereas none of the NAS-treated animals died. To explore the ability of these animals to mount an immune response to influenza, we further analyzed the percentages of germinal center cells in the spleen. The analysis of splenic cells indicates that germinal center maturation is also not affected by NAS treatment (Fig. S5). These data demonstrate that PAMAM-G3 treatment does not suppress the antiviral immune response in lupus-prone animals and suggest that, if adequately developed, NASs may become inherently safer, antiinflammatory agents.
Fig. 4.
PAMAM-G3 treatment does not suppress the immune system of NZBW F1 animals during PR8 influenza infection. NZBW F1 mice were intranasally infected with PR8 influenza and injected s.c. twice per week with PBS or PAMAM-G3 (20 mg/kg). Mice were monitored for survival (A) and weight loss (B).
Fig. S5.
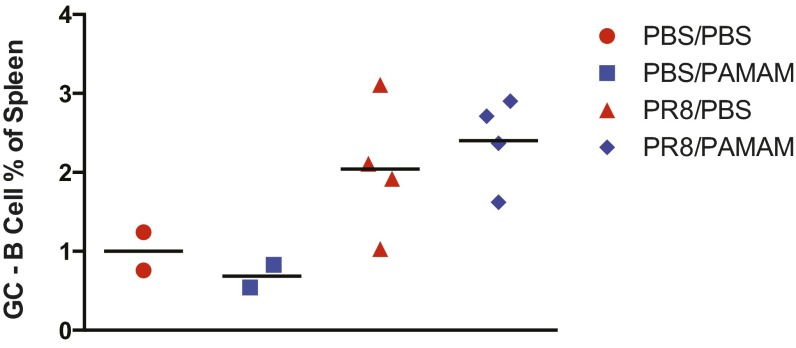
PAMAM-G3 treatment does not affect the ability of NZBW F1 mice to mount a germinal center response after a sublethal dose of PR8 influenza treatment. Spleens from PR8 influenza-infected NZBW F1 mice were isolated 14 d posttreatment with PBS or PAMAM-G3. Percentages of GL7+B220+ B cells were determined by flow cytometry. Data are representative of two independent experiments. n = 4.
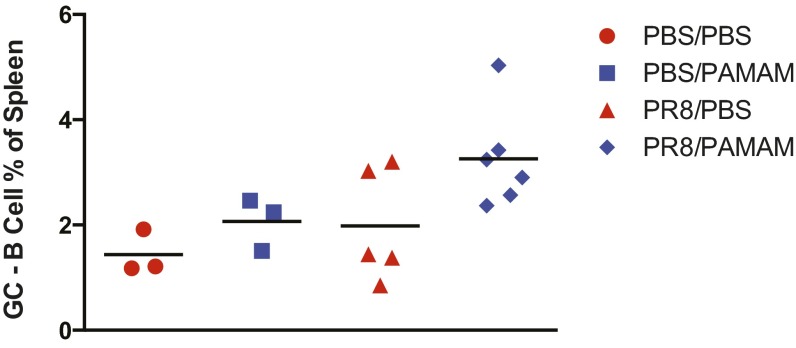
Our observation that NAS treatment may improve survival of lupus-prone mice when the animals are challenged at low mLDs, led us to investigate whether PAMAM-G3 might have beneficial effects on normal mice challenged with higher doses of influenza. Therefore, C57BL6/J mice were infected with a mLD50 of influenza A virus PR8 (H1N1) and treated with PAMAM-G3 at the time of viral challenge (20 mg/kg, twice per week). Remarkably, as shown in Fig. 5A, NAS treatment significantly improved survival following flu infection, reducing mortality from 75% to only 14% (P = 0.0126). Similarly, morbidity was dramatically improved as seen by a significant reduction in weight loss following viral challenge (Fig. 5B). To explore the ability of these animals to mount an immune response to influenza, we measured the level of neutralizing Abs to PR8 in the serum (Fig. 5C). These studies revealed that anti-influenza Ab titers are not impacted by NAS treatment. Furthermore, the analysis of splenic cells indicates that germinal center maturation is also not affected by NAS treatment (Fig. S6). These results suggest that, by binding extracellular nucleic acid debris released from virally infected, dying cells, NASs can limit viral-induced, acute pathological inflammation.
Fig. 5.
PAMAM-G3 treatment protects C57BL6/J mice from lethal PR8 influenza infection. (A) C57BL6/J mice were intranasally infected with PR8 influenza (PR8) or treated with saline (mock) and injected intraperitoneally twice per week with PBS or PAMAM-G3 (20 mg/kg). Mice were monitored for survival. n = 7 per group. Graphs are representative of at least three independent experiments. **P < 0.01. (B) Treated mice were monitored for weight loss throughout the study. (C) Anti-influenza neutralizing Ab titers from infected mice were analyzed by microneutralization assay.
Fig. S6.
PAMAM-G3 treatment does not affect the ability of wild-type mice to mount a germinal center response after lethal dose of PR8 influenza treatment. Spleens from PR8 influenza-infected C57BL/6 mice were isolated 14 d post treatment with PBS or PAMAM-G3. Percentages of GL7+B220+ B cells were determined by flow cytometry. Data are representative of two independent experiments. n = 5.
Discussion
Inflammation is a complex biological process that is necessary for clearance of pathogens. However, when acute inflammation turns chronic, it can lead to inflammatory disorders that are hard to control. Dead or dying cells release RNA and DNA into circulation. If these self-nucleic acids are not properly cleared, they can trigger activation of endosomal TLRs such as TLR7, -8, and -9 (37–39). This in turn results in further downstream activation of signaling pathways and production of proinflammatory cytokines (15, 40). In fact, multiple autoimmune disorders are characterized by elevated levels of circulating proinflammatory cytokines and autoantibodies (41–43). To date, numerous studies and clinical trials have focused on addressing circulating self-RNA and DNA, TLR activation, proinflammatory cytokines and circulating autoantibodies (16). Many of these drugs act on a single component or cell type of the inflammatory response, have short-term effects, and do not break the TLR activation cycle (44). Additionally, a number of these compounds are associated with increased susceptibility to infection and decreased pathogen clearance.
The goal of our study was to break the cycle of aberrant inflammation by targeting self-nucleic acids before their binding of endosomal TLRs. To address our goals, we used a newly identified class of compounds, NASs, which bind circulating nucleic acids and block TLR activation. Treatment of immune cells from both C57/Bl6 wild-type animals and NZBW F1 lupus-prone animals with nucleic acid agonists in the presence of NASs resulted in diminished proinflammatory cytokine production (IL-6 and TNF-α) in vitro. Cell activation through nonendosomal TLRs remained intact, thus further demonstrating the specificity of our compounds for nucleic acids. Importantly, these compounds inhibited nucleic acid-driven TLR activation in cultures of DCs derived from SLE-prone animals, suggesting that these compounds can potentially be effective in an autoimmune disease setting.
NASs administered local and systemically in lupus-prone animals improved disease outcomes in these animals. Endogenous nucleic acid-driven inflammation was diminished in the presence of NASs. In CLE models, we observed reduced skin inflammation, which resulted in improved disease pathology. Moreover, long-term SLE studies in the presence of NASs demonstrated the potential of these compounds to reduce levels of circulating autoantibodies as well as decreased organ damage due to uncontrolled inflammation.
Chronic and unresolved inflammation is often associated with reduced platelet counts in patients as disease severity is increased (35, 36, 45). Here, we demonstrate that treatment of lupus-prone mice with NASs rescues platelet depletion, thus making these compounds an important therapeutic agent, capable of acting on several different components of the inflammatory cascade as well as several different cell types.
TLR targeting has been attempted using several inhibitory compounds that bind directly to TLR7 and TLR9 (5–7, 46, 47). In fact, some of these are currently being tested in clinical trials (clinicaltrials.gov, 2016). Although direct inhibition of endosomal TLRs can in principle result in improved autoimmune disease outcomes, there is room for improvement. Nucleic acids are capable of activating a cohort of endosomal receptors, including but not limited to TRL7 and TLR9 (48, 49), thus rendering these compounds partially effective in multifaceted autoimmune disorders that rely on multiple types of receptors. Moreover, TLR7 gene mutations play an important role in disease severity, and any therapies that directly target these receptors could potentially fail due to decreased mutant receptor binding (50–52). Our strategy does not rely on receptor binding, therefore, addressing a number of these concerns. We used PAMAM-G3 as the NAS in these studies due to its 32 surface amines that allow for high-affinity binding of nucleic acids, whereas lower-generation PAMAM dendrimers were not as effective at inhibiting nucleic acid-mediated TLR activation in our previous in vitro studies with model TLR ligands (22). Additionally, generation-5 PAMAM has revealed increased toxicity in our previous and current studies at a similar dosing regimen (21, 22). However, our studies suggest that exploration of higher-generation dendrimers with biodegradable properties or other NAS is warranted as they may further improve treatment outcomes. Moreover, a drug delivery device could prove to be an important strategy to deliver therapeutic agents slowly and uniformly and thereby increase their efficacy while eliminating any toxicity.
Lastly, our study addresses immunosuppression: a very important aspect of all therapeutic agents attempting to target aberrant inflammation. Chronic treatment with antiinflammatory agents can lead to overall immune suppression and increased susceptibility to infections (53, 54). To determine whether our therapeutic approach impacts immune responses to pathogens, we infected NAS-treated animals with PR8 influenza to mimic human flu infection. We did not observe immune suppression in treated animals, as NAS-treated lupus-prone mice were able to recover similarly to untreated controls. To our surprise, C57BL6 animals that received lethal doses of PR8 in the presence of NAS did not succumb to infection at the same rate as the control-treated animals. These findings suggest that NASs may have broader applications in not only controlling aberrant inflammation but in also improving the immune response to pathogens.
Thus, NASs represent a class of agents to potentially treat SLE as well as a wide variety of infectious diseases, particularly those caused by highly pathogenic viruses such as pandemic influenza and Ebola. Their potential therapeutic utility should now be explored.
Materials and Methods
Mice.
C57BL/6, NZBW F1, and MRLlpr were obtained from The Jackson Laboratory. Mice were housed in a pathogen-free barrier facility at Duke University. Only male mice were used in all of our studies. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (55) and were approved by the Duke University Institutional Animal Care and Use Committee (Protocol A011-11-01).
SLE and Flu Studies.
Tape stripping.
Tape stripping was performed on the dorsal area of the mice, after shaving, with standard-size bandages, 20 strokes. PAMAM-G3 (20 mg/kg) (Sigma-Aldrich) was administered at the time of tape stripping s.c. and every 3 d after injury. Animals were sacrificed at 14 d following injury. All skin samples were then harvested and fixed in 10% (vol/vol) formalin for future histological analysis.
SLE long-term treatment.
MRL-lpr male mice were injected with PAMAM-G3 (20 mg/kg) twice a week for a period of 8–10 wk starting at 10 wk of age. Mice were then sacrificed, and blood and tissue were collected for further analysis.
PR8 infections.
Mouse-adapted virus strain, influenza A/Puerto Rico/8/34 (H1N1; PR8), was obtained from Charles River. Ten-week-old mice were anesthetized with vaporized isoflurane. Virus was administered intranasally in a total volume of 40 μL of sterile pharmaceutical-grade saline. Control mice were mock treated with pharmaceutical grade saline only. PAMAM-G3 was injected i.p. at 20 mg/kg in a total volume to 200 μL of pharmaceutical-grade saline. Weight loss and survival of infected mice were followed over a period of 14 d. Mice that lost 15% or more of their body weight were euthanized and recorded as dead per Duke University Institutional Animal Care and Use Committee guidelines.
Cell Culture.
B-cell activation.
B cells from spleens for NZBW F1 animals were purified by negative selection. Stimulation assays were performed as previously described (20).
DC culture.
Murine bone marrow DCs were isolated from C57/Bl6 and NZBW F1 mice and were cultured in the presence of GM-CSF (PeproTech) and IL-4 (PeproTech) as previously described (56). Stimulation assays were performed as previously described (20).
Neutralization Assay.
Virus microneutralization assay was performed as described previously (57) with modifications. Briefly, virus and serum dilutions were performed as described and then mixed with 100 µL of freshly trypsinized MDCK-London cells containing 1.5 × 104 cells in 96-well cell culture-treated plates. Negative controls consisted of cells alone, whereas positive controls contained virally infected cells. Plates were incubated for 20 h before fixation with acetone. Endogenous biotin in wells was blocked with PBS containing 0.1% avidin (Life Technologies) for 15 min followed by washes, and any bound avidin was blocked with PBS containing 0.01% biotin (Sigma Aldrich) for 15 min. Plates were analyzed for positive infection via ELISA. Mouse monoclonal biotinylated anti-NP antibodies MAB8257B and MAB8258B (Millipore) (dilution, 1:6,000) and HRP–streptavidin conjugate (BD Biosciences) (dilution, 1:4,000) were used in the ELISA. Color was developed using OPD substrate (Sigma-Aldrich) in citrate buffer (Sigma-Aldrich), and optical density was measured at 490 nm in plate reader (Molecular Devices). The highest serum dilution that generated >50% specific signal was determined to be the neutralization titer: 50% specific signal = (OD490 virus control − OD490 cell control)/2 + OD490 cell control.
Determination of Clinical Disease.
Skin lesion scoring was conducted in a blinded fashion by a trained veterinarian pathologist as previously described (28, 29). Briefly, skin samples were collected, fixed in 10% (vol/vol) formalin, paraffin embedded (FFPE), and further processed for H&E staining. Epidermal thickness, degree of ulceration, intraepithelial inflammation, dermal inflammation, and panniculus inflammation were assessed and graded on a scale from 0 to 3: 0, normal skin architecture; 1, mild inflammation with slight epidermal hyperplasia; 2, moderate inflammation with noticeable epidermal hyperplasia; 3, severe inflammation with marked epidermal hyperplasia. All parameters were scored separately and summed to reach a total disease score.
For kidney disease, kidneys were collected and further processed as FFPE samples for H&E staining or snap frozen in OCT for immunofluorescence staining. Glomerulonephritis scoring was done as previously described in a blinded fashion by a trained veterinarian pathologist (7). Briefly, kidneys were scored for glomerulonephritis on a scale of 1–4: 1, normal; 2, mild; 3, moderate; and 4, severe. This scoring takes into account glomerular changes, interstitial changes, and severity of lymphoplasmatic infiltration into the kidney.
Immunofluorescence.
Slides coated with Crithidia luciliae and HEp-2 cells (Scimedx) were rehydrated with PBS for 30 min. Samples were then blocked for 2 h [PBS, 0.1% Tween 20, 5% (vol/vol) goat serum, and 1% rat anti-mouse CD16/CD32]. Serum samples were subsequently added to the slides at various dilutions (1:40 to 1:360). Serum Ab levels were detected using secondary Ab goat anti-mouse IgG FITC. Kidneys from treated animals were processed as described above. Five-micrometer sections were then stained with anti-complement Ab. All slides were mounted, and images were acquired using a Zeiss Axiovert 500 confocal immunofluorescent microscope. Images were analyzed for staining intensity using ImageJ.
Antibodies and FACS.
Monoclonal Abs included the following: B220, CD45.2, CD4, CD8, IFN-γ, CD3, CD11c, and GL7 (eBioscience). Single-cell suspensions of cultured cells were counted, and 106 cells were suspended in FACS buffer [PBS plus 2% (vol/vol) FBS] and stained with various Ab combinations. In addition, skin samples collected at 24 h postinjury were enzymatically digested with 0.28 u/mL Liberase 3 (Roche) for 30 min at 37 °C, and then treated with DNase, filtered, and stained with various antibodies. Flow cytometry was performed on a Gallios flow cytometer and FACSCanto. All data were analyzed with FlowJo software.
Statistical Analysis.
Statistical significance was determined with two-tailed Student’s t test or ANOVA. Log-rank Mantel–Cox test was performed on all survival curve graphs. All P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by National Institutes of Health Grants R56 AI093900, R01 HL06522, and P01 HL11626 (B.A.S.); the Coulter Foundation (to B.A.S.); and National Institutes of Health Fellowship F32 DK094543 (E.K.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607011113/-/DCSupplemental.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85(3):303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Kirou KA, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 3.Salloum R, Niewold TB. Interferon regulatory factors in human lupus pathogenesis. Transl Res. 2011;157(6):326–331. doi: 10.1016/j.trsl.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niewold TB. Interferon alpha as a primary pathogenic factor in human lupus. J Interferon Cytokine Res. 2011;31(12):887–892. doi: 10.1089/jir.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Kandimalla ER, et al. Design, synthesis and biological evaluation of novel antagonist compounds of Toll-like receptors 7, 8 and 9. Nucleic Acids Res. 2013;41(6):3947–3961. doi: 10.1093/nar/gkt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37(12):3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 8.Duramad O, et al. Inhibitors of TLR-9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J Immunol. 2005;174(9):5193–5200. doi: 10.4049/jimmunol.174.9.5193. [DOI] [PubMed] [Google Scholar]
- 9.Fox R. Anti-malarial drugs: Possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus. 1996;5(Suppl 1):S4–S10. [PubMed] [Google Scholar]
- 10.Kuznik A, et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: The role of Toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 15.Celhar T, Magalhães R, Fairhurst AM. TLR7 and TLR9 in SLE: When sensing self goes wrong. Immunol Res. 2012;53(1-3):58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 16.Suurmond J, Zou YR, Kim SJ, Diamond B. Therapeutics to block autoantibody initiation and propagation in systemic lupus erythematosus and rheumatoid arthritis. Sci Transl Med. 2015;7(280):280ps5. doi: 10.1126/scitranslmed.aaa3809. [DOI] [PubMed] [Google Scholar]
- 17.Junt T, Barchet W. Translating nucleic acid-sensing pathways into therapies. Nat Rev Immunol. 2015;15(9):529–544. doi: 10.1038/nri3875. [DOI] [PubMed] [Google Scholar]
- 18.Deng Y, et al. Argentine Collaborative Group BIOLUPUS and GENLES Networks MicroRNA-3148 modulates allelic expression of Toll-like receptor 7 variant associated with systemic lupus erythematosus. PLoS Genet. 2013;9(2):e1003336. doi: 10.1371/journal.pgen.1003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, et al. Increased ribonuclease expression reduces inflammation and prolongs survival in TLR7 transgenic mice. J Immunol. 2013;190(6):2536–2543. doi: 10.4049/jimmunol.1202689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holl EK, Shumansky KL, Pitoc G, Ramsburg E, Sullenger BA. Nucleic acid scavenging polymers inhibit extracellular DNA-mediated innate immune activation without inhibiting anti-viral responses. PLoS One. 2013;8(7):e69413. doi: 10.1371/journal.pone.0069413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain S, et al. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc Natl Acad Sci USA. 2012;109(32):12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, et al. Nucleic acid-binding polymers as anti-inflammatory agents. Proc Natl Acad Sci USA. 2011;108(34):14055–14060. doi: 10.1073/pnas.1105777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 24.Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2005;57(15):2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53(6):424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muro Y. Antinuclear antibodies. Autoimmunity. 2005;38(1):3–9. doi: 10.1080/08916930400024612. [DOI] [PubMed] [Google Scholar]
- 27.Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- 28.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207(13):2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J Clin Invest. 2009;119(1):47–60. doi: 10.1172/JCI32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawar RD, et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18(6):1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 31.Giles JR, Kashgarian M, Koni PA, Shlomchik MJ. B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J Immunol. 2015;195(6):2571–2579. doi: 10.4049/jimmunol.1500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stearns NA, Lee J, Leong KW, Sullenger BA, Pisetsky DS. The inhibition of anti-DNA binding to DNA by nucleic acid binding polymers. PLoS One. 2012;7(7):e40862. doi: 10.1371/journal.pone.0040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickerson KM, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Habets KL, Huizinga TW, Toes RE. Platelets and autoimmunity. Eur J Clin Invest. 2013;43(7):746–757. doi: 10.1111/eci.12101. [DOI] [PubMed] [Google Scholar]
- 36.Boilard E, Blanco P, Nigrovic PA. Platelets: Active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 37.Blasius AL, Beutler B. Intracellular Toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202(8):1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauhan SK, Singh VV, Rai R, Rai M, Rai G. Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J Clin Immunol. 2013;33(5):954–964. doi: 10.1007/s10875-013-9887-0. [DOI] [PubMed] [Google Scholar]
- 40.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 41.Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J Clin Invest. 2015;125(6):2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carneiro JR, et al. IL-2, IL-5, TNF-α and IFN-γ mRNA expression in epidermal keratinocytes of systemic lupus erythematosus skin lesions. Clinics (Sao Paulo) 2011;66(1):77–82. doi: 10.1590/S1807-59322011000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karonitsch T, et al. Activation of the interferon-gamma signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 2009;60(5):1463–1471. doi: 10.1002/art.24449. [DOI] [PubMed] [Google Scholar]
- 44.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: Challenges to treatment. Lancet. 2013;382(9894):809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 45.Hepburn AL, Narat S, Mason JC. The management of peripheral blood cytopenias in systemic lupus erythematosus. Rheumatology (Oxford) 2010;49(12):2243–2254. doi: 10.1093/rheumatology/keq269. [DOI] [PubMed] [Google Scholar]
- 46.Zhu FG, et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 2013;46(7):419–428. doi: 10.3109/08916934.2013.798651. [DOI] [PubMed] [Google Scholar]
- 47.Jiang W, et al. A Toll-like receptor 7, 8, and 9 antagonist inhibits Th1 and Th17 responses and inflammasome activation in a model of IL-23-induced psoriasis. J Invest Dermatol. 2013;133(7):1777–1784. doi: 10.1038/jid.2013.57. [DOI] [PubMed] [Google Scholar]
- 48.Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17(2):57–64. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo H, Callaway JB, Ting JP. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki A, et al. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: A case-control association study. Arthritis Res Ther. 2011;13(2):R41. doi: 10.1186/ar3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CM, et al. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci Rep. 2014;4:3792. doi: 10.1038/srep03792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen N, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci USA. 2010;107(36):15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: Bridging the basic immunology with clinical application. Immunity. 2006;24(3):233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Davies RJ, et al. Rituximab in the treatment of resistant lupus nephritis: Therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. 2013;22(6):574–582. doi: 10.1177/0961203313483376. [DOI] [PubMed] [Google Scholar]
- 55. National Research Council (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
- 56.van Deventer HW, et al. Transfection of macrophage inflammatory protein 1 alpha into B16 F10 melanoma cells inhibits growth of pulmonary metastases but not subcutaneous tumors. J Immunol. 2002;169(3):1634–1639. doi: 10.4049/jimmunol.169.3.1634. [DOI] [PubMed] [Google Scholar]
- 57.Koutsonanos DG, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]