Significance
Nonclassical monocytes patrol the microvascular lumen in numerous organs in behavior thought to represent a form of immune surveillance. However, the mechanisms whereby they promote inflammatory responses are unclear. Here, we show using in vivo imaging that, in the unique microvasculature of the glomerulus in the kidney, monocytes constitutively undergo interactions with intravascular migratory neutrophils. Upon induction of glomerular inflammation, neutrophils that interact with monocytes show increased retention in glomerular capillaries and increased propensity to generate reactive oxygen species, leading to renal injury. These findings of immune cell interactions occurring within the glomerular microvasculature indicate that cell–cell contact between neutrophils and nonclassical monocytes is a previously unrecognized intravascular inflammatory mechanism underpinning glomerular injury.
Keywords: monocyte–neutrophil interaction, inflammation, glomerulonephritis, reactive oxygen species, tumor necrosis factor
Abstract
Nonclassical monocytes undergo intravascular patrolling in blood vessels, positioning them ideally to coordinate responses to inflammatory stimuli. Under some circumstances, the actions of monocytes have been shown to involve promotion of neutrophil recruitment. However, the mechanisms whereby patrolling monocytes control the actions of neutrophils in the circulation are unclear. Here, we examined the contributions of monocytes to antibody- and neutrophil-dependent inflammation in a model of in situ immune complex-mediated glomerulonephritis. Multiphoton and spinning disk confocal intravital microscopy revealed that monocytes patrol both uninflamed and inflamed glomeruli using β2 and α4 integrins and CX3CR1. Monocyte depletion reduced glomerular injury, demonstrating that these cells promote inappropriate inflammation in this setting. Monocyte depletion also resulted in reductions in neutrophil recruitment and dwell time in glomerular capillaries and in reactive oxygen species (ROS) generation by neutrophils, suggesting a role for cross-talk between monocytes and neutrophils in induction of glomerulonephritis. Consistent with this hypothesis, patrolling monocytes and neutrophils underwent prolonged interactions in glomerular capillaries, with the duration of these interactions increasing during inflammation. Moreover, neutrophils that interacted with monocytes showed increased retention and a greater propensity for ROS generation in the glomerulus. Also, renal patrolling monocytes, but not neutrophils, produced TNF during inflammation, and TNF inhibition reduced neutrophil dwell time and ROS production, as well as renal injury. These findings show that monocytes and neutrophils undergo interactions within the glomerular microvasculature. Moreover, evidence indicates that, in response to an inflammatory stimulus, these interactions allow monocytes to promote neutrophil recruitment and activation within the glomerular microvasculature, leading to neutrophil-dependent tissue injury.
Neutrophil recruitment is a crucial event in the response to tissue injury and host defense against pathogens. However, if dysregulated, it can also cause significant tissue injury. Inappropriate recruitment and activation of neutrophils are recognized as major contributors to organ damage in numerous inflammatory diseases, including inflammatory arthritis, acute respiratory distress syndrome, systemic lupus erythematosus, and acute glomerulonephritis (1–4). Although our understanding of the molecular basis of neutrophil recruitment is well-developed, less is known about the interactions of neutrophils with other circulating immune cells during the development of the innate inflammatory response. However, over the last decade, evidence has emerged that neutrophil recruitment and function during inflammation can be influenced by another circulating immune cell—the monocyte (5–10).
Monocytes exist in two major populations, termed classical and nonclassical, as defined by expression of specific surface markers. In mice, these populations are CC chemokine receptor 2 (CCR2)hi CX3C chemokine receptor 1 (CX3CR1)lo Ly6C+ (classical) and CX3CR1hi CCR2− Ly6C− (nonclassical), with the corresponding populations in humans being CD14hi CD16− and CD14lo CD16hi, respectively. A third low abundance population, termed intermediate, also exists, characterized in mice by intermediate Ly6C expression and in humans as CD14hi CD16+ (11–13). Classical monocytes are selectively recruited to inflamed tissues and lymph nodes partly in a CCR2-dependent manner, where they produce inflammatory and antimicrobial factors and contribute to local and systemic inflammation (12–16). In contrast, CX3CR1hi Ly6C− monocytes lack CCR2 and are present in both resting and inflammatory tissues (12, 15). Evidence indicates that nonclassical monocytes perform a specialized form of immune homeostasis, serving as initiators of acute inflammatory responses and contributing to tissue remodeling (5, 6, 13, 17–19). A unique aspect of the function of these cells is their capacity to undergo extensive intravascular crawling or “patrolling,” a constitutive phenomenon that has now been observed in a wide range of organs (5, 6, 20–22). A growing body of evidence indicates that this form of immune surveillance allows nonclassical monocytes to respond rapidly to inflammatory stimuli and to direct the responses of other immune cells in the circulation, particularly neutrophils (5, 6). However, the nature and mechanisms of this response are not fully understood. Moreover, the mode of action of nonclassical monocytes in specialized microvascular beds, in which the mechanisms of leukocyte recruitment do not follow the conventional paradigm, such as the glomerulus (20, 23), has not been investigated.
The glomerulus in the kidney is a key target of damaging inflammation in a wide range of conditions (2). Indeed, glomerulonephritis is a leading cause of end-stage renal failure. Furthermore, although capillaries are typically not major sites of leukocyte recruitment, leukocyte recruitment to the glomerular capillaries plays a central role in glomerular injury and renal dysfunction (23, 24). We recently used multiphoton imaging to demonstrate that leukocyte recruitment to the glomerulus occurs via a distinct paradigm (20). In the absence of inflammation, neutrophils underwent constitutive intravascular retention and crawling within glomerular capillaries. Upon induction of glomerular inflammation, the major response of the neutrophil was to significantly increase the duration of its retention in the glomerular capillaries (referred to as dwell time), which, however, did not subsequently progress to transendothelial migration. In contrast, while retained intravascularly, neutrophils generated reactive oxygen species (ROS) that were responsible for glomerular injury and renal dysfunction. In parallel, CX3CR1hi monocytes were also observed to patrol the glomerular capillaries and to significantly increase their dwell time during glomerular inflammation. However, while this study showed that both these leukocyte subsets patrol the glomerular capillaries, their functional relationships and whether these cells undergo interactions within the glomerular microvasculature have not been investigated. Therefore, the aim of this study was to investigate the contribution of patrolling monocytes to neutrophil-dependent glomerulonephritis. These experiments show that patrolling monocytes interact with neutrophils within the glomerular microvasculature and, during glomerular inflammation, promote neutrophil retention and activation that underlie glomerular dysfunction.
Results
Monocytes Patrol Uninflamed Glomerular Capillaries via CX3CR1 and β2 and α4 Integrins.
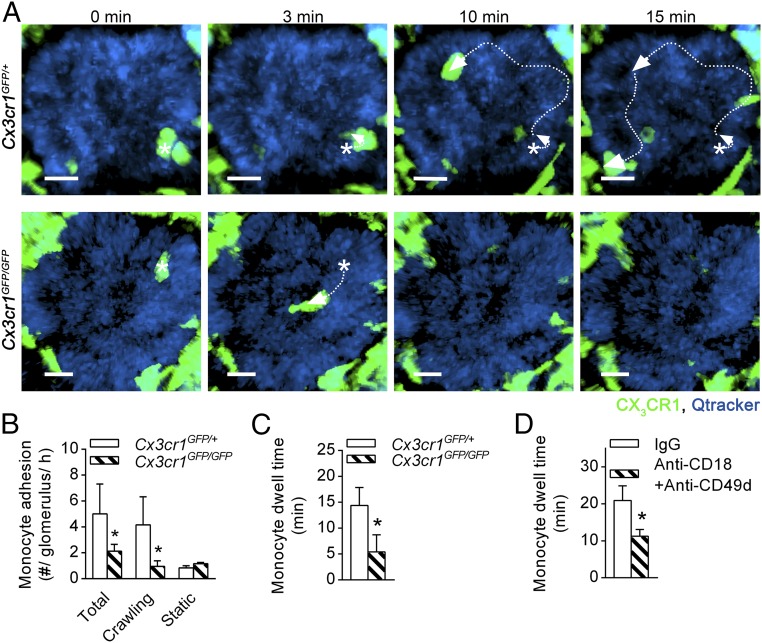
Monocytes constitutively migrate within glomerular capillaries in vivo (20). To determine the mechanism of monocyte migration within the glomerular microvasculature, Cx3cr1GFP/+ and Cx3cr1GFP/GFP mice, in which monocytes are visible via GFP expression (5, 25), were examined by multiphoton microscopy. Similar to previously published data, in Cx3cr1GFP/+ mice, monocytes were found to undergo extended periods of intravascular retention and migration in glomerular capillaries in the absence of inflammatory stimulation (Fig. 1A and Movie S1). The majority of monocytes retained in glomeruli were migratory, and their duration of retention averaged ∼15 min (Fig. 1 B and C). In mice, the two major populations of monocytes are the Ly6C+ classical and the Ly6C− nonclassical. To clarify which population of monocytes underwent migration in the glomerular capillaries, we performed parallel experiments using spinning disk confocal microscopy (SDCM), using anti–Gr-1 in Cx3Cr1GFP/+ mice. Anti–Gr-1 detects both Ly6C and the neutrophil-restricted antigen Ly6G, allowing the differentiation of classical (GFP+, Gr-1+) and nonclassical (GFP+, Gr-1−) monocytes. In these experiments, Gr-1+ GFP+ monocytes were very rare, being detected at the rate of ∼0.1 per glomerulus per hour. In contrast, the vast majority of monocytes, migrating through glomeruli at a rate of ∼5 per glomerulus per hour, were Gr-1−. These findings indicate that the monocytes crawling in the glomerular capillaries were almost exclusively Ly6C− nonclassical patrolling monocytes.
Fig. 1.
Monocyte patrolling in uninflamed glomerular capillaries is dependent on CX3CR1 and β2 and α4 integrins. The role of surface receptors in monocyte trafficking within uninflamed glomerular capillaries was investigated in Cx3cr1GFP mice using intravital multiphoton microscopy. (A) Representative multiphoton image sequences of an untreated Cx3cr1GFP/+ mouse (Top) and Cx3cr1GFP/GFP mouse (Bottom) illustrating migration of a GFP+ monocyte (green) within glomerular capillaries (blue; labeled with Qtracker-655). Asterisks indicate starting position, and arrows indicate the path of migration. Time stamp is shown above the images. (Scale bar: 10 μm.) See also Movie S1. (B and C) The number of monocytes adhering in glomeruli per hour shown for total, crawling, and static cells (B) and monocyte dwell time (of total cells) (C) was assessed in untreated Cx3cr1GFP/+ (n = 8) and Cx3cr1GFP/GFP mice (n = 5). (D) Monocyte dwell time was compared in Cx3cr1GFP/+ mice pretreated with anti-CD18 and anti-CD49d blocking antibodies together (n = 4), or the respective isotype controls (n = 6). Data are presented as mean ± SEM; *P < 0.05 vs. corresponding control group.
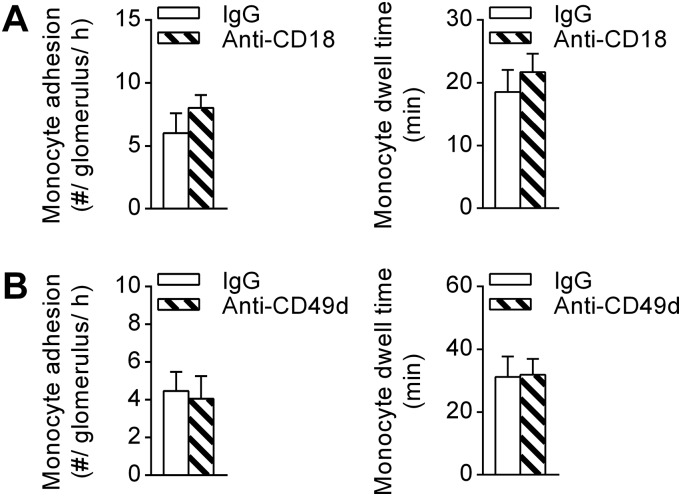
Mice deficient in CX3CR1 (Cx3cr1GFP/GFP mice) showed a significant reduction in the number of retained monocytes, compared with heterozygous Cx3cr1GFP/+ control animals, solely via reduction in the number of crawling cells (Fig. 1B). This observation may have stemmed from a reduction in the number of nonclassical monocytes in the blood of Cx3cr1GFP/GFP mice as previously reported (26, 27). Nevertheless, the dwell time of recruited monocytes was also significantly reduced in Cx3cr1GFP/GFP mice (Fig. 1 A and C and Movie S1), providing clear evidence of a role for CX3CR1 in retention of monocytes in glomeruli. Inhibition of CD18 (integrin subunit β2) or CD49d (integrin subunit α4) did not reduce monocyte trafficking during steady-state conditions (Fig. S1). In contrast, blocking both β2 and α4 integrins significantly reduced monocyte dwell time (Fig. 1D). These data indicate that patrolling monocytes use CX3CR1 and both β2 and α4 integrins to undergo constitutive intravascular retention and migration in the glomerulus.
Fig. S1.
Inhibition of either β2 or α4 integrins alone is insufficient to inhibit monocyte patrolling in uninflamed glomerular capillaries. (A and B) The number of monocytes adhering in glomeruli (Left) and monocyte dwell time (Right) were assessed in uninflamed Cx3cr1GFP/+ mice pretreated with anti-CD18 (n = 5) (A) or anti-CD49d (n = 4) (B) blocking antibodies or the respective isotype controls (n = 4–5). Data are shown as mean ± SEM.
CX3CR1, LFA-1, and Mac-1 Mediate Distinct Steps of Glomerular Monocyte Trafficking During Inflammation.
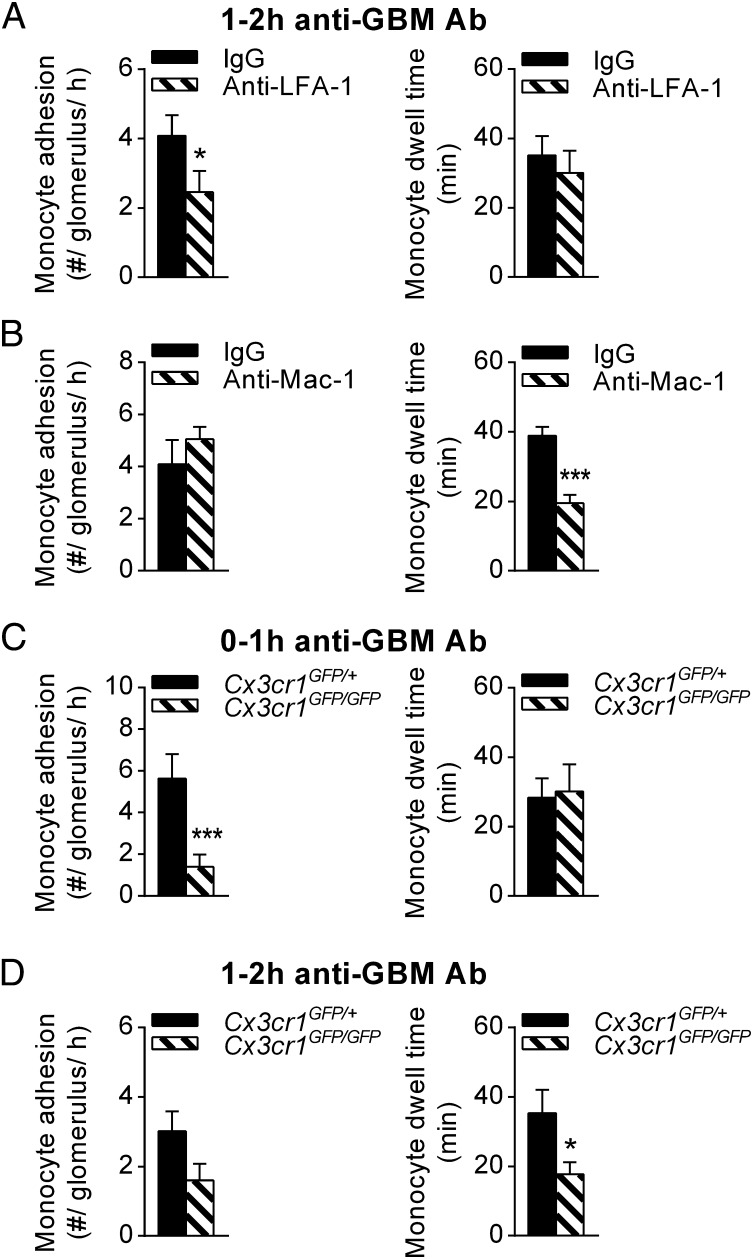
We previously demonstrated that monocyte dwell time increases with inflammation (20), and this finding is replicated in the present study in that dwell time was found to increase from ∼20 min to ∼40 min during the anti-GBM Ab response (compare Fig. 1C with Fig. 2A). Therefore, we next used multiphoton microscopy to decipher the mechanisms of glomerular monocyte trafficking under inflammatory conditions. Glomerular inflammation was induced using the anti-glomerular basement membrane antibody (anti-GBM Ab) model of in situ immune complex-mediated inflammation. Inhibition of LFA-1 resulted in a significant reduction in monocyte attachment to glomerular capillaries 1–2 h after anti-GBM Ab administration. However, LFA-1 inhibition did not alter the dwell time of the remaining monocytes (Fig. 2A). In contrast, inhibition of Mac-1 had no effect on the number of adherent monocytes but diminished monocyte dwell time by ∼50% (Fig. 2B). These data indicate that LFA-1, but not Mac-1, is required for initial monocyte attachment whereas Mac-1 is more important for intravascular monocyte retention during inflammation.
Fig. 2.
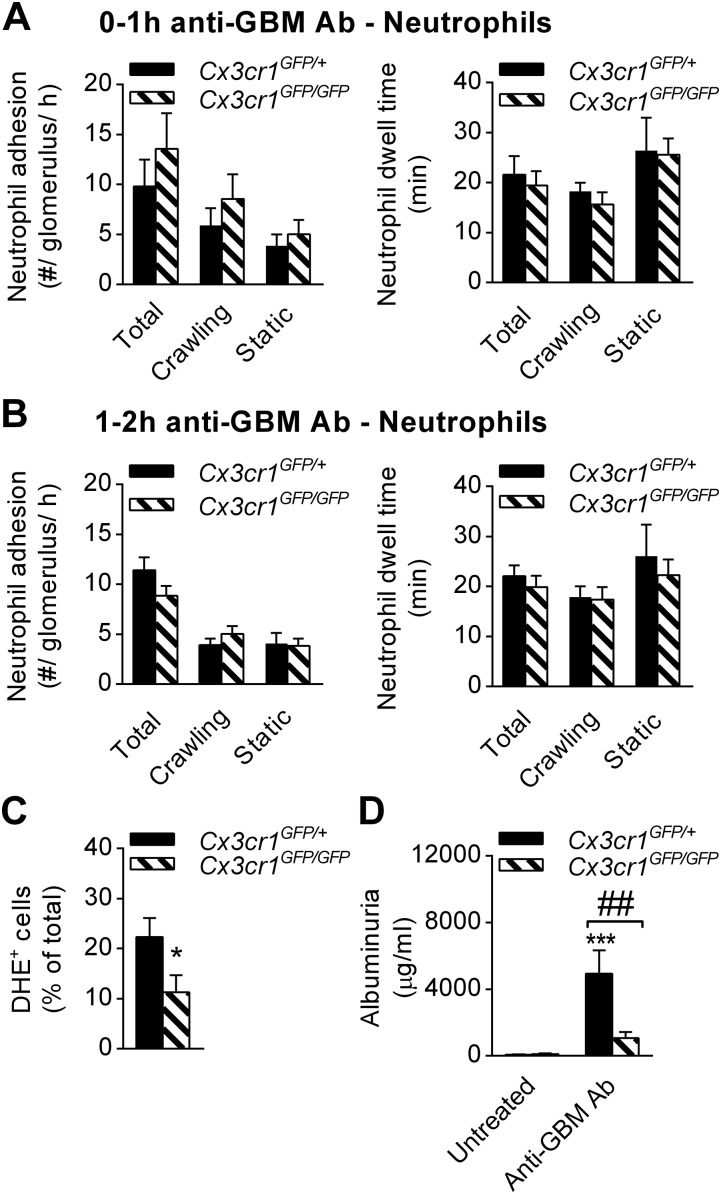
Monocyte trafficking in inflamed glomerular capillaries is dependent on CX3CR1, LFA-1 and Mac-1. The role of surface receptors in monocyte trafficking within inflamed glomerular capillaries was investigated in Cx3cr1GFP/+ and Cx3cr1GFP/GFP mice using intravital multiphoton microscopy. (A and B) Monocyte adhesion (Left), and dwell time (Right) were quantified 1–2 h after anti-GBM Ab injection in mice pretreated with either anti-LFA-1 (n = 6) (A), anti-Mac-1 (n = 6) (B), or the respective isotype controls (n = 6 and 4, respectively). (C and D) Contribution of CX3CR1 to glomerular monocyte patrolling during inflammation. Monocyte adhesion and dwell time are shown for Cx3cr1GFP/+ (n = 6) and Cx3cr1GFP/GFP mice (n = 6) 0–1 h (C) or 1–2 h (D) after anti-GBM Ab injection. Data are presented as mean ± SEM; *P < 0.05, ***P < 0.001 vs. corresponding control group.
The actions of CX3CR1 varied according to the phase of the response. In the first hour of inflammation, there were fewer monocytes recruited to glomerular capillaries in Cx3cr1GFP/GFP mice compared with Cx3cr1GFP/+ control animals whereas monocyte dwell time remained unchanged (Fig. 2C). In contrast, in the second hour of inflammation (1–2 h after anti-GBM Ab injection), monocyte dwell time was markedly reduced in Cx3cr1GFP/GFP mice whereas the change in number of adherent monocytes was not significant (Fig. 2D). Together, these data indicate that the initial attachment of monocytes to glomerular capillaries during inflammation requires LFA-1 and CX3CR1, but not Mac-1, whereas Mac-1 and CX3CR1 were crucial for prolonged dwell times of monocyte patrolling in the second hour of the response.
Monocytes Contribute to Acute Glomerular Injury by Modulating Neutrophil Responses.
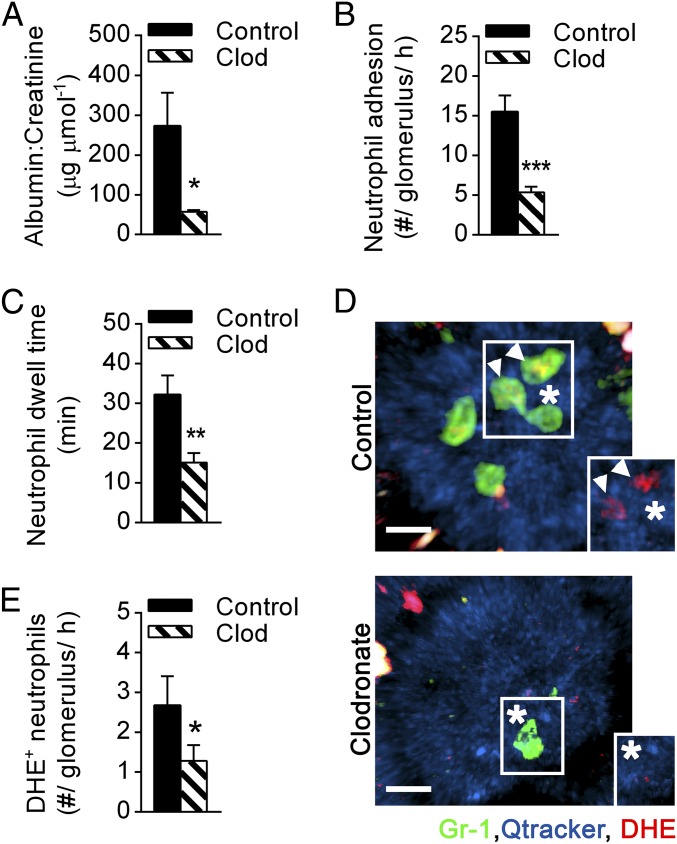
To investigate the functional role of monocytes in acute glomerulonephritis, we assessed the effect of clodronate liposome-mediated monocyte depletion on pathological albuminuria, a clinically relevant marker of functional glomerular injury. Notably, monocyte depletion prevented development of albuminuria in response to anti-GBM Ab (Fig. 3A), indicating a critical contribution of monocytes to glomerular injury in this model.
Fig. 3.
Monocyte depletion diminishes renal injury and neutrophil responses. C57BL/6 WT mice were pretreated with clodronate liposomes (clod) for monocyte depletion or control liposomes (control) and subsequently underwent the anti-GBM Ab model. (A) Urinary albumin/creatinine ratio in clodronate (n = 6) or control liposome-treated mice (n = 5) after anti-GBM Ab administration. (B and C) Neutrophil adhesion (B) and dwell time (C) in monocyte-depleted (clodronate, n = 5) and control mice (n = 6–7, respectively) 1–2 h after anti-GBM Ab injection as determined using multiphoton intravital microscopy. (D) Representative multiphoton images of control liposome (Top) or clodronate liposome-treated mice (Bottom) 1–2 h after anti-GBM Ab administration, illustrating neutrophils (green, Gr-1) within glomerular capillaries (blue, Qtracker) being oxidant-positive (red, DHE) (denoted by arrowheads) or oxidant-negative (denoted by asterisks). (Insets) Highlighted neutrophils in the absence of Gr-1 staining. (Scale bar: 10 μm.) See also Movies S2 and S3. (E) Number of DHE+ neutrophils in control liposome-treated (n = 5) and clodronate liposome-treated mice (n = 6) 1–2 h after anti-GBM Ab administration. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding control group.
Given that glomerular injury in this model is highly neutrophil-dependent (20), we next investigated the effect of monocyte depletion on neutrophil behavior. Monocyte-depleted mice showed an ∼60% reduction in neutrophil recruitment to glomerular capillaries (Fig. 3B and Movies S2 and S3), as well as a significant reduction in neutrophil dwell time (Fig. 3C). These responses were not due to an unanticipated effect of clodronate on the proportion of circulating neutrophils because neutrophil numbers in the blood were not reduced in clodronate-treated mice (control, 18 ± 3% vs. clodronate, 22 ± 5%; n = 3 per group), consistent with previous reports (28, 29). We have also previously demonstrated that Nox2-dependent generation of ROS by intraglomerular neutrophils is critical to glomerular injury in response to anti-GBM Ab (20). Here, we observed that monocyte depletion also resulted in a reduction in the number of ROS-producing neutrophils, as detected using the ROS-sensitive fluorochrome dihydroethidium (DHE) (Fig. 3 D and E and Movies S2 and S3). This finding indicates a role for monocytes in promoting neutrophil activation in the glomerulus. Moreover, whereas DHE+ cells are typically retained in the glomerulus for longer than DHE− neutrophils, monocyte depletion attenuated the dwell time of both DHE+ and DHE− populations (Fig. S2). Taken together, these results demonstrate that monocytes regulate neutrophil behavior and function in the acutely inflamed glomerulus, identifying this mechanism as a mechanism whereby monocytes promote glomerular injury.
Fig. S2.
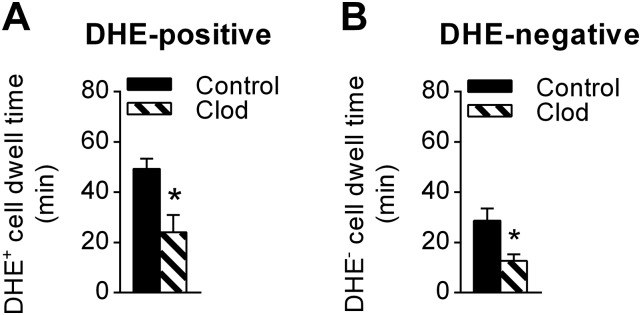
Dwell time for ROS-producing neutrophils is longer compared with ROS-negative cells. C57BL/6 WT mice were pretreated with clodronate liposomes for monocyte depletion (n = 7) or control liposomes (n = 6). Dwell times of adherent DHE+ (A) and DHE− neutrophils (B) were compared 1–2 h after anti-GBM Ab administration. Data are shown as mean ± SEM; *P < 0.05 vs. control liposome mice.
Having demonstrated that mice deficient in CX3CR1 displayed impaired monocyte recruitment to the inflamed glomerulus (Fig. 2 C and D), we also investigated the behavior of neutrophils in these mice. In contrast to monocyte-depleted mice, deletion of CX3CR1 did not reduce neutrophil adhesion or dwell time after induction of inflammation, compared with Cx3cr1GFP/+ mice (Fig. S3 A and B). However, the percentage of ROS-producing neutrophils was reduced in Cx3cr1GFP/GFP mice (Fig. S3C), and this effect correlated with reduced renal injury as determined by albuminuria (Fig. S3D). Therefore, absence of CX3CR1 and consequent reduced monocyte retention were also associated with reduced neutrophil activation in the acutely-inflamed glomerulus.
Fig. S3.
ROS production by neutrophils is partly CX3CR1-dependent. The role of CX3CR1 in neutrophil recruitment and activation in inflamed glomerular capillaries was investigated using Cx3cr1GFP/GFP mice. (A and B) The number of neutrophils adhering in glomeruli per hour (Left) and neutrophil dwell time (Right) were compared in Cx3cr1GFP/+ and Cx3cr1GFP/GFP mice 0–1 h (A) (n = 6 each) and 1–2 h (B) (n = 12 and 9, respectively) after anti-GBM Ab injection. (C) Percentage of DHE+ neutrophils in Cx3cr1GFP/+ mice (n = 10) and Cx3cr1GFP/GFP mice (n = 9) 1–2 h after anti-GBM Ab administration. (D) Urinary albumin levels in Cx3cr1GFP/+ mice (n = 10) and Cx3cr1GFP/GFP mice (n = 9) after anti-GBM Ab administration. Data are shown as mean ± SEM. (C) *P < 0.05 vs. Cx3cr1GFP/+. (D) ***P < 0.001 vs. untreated; ##P < 0.01 vs. Cx3cr1GFP/+.
Monocytes and Neutrophils Interact in Glomerular Capillaries, and Inflammation Increases the Duration of These Interactions.
To interrogate the mechanism whereby monocytes regulate neutrophil function during glomerular inflammation, we next investigated the possibility that monocytes and neutrophils interact in the glomerular microvasculature. For these experiments, we used SDCM to facilitate simultaneous imaging of GFP+ monocytes and phycoerythrin (PE) or Dylight650 anti–Gr-1–labeled neutrophils in Cx3cr1GFP/+ mice. Imaging of Cx3cr1GFP/+ mice via SDCM showed similar neutrophil and monocyte numbers and dwell times under control and inflammatory conditions, compared with our findings obtained with multiphoton microscopy (Fig. S4 and ref. 20). In the absence of inflammation, neutrophils (exclusively Gr-1+ cells) formed contacts with monocytes (exclusively GFP+) present in glomerular capillaries. This behavior was observed in both posthydronephrotic and intact kidneys (Fig. 4 A and B and Movies S4 and S5), indicating that this behavior was unrelated to induction of hydronephrosis. Analysis of monocyte–neutrophil interactions demonstrated that ∼35% of all neutrophils (Fig. 4C) and ∼50% of all monocytes (Fig. 4D) retained in glomerular capillaries underwent interactions with the other cell type. In uninflamed glomeruli, these interactions lasted on average for 3 min (Fig. 4E). Neutrophils typically underwent physical contact with one monocyte during their retention in glomerular capillaries whereas each monocyte interacted with approximately two neutrophils (Fig. 4 F and G).
Fig. S4.
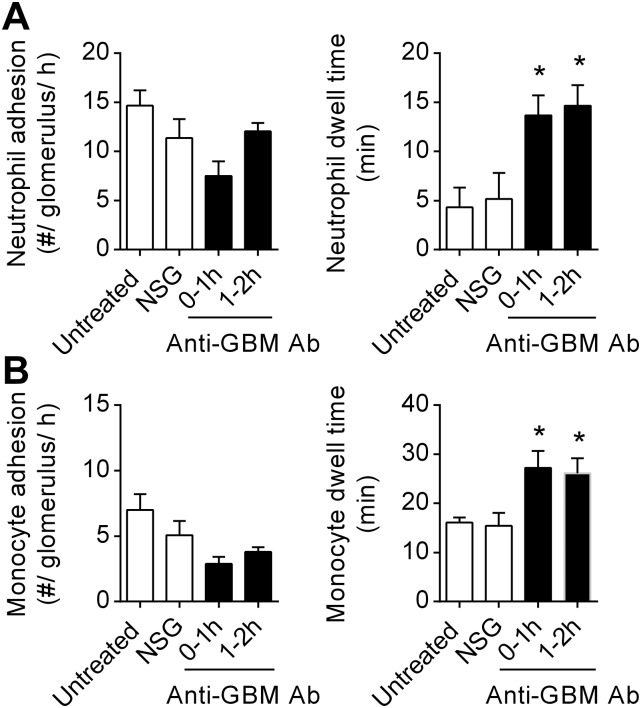
Neutrophil and monocyte dwell time in resting and inflamed glomerular capillaries, as assessed by SDCM. Neutrophil (A) and monocyte (B) recruitment were assessed in untreated Cx3cr1GFP/+ mice (n = 3), 0–1 h after NSG (n = 7), or 0–1 (n = 7) or 1–2 h after anti-GBM Ab injection (n = 8). Data are shown for the number of adherent neutrophils and monocytes (Left) and neutrophil and monocyte dwell times (Right). Data are presented as mean ± SEM; *P < 0.05 vs. NSG.
Fig. 4.
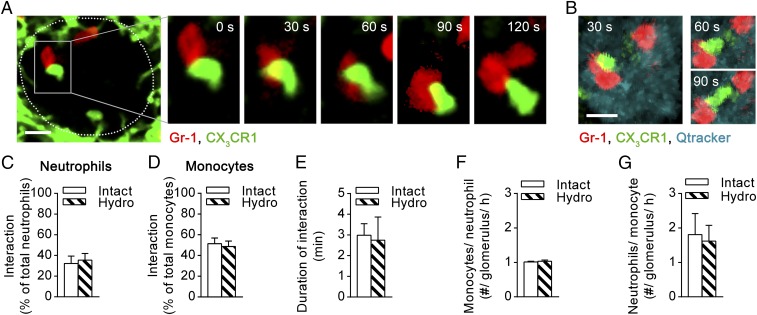
Neutrophils and monocytes undergo interactions in glomerular capillaries of uninflamed kidneys. Interactions between monocytes and neutrophils in glomerular capillaries were determined in untreated Cx3cr1GFP/+ mice using intravital microscopy. (A) Representative time-lapse SDCM images of a glomerulus (outlined) in a posthydronephrotic kidney. The region within the Inset (in the Left image) is enlarged in the Right panels to highlight a GFP+ monocyte (green) undergoing prolonged interaction with a neutrophil (red, Gr-1). Time stamp is shown at the top of the image. (Scale bar: 10 μm.) See also Movie S4. (B) Representative time-lapse multiphoton images of an intact kidney showing a neutrophil (red, Gr-1) within glomerular capillaries (blue, Qtracker) interacting with a GFP+ monocyte (green). (Scale bar: 10 μm.) See also Movie S5. (C–G) Percentage of interacting neutrophils (C) and monocytes (D), mean duration of interaction (E), and the number of monocytes interacting with neutrophils (F) and number of neutrophils interacting with monocytes (G) were quantified in the posthydronephrotic kidney (“Hydro,” n = 3) and intact kidney (“Intact,” n = 3). Data are presented as mean ± SEM.
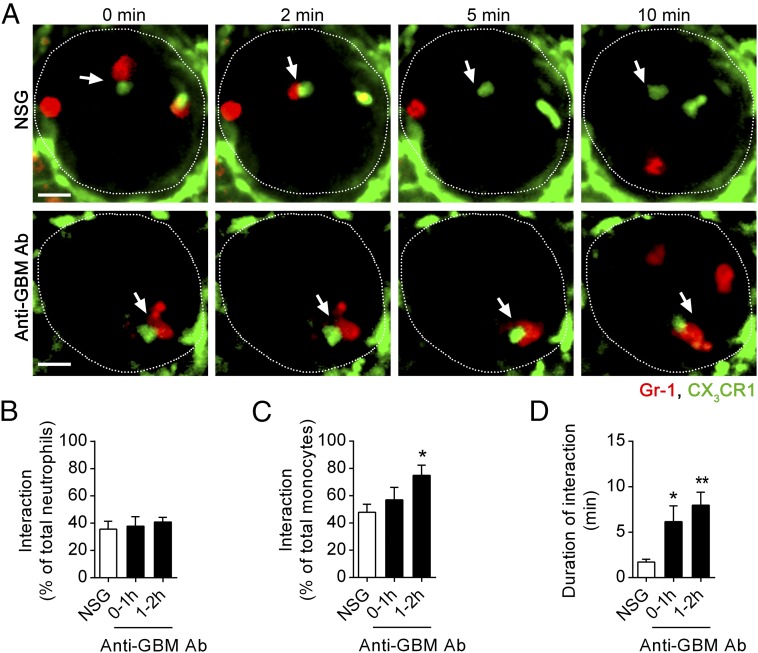
We next investigated whether these interactions were altered during glomerular inflammation, comparing control, normal sheep globulin (NSG)-treated, and anti-GBM Ab–treated mice. As seen in untreated mice, monocyte–neutrophil interactions were frequent in NSG-treated mice (Fig. 5 A–C), with a typical duration of 2–3 min (Fig. 5 A and D). During inflammation, the duration of interactions between neutrophils and monocytes increased to over 6 or 7 min, 0–1 h, or 1–2 h after injection of anti-GBM Ab, respectively (Fig. 5 A and D and Movie S6). Although the percentage of neutrophils interacting with monocytes within glomerular capillaries was similar under control and inflammatory conditions (Fig. 5B), a greater proportion of monocytes underwent interactions with neutrophils 1–2 h after anti-GBM Ab administration (Fig. 5C). Finally, we determined the timing of these interactions relative to the entry of the neutrophil into the glomerulus, which revealed that, in inflamed glomeruli, neutrophils form contacts with monocytes mainly, but not exclusively, early in their retention in the glomerulus (Fig. S5).
Fig. 5.
Monocyte–neutrophil interactions are prolonged in inflamed glomeruli. Monocyte–neutrophil interactions were examined in Cx3cr1GFP/+ mice treated with NSG control or anti-GBM Ab for 0–1 or 1–2 h, using SDCM. (A) Representative SDCM images of a glomerulus (outlined) from a mouse treated either with NSG (Top) or anti-GBM Ab (Bottom) for 1–2 h. Images illustrate the difference in the duration of interactions (indicated by arrows) between GFP+ monocytes (green) and neutrophils (red, Gr-1). Time stamp is shown at the top of images. (Scale bar: 10 μm.) See also Movie S6. (B–D) Percentage of adherent neutrophils interacting with monocytes (B) and adherent monocytes interacting with neutrophils (C), and duration of interaction (D) in mice treated with NSG for 0–1 h (n = 7) or anti-GBM Ab for 0–1 h (n = 7) or 1–2 h (n = 8). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01 vs. NSG control group.
Fig. S5.
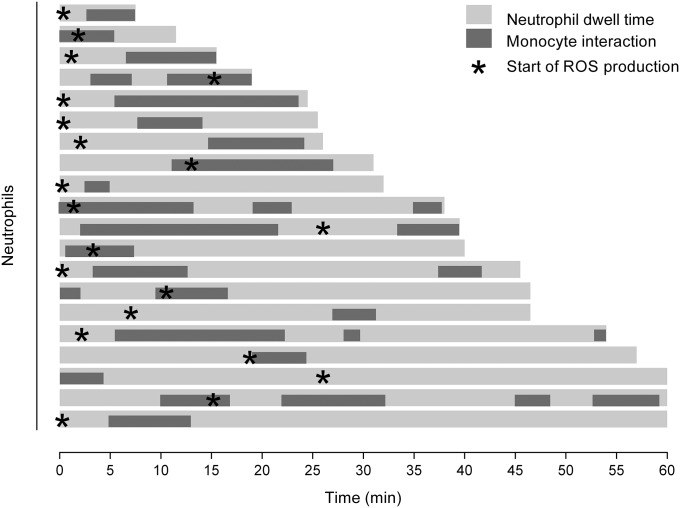
Timing of monocyte/neutrophil interactions and neutrophil ROS production relative to the timing of neutrophil entry into the glomerulus. Graphical representation of the timing of monocyte–neutrophil interactions after entry of the neutrophil into the glomerulus during the anti-GBM Ab response. Each light gray line represents the dwell time for an individual neutrophil, with the periods of interaction with monocytes represented by dark gray bars. Asterisks denote the time at which a DHE+ signal is first detected. Data represent exclusively DHE+ neutrophils that undergo an interaction with one or monocytes and are derived from eight mice examined 1–2 h after anti-GBM Ab administration.
Monocyte–Neutrophil Interactions Are Associated with Prolonged Neutrophil Dwell Time and Neutrophil Activation.
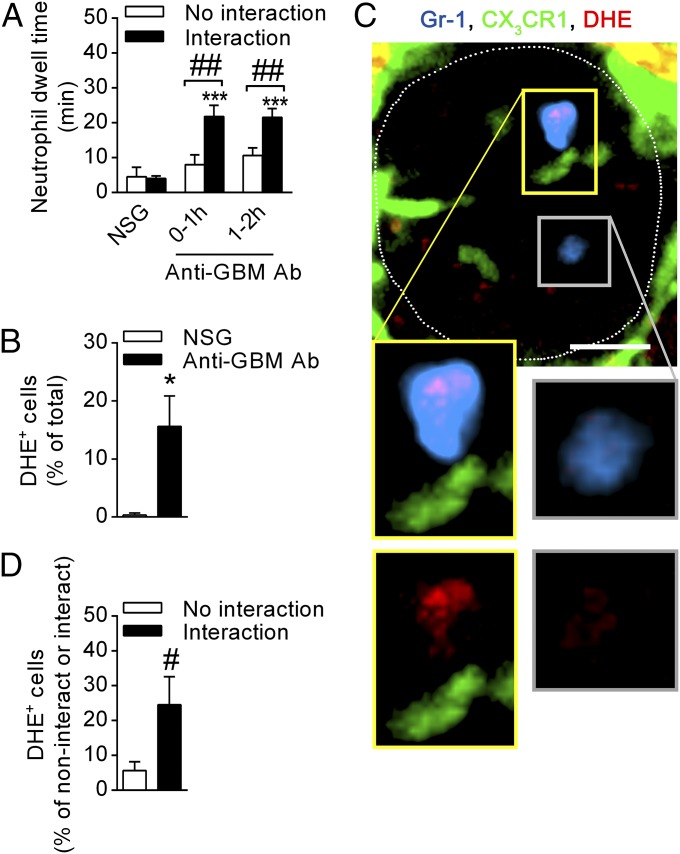
The above observations raised the possibility of a relationship between monocyte–neutrophil interactions and neutrophil activation during acute glomerulonephritis. To assess this hypothesis, we compared the responses of neutrophils that underwent interaction with monocytes with the responses of neutrophils that did not. Under resting conditions (NSG), dwell time was comparable between those that had and had not undergone interaction with a monocyte (Fig. 6A). In contrast, during inflammation, neutrophils that interacted with monocytes showed significantly prolonged dwell times 0–1 and 1–2 h after anti-GBM Ab injection whereas noninteracting neutrophils displayed similar dwell times to basal levels. These results are consistent with the hypothesis that interaction with monocytes regulates neutrophil responses in acute glomerulonephritis.
Fig. 6.
Monocyte–neutrophil interactions are associated with prolonged neutrophil dwell time and increased ROS production. Neutrophil behavior in relation to monocyte–neutrophil interactions was determined using SDCM in Cx3cr1GFP/+ mice, after NSG or anti-GBM Ab administration. (A) Neutrophil dwell time shown for neutrophils that did or did not undergo interaction with monocytes 0–1 h after NSG (n = 7), or 0–1 h (n = 7) or 1–2 h after anti-GBM Ab injection (n = 8). ***P < 0.001 vs. NSG; ##P < 0.01 vs. “No interaction.” (B–D) Data from separate experiments analyzing ROS production in interacting and noninteracting neutrophils. (B) Percentage of adherent neutrophils that were DHE+ 1–2 h after treatment with either NSG (n = 3) or anti-GBM Ab (n = 8). (C) SDCM images of a glomerulus (outlined) in a Cx3cr1GFP/+ mouse 1–2 h after anti-GBM Ab administration showing an oxidant-positive (red, DHE) neutrophil (blue, Gr-1) in close proximity to a GFP+ monocyte (green). An oxidant-negative neutrophil is also present. Insets in the Top image are enlarged in the Bottom panels and show neutrophils in the presence or absence of Gr-1 staining, for either an interacting neutrophil (panels outlined in yellow) and a noninteracting neutrophil (outlined in gray). (Scale bar: 10 μm.) See also Movie S7. (D) Percentage of DHE+ neutrophils that did or did not undergo interaction with monocytes 1–2 h after anti-GBM Ab (n = 8). Data are presented as mean ± SEM; *P < 0.05 vs. NSG, #P < 0.05 vs. “No interaction.”
To examine this hypothesis further, we performed additional experiments to compare ROS generation by interacting and noninteracting neutrophils. One to 2 h after anti-GBM Ab administration, ∼15% of intraglomerular neutrophils produced ROS as assessed by DHE-derived fluorescence (Fig. 6B), consistent with previous observations (20). Interestingly, neutrophils that underwent interactions with monocytes were fivefold more likely to produce ROS, compared with neutrophils that did not interact with monocytes (Fig. 6 C and D and Movie S7). In addition, comparison of monocyte/neutrophil interactions between DHE+ and DHE− neutrophils revealed that the mean duration of the interactions was significantly greater for DHE+ cells compared with DHE− cells (Fig. S6). However, analysis of the timing of initiation of ROS production by neutrophils after entering the glomerulus indicated that, in ∼50% of cases, neutrophils became DHE+ before undergoing a detectable interaction with a monocyte (Fig. S5). These findings provide evidence that interaction with monocytes can promote neutrophil retention and activation in the glomerulus but indicate that this interaction is not absolutely required for induction of ROS generation.
Fig. S6.
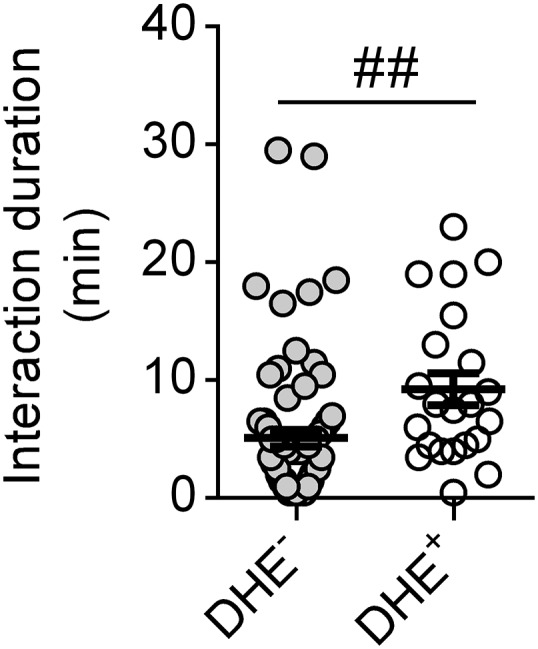
Comparison of the duration of monocyte/neutrophil interaction between DHE+ and DHE− neutrophils. The duration of monocyte/neutrophil interactions was determined in mice undergoing the anti-GBM Ab model, 1–2 h after anti-GBM Ab administration, and compared for DHE− and DHE+ neutrophils. Data points represent individual neutrophils and are derived from n = 8 mice. Mean ± SEM are also displayed; ##P < 0.01.
TNF Is Up-Regulated in Monocytes in the Inflamed Kidney and Regulates Neutrophil Activation and Glomerular Injury.
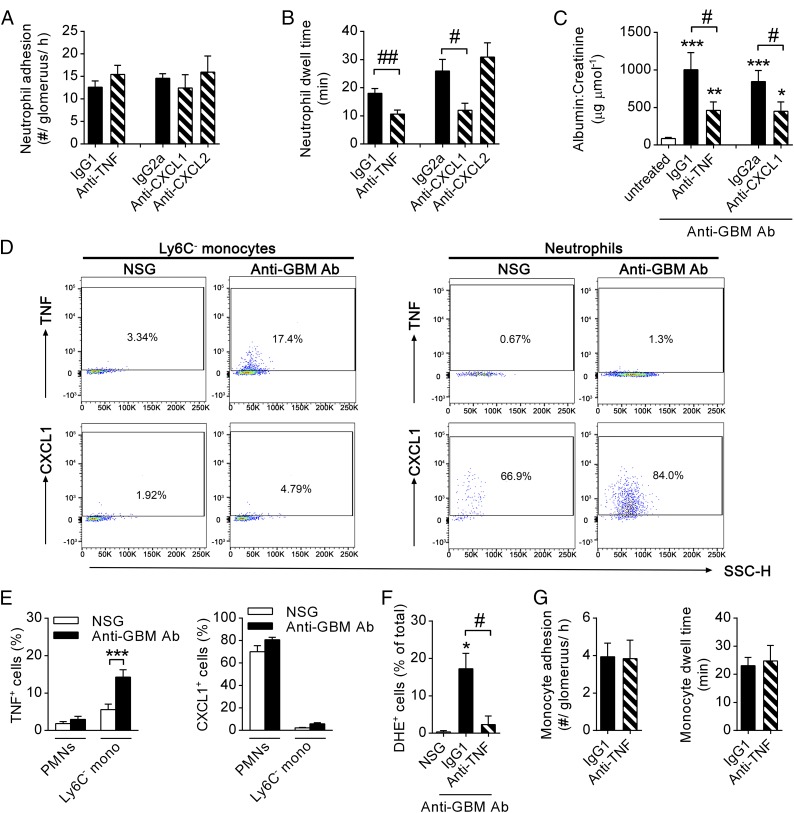
To investigate the molecular basis of monocyte-mediated promotion of neutrophil activation within the glomerular capillaries, we examined monocyte-derived cytokines with the potential to contribute to this process. Because CX3CR1hi Ly6C− monocytes (the monocyte subset observed to patrol the glomerular capillaries) have been demonstrated to be a source of potent neutrophil activators, such as TNF, CXC ligand (CXCL)1, and CXCL2 (5, 6, 30), we investigated the impact of these proinflammatory mediators on neutrophil function in our model. Although inhibition of TNF or CXCL1 did not change the number of adherent neutrophils in glomerular capillaries (Fig. 7A), neutrophil dwell time was markedly reduced compared with isotype control-treated mice (Fig. 7B). In contrast, inhibition of CXCL2 had no effect on neutrophil adhesion or dwell time (Fig. 7 A and B).
Fig. 7.
TNF and CXCL1 promote neutrophil functions, and TNF is up-regulated in patrolling monocytes, but not neutrophils, during inflammation. (A and B) Numbers of neutrophils adhering (A) and neutrophil dwell time (B) in WT C57BL/6 mice undergoing the anti-GBM Ab model after pretreatment with either anti-TNF (n = 10), anti-CXCL1 (n = 4), and anti-CXCL2 (n = 4), or appropriate isotype controls (n = 10 and 4, respectively) as determined using SDCM (anti-TNF) or multiphoton intravital microscopy (anti-CXCL1/2) 1–2 h after anti-GBM injection. (C) Urinary albumin/creatinine ratio in untreated C57BL/6 mice (n = 19) or in anti-GBM Ab-treated mice after pretreatment with either isotype control Ab (either IgG1, n = 12 or IgG2a, n = 10), anti-TNF (n = 12), or anti-CXCL1 (n = 10). (D) Representative flow cytometry dot blots of intracellular TNF and CXCL1 in CD45+ CD11c− Gr-1− CX3CR1+ monocytes and CD45+ CD11c− Gr-1+ CX3CR1− neutrophils from kidney homogenates of Cx3cr1GFP/+ mice treated with NSG or anti-GBM Ab. Gates for TNF and CXCL1 were defined based on isotype controls. Numbers within the blots indicate the percentage of TNF- or CXCL1-expressing cells. (E) Percentage of TNF+ or CXCL1+ renal neutrophils (PMNs) or Ly6C− monocytes from NSG or anti-GBM Ab–treated mice as shown in D (n = 7). (F and G) Percentage of DHE+ neutrophils (F), and monocyte adhesion and dwell time (G) in anti-TNF–treated (n = 5) or isotype control-treated Cx3cr1GFP/+ mice (n = 6 in F or n = 4 in G, respectively) 1–2 h after anti-GBM Ab, or after NSG injection (n = 3). *P < 0.05, ***P < 0.001 vs. untreated/NSG; #P < 0.05, ##P < 0.01 vs. isotype control.
We next examined the functional roles of TNF and CXCL1 in this model of in situ immune complex-mediated glomerulonephritis. Inhibition of TNF resulted in a significant inhibition of renal injury, as shown by a >50% reduction in urinary albumin:creatinine ratio (Fig. 7C), consistent with previously published results in similar models (31, 32). Similarly, inhibition of CXCL1 also resulted in a significant reduction in urinary albumin:creatinine ratio (Fig. 7C). These data indicate a contribution of both TNF and CXCL1, but not CXCL2, to neutrophil activation and glomerular injury in this model. To test the hypothesis that monocytes are responsible for the generation of these molecules, we assessed expression of TNF and CXCL1 by renal CX3CR1hi Ly6C− monocytes and neutrophils in response to anti-GBM Ab treatment. Only low frequencies of TNF-producing CX3CR1hi Ly6C− monocytes could be observed after NSG administration as detected via flow cytometry. In contrast, CX3CR1hi Ly6C− monocytes isolated from the kidney 2 h after anti-GBM Ab treatment displayed up-regulated TNF expression (Fig. 7 D and E). Notably, renal neutrophils did not produce TNF. In contrast, CXCL1 production was almost completely absent in CX3CR1hi Ly6C− monocytes but instead could be detected in the majority of neutrophils in both control (NSG) and inflamed (anti-GBM Ab) kidneys (Fig. 7 D and E).
To further investigate the impact of endogenous TNF on neutrophil function in our model, neutrophil ROS production was analyzed in Cx3cr1GFP/+ mice treated with anti-TNF blocking antibodies. Despite unaltered numbers of adhering neutrophils (Fig. 7A), inhibition of TNF resulted in a >80% reduction in the number of DHE+ neutrophils during the second hour of the anti-GBM Ab response (Fig. 7F). Of interest, monocyte recruitment was not affected in these mice and was comparable with mice injected with control IgG antibody (Fig. 7G). Together, these data demonstrate that anti-GBM Ab–induced inflammation triggers TNF production by monocytes in the kidney and that TNF contributes to renal injury by mediating prolonged neutrophil dwell time and neutrophil activation.
Discussion
The glomerulus is a unique high shear, high flow microvasculature that, despite comprising capillaries, is susceptible to leukocyte-mediated injury. We have previously observed, using advanced in vivo imaging, that both monocytes and neutrophils constitutively patrol the glomerular microvessels (20). However, whether these cells interact during development of glomerular inflammation has not been examined previously. Here, we demonstrate using monocyte depletion strategies that, during inflammation, monocytes facilitate neutrophil recruitment, retention, and ROS production in glomerular capillaries in addition to glomerular injury, providing evidence of a functional interaction between these populations. In addition, in vivo imaging revealed that, while constitutively migrating within the glomerular microvasculature, monocytes and neutrophils regularly undergo cell–cell contacts of several minutes' duration. Inflammation results in a significant increase in the duration of these interactions. Furthermore, neutrophils that interact with monocytes are subsequently more likely to be retained in the glomerulus, undergo activation resulting in ROS generation, and subsequently contribute to glomerular injury. These findings demonstrate that intravascular neutrophil–monocyte interactions occur in the unique microvasculature of the glomerulus and provide evidence that these interactions contribute to neutrophil-mediated glomerular injury and renal dysfunction.
Macrophages have been implicated in experimental acute glomerulonephritis previously (33–37), including in studies showing that passively transferred macrophages can promote acute glomerulonephritis (38). However, studies have not differentiated between the roles of the macrophage and the monocyte. Here, we show that nonclassical monocytes constitutively migrate within the glomerular capillaries and increase the duration of their glomerular retention in response to inflammatory stimulation, while remaining in the glomerular capillary lumen. During this time they interact with neutrophils and promote the proinflammatory actions of these cells. Depletion of monocytes from the circulation reduces neutrophil retention and activation, as well as albuminuria, indicating a role for monocytes in promoting acute neutrophil-dependent glomerular injury. Taken together, these observations support the concept that monocytes play a critical role in this form of glomerular disease.
The interventions used in this study do not specifically target either classical or nonclassical monocytes, and therefore these experiments do not define a specific role for either of these subsets. However, use of spinning disk confocal intravital microscopy enabled us to detect cells expressing CX3CR1 and Gr-1, which incorporates the Ly6C epitope, and therefore differentiate classical (Ly6C+, CX3CR1+) and nonclassical monocytes (Ly6C−, CX3CR1+). Using this approach, we found that the vast majority of monocytes patrolling in glomerular capillaries were Gr-1− and therefore Ly6C−. These observations indicate that the monocyte population interacting with neutrophils in the glomerulus, and therefore responsible for the monocyte-dependent phenotype observed in this study, were the nonclassical patrolling monocytes.
Previous studies have found evidence supporting a role for various monocyte populations in promoting neutrophil recruitment in other inflammatory settings. CCR2-expressing classical monocytes have been implicated as promoting neutrophil recruitment to the lung, in response to CCL2 and LPS, and to the joint, in an acute, antibody-dependent model of arthritis (9, 10), but not in response to local bacterial infection. These studies indicate that classical monocytes can promote neutrophil entry into sites of aseptic inflammation. Similarly, after intratracheal administration of bacteria, neutrophils were found to exit the pulmonary vasculature and cluster adjacent to recently transmigrated Ly6C+ monocytes in the lung interstitium, a response that was eliminated by clodronate-mediated monocyte depletion (8). These studies indicate that classical monocytes can also affect neutrophil recruitment after their extravasation. Nonclassical patrolling monocytes have also been shown to affect neutrophil behavior. In response to peritoneal Listeria monocytogenes infection, CX3CR1hi monocytes rapidly transmigrate and up-regulate expression of proinflammatory genes with the capacity to induce neutrophil recruitment, such as those encoding TNF and IL-1 (5). More recently, Carlin et al. showed that nonclassical monocytes constitutively patrol the microvasculature of the renal interstitium (6). Upon activation by exposure to a TLR7 ligand, these cells induced recruitment and clustering of neutrophils that subsequently mediated a localized inflammatory response. Here, we extend this work using a model of an important renal disease to demonstrate that, in the glomerular microvasculature, nonclassical monocytes undergo regular interactions with intravascular neutrophils, including in the absence of inflammation, and that this response is associated with neutrophil activation and ROS generation.
It was notable that, whereas clodronate treatment reduced neutrophil adhesion, the proportion of neutrophils that became DHE+ was not altered by this intervention (Fig. 3). In contrast, deletion of Cx3Cr1 resulted in a 50% reduction in the percentage of DHE+ neutrophils (Fig. S3). Furthermore, in contrast to the effect of clodronate, in Cx3Cr1GFP/GFP mice, neutrophil adhesion and dwell time were not modified whereas albuminuria was significantly attenuated. These findings indicate that these interventions have different mechanisms of action. We speculate, given the capacity of chemokine receptor-mediated signaling to promote leukocyte activation, that absence of this signal in Cx3Cr1GFP/GFP mice modifies the capacity of the monocyte to promote neutrophil activation independent of its capacity to promote recruitment.
Monocytes are capable of generating a wide range of inflammatory mediators with the potential to promote leukocyte recruitment (5, 6). Of these mediators, TNF has been previously identified as a critical mediator in in situ immune complex-mediated glomerulonephritis, via its promotion of adhesion molecule expression and glomerular barrier breakdown (31, 32). TNF is also a potent and rapid inducer of ROS production via stimulation of NAPDH oxidase activity in neutrophils (39), a pathway we have previously shown to be responsible for glomerular dysfunction in acute glomerular inflammation (20). Here, we show that TNF also promotes neutrophil retention in the acutely inflamed glomerulus. Depending on the inflammatory stimulus, TNF can be expressed by a range of cell types in the kidney. In inflammatory conditions affecting the glomerulus, these cell types include endothelial cells, mesangial cells, and podocytes, in addition to infiltrating immune cells (40). The present experiments show that, in this form of acute glomerular inflammation, monocytes are major producers of TNF although these findings do not exclude the possibility that TNF is also expressed by cells other than patrolling monocytes, including intrinsic renal cells. Nevertheless, we also demonstrated that monocyte depletion was sufficient to restore glomerular function, supporting the concept that monocyte-derived inflammatory mediators are central to this response. Inhibition of CXCL1, another product of activated monocytes capable of promoting neutrophil recruitment, also reduced glomerular neutrophil retention and protected against glomerular barrier dysfunction. However, in these experiments, monocytes did not produce significant amounts of CXCL1. These data support a model by which monocyte-derived TNF influences the inflammatory responses of intravascular neutrophils in the glomerulus but also show that additional prorecruitment mediators that are active in this response are generated from other sources.
It is important to note that these data indicate that interaction with monocytes is not absolutely required for neutrophil ROS generation. Clodronate-mediated monocyte depletion results in only a 50% decrease in the number of ROS-producing neutrophils, demonstrating that neutrophil ROS production is possible when monocyte numbers are significantly reduced. Similarly, some neutrophils that are not seen to interact with monocytes become DHE+, and indeed some neutrophils become ROS-positive very rapidly after entering the glomerulus and before coming into contact with monocytes. Nevertheless, these data indicate that monocytes do promote neutrophil ROS generation although further investigation is required as to the exact mechanism underlying this response.
Previous studies have examined the molecular basis of intraluminal crawling of patrolling monocytes in organs such as the skin, mesentery, and muscle (5, 6, 22). Constitutive monocyte migration in these locations has been found to be dependent on LFA-1 and CX3CR1. Here, we observed that, in the glomerular capillaries, monocyte retention was reduced in mice deficient in CX3CR1, consistent with a role for the CX3CR1/CX3CL1 pathway in the glomerulus. In contrast, it was necessary to inhibit both β2 and α4 integrins to reduce monocyte retention. Under inflammatory conditions in vessels of the renal interstitium and cremaster muscle, monocytes have been shown to use both LFA-1 and Mac-1 for crawling and, in some circumstances, VLA-4 for adhesion (6, 22). In the glomerulus, LFA-1 and Mac-1 showed distinct roles, in that LFA-1 was more important for initial recruitment of monocytes whereas Mac-1 contributed to their retention. CX3CR1 also contributed to monocyte retention, particularly after the first hour of the response. Taken together, these studies show that the molecular basis of monocyte crawling in the glomerulus is different from that documented in other vascular beds, particularly in the absence of inflammation. These observations support our previous findings that the mechanisms of leukocyte recruitment to the glomerular microvasculature are functionally distinct from those in most other vascular beds (20, 23, 41). For example, neutrophils undergo immediate arrest in glomerular capillaries, without a requirement for a prior rolling step, while retaining a functional role for P-selectin, an adhesion molecule traditionally involved in leukocyte rolling (23). In addition, neutrophils respond to inflammation primarily by increasing the duration of retention in the glomerular microvasculature without undergoing transmigration (20). The present findings provide further evidence of the distinct nature of leukocyte recruitment in this location.
In conclusion, monocytes play a key role in promoting the proinflammatory actions of intravascular neutrophils in the acutely inflamed glomerulus. This role is associated with the constitutive migration of both of these populations in the glomerular capillaries, behaviors that allow these cells to regularly encounter each other on a random basis. Under physiological conditions, monocyte patrolling may be important in maintaining homeostasis in the glomerulus. However, the current studies demonstrate that this ongoing immune surveillance by monocytes also underpins inappropriate glomerular inflammation in response to nephritogenic stimuli, such as immune complexes, and mediates glomerular dysfunction under these conditions.
Materials and Methods
Mice.
Cx3cr1GFP/+ and Cx3cr1GFP/GFP mice, expressing GFP under the CX3CR1 promotor (25), on a C57BL/6 background were bred in-house. C57BL/6 WT mice were obtained from Monash Animal Services. Mice were maintained on a standard chow diet. Male mice were used for most experiments. For imaging the intact kidney, both male and female mice were used. All experiments were approved by the Monash Medical Centre B Animal Ethics Committee.
Antibodies and Reagents.
Polyclonal sheep anti-mouse GBM Abs were prepared as described previously (23). Normal sheep globulin (NSG) as a control was prepared from nonimmune sheep serum using an identical protocol. Anti-mouse Gr-1 (clone RB6-8C5) was grown from hybridoma or purchased from eBioscience and conjugated to Alexa-488, E450, or PE. CD11c-PECy7 (N418) was also from eBioscience. Anti-mouse CD45.2-PerCPC5.5 (104) and the blocking antibodies anti-CD18 (GAME-46), anti-LFA-1 (M17/4), and anti-Mac-1 (5C6) were purchased from BD Bioscience. Anti-α4 integrin (PS/2) was grown in-house; anti-CXCL1 antibody (48415) and anti-CXCL2 antibody (40605) were from R&D Systems; and purified and PE-conjugated anti-TNF antibody (MP6-XT22) was from eBioscience. Purified (Bio X Cell) or PE-conjugated (eBioscience) rat IgG1, rat IgG2a, and rat IgG2b were used as control antibodies. Purified anti-mouse Gr-1, anti-mouse CXCL1, and rat IgG2a were conjugated to DyLight-650 using a commercially available kit (Thermo Fisher Scientific). DHE and Qtracker nontargeted Quantum Dots-655 were from Thermo Fisher Scientific. For monocyte depletion experiments, clodronate liposomes and control PBS liposomes were purchased from clodronateliposomes.com. Brefeldin A (Sigma-Aldrich) was used for intracellular cytokine detection. Collagenase D and DNase I were purchased from Roche Life Sciences.
Induction of Glomerular Inflammation.
To induce glomerulonephritis, anti-GBM Ab (15 mg in 300 μL of saline) was administered intravenously. As control for these experiments, NSG was used at the same dose. For imaging experiments, kidneys were examined either 0–1 or 1–2 h after anti-GBM Ab administration.
Renal Intravital Imaging.
Male mice subjected to unilateral ureteric ligation as previously described (23) were used between 16 and 18 wk of age, 12 wk after undergoing ureteric ligation. For intravital imaging, the posthydronephrotic kidney was prepared as previously described (20, 23, 42). Briefly, mice were anesthetized by initial i.p. injection of ketamine hydrochloride (150 mg/kg; Troy Laboratories) and xylazine (10 mg/kg; Pfizer) in saline, and the left jugular vein was catheterized for administration of further anesthetic, blocking antibodies, fluorochromes, and reagents. Mice were maintained on a heated platform at a constant temperature of 37 °C. The kidney was exteriorized, drained of urine, and extended over a heated viewing platform using 4/0 silk tied to the kidney capsule. The kidney was superfused with saline and covered with a coverslip. To visualize neutrophils, mice received anti–Gr-1 (2 μg; conjugated to Alexa-488, E450, PE, or DyLight 650) i.v. immediately before imaging. For multiphoton microscopy, the vasculature was labeled with Qtracker nontargeted Quantum Dots-655 (10 μL of 1:10 dilution i.v.).
Preparation of the Intact Kidney for in Vivo Imaging.
To image intact kidneys, 3- to 4-wk-old Cx3cr1GFP/+ mice were prepared as previously described (20). Mice were anesthetized, catheterized, and maintained on a heated platform as described above. The left kidney was exteriorized through a dorsal incision and immobilized in a heated well incorporated into a custom-built stage, where it was covered with saline and a coverslip. Superficial glomeruli located 50–100 μm below the surface of the kidney were visualized with the vascular label Qtracker-655 (10 μL of 1:10 dilution i.v.). To visualize neutrophils, mice received 2 μg of anti–Gr-1-PE i.v. immediately before imaging via multiphoton microscopy.
Multiphoton Microscopy.
In experiments involving the analysis of either monocytes or neutrophils, or imaging of the intact kidney, the renal microvasculature was examined using a Leica SP5 multiphoton confocal microscope (Leica Microsystems), incorporating a 20× water-dipping objective (N.A. 1.0) and a Spectra Physics MaiTai pulsed infrared laser (NewSpec) as previously described (20). Z-stack images of at least three glomeruli per mouse (within one field of view) were captured for 1 h. Images were collected every 30 s at a resolution of 512 × 512 pixels and a 6-μm step size. In experiments involving GFP (Cx3cr1GFP/+ or Cx3cr1GFP/GFP mice), images were acquired at 900 nm excitation; otherwise, 810-nm excitation was used. Emitted fluorescence was detected by nondescanned detectors with 432–482 nm, 500–550 nm, 575–605 nm, and 625–675 nm emission filters. Predefined settings for laser power and detector gain were used for all experiments.
Spinning Disk Confocal Microscopy.
Most experiments involving the simultaneous analysis of monocytes and neutrophils were performed using an UltraVIEW VoX spinning disk microscope system (PerkinElmer) mounted on an upright intravital microscope (Axioplan 2 Imaging; Carl Zeiss) equipped with a 20× water-dipping objective (N.A. 0.5) (43). The preparation was excited with 488-, 561-, and 640-nm lasers. Images of at least three glomeruli per mouse (within one field of view) were collected every 30 s for 1 h using a 512 × 512-pixel electron-multiplying charge-coupled device camera (C9100-13; Hamamatsu). Exposure times between 0.05 and 0.4 seconds for each channel were used. Data acquisition was controlled via Volocity (PerkinElmer).
Integrin, CXCL1, CXCL2, and TNF Inhibition.
For examination of the function of adhesion molecules or chemokines/cytokines, antibodies against CD18 (1 mg/kg), LFA-1 (5.6 mg/kg), Mac-1 (3.3 mg/kg), α4 integrin (2 mg/kg), TNF (3 mg/kg), CXCL1 (3.3 mg/kg), or CXCL2 (3.3 mg/kg) or the same dose of the respective isotype controls was injected i.v. 5 min before administration of anti-GBM Ab or, if untreated, 5 min before imaging.
Monocyte Depletion.
WT or Cx3cr1GFP/+ mice were depleted of circulating monocytes using clodronate as previously described (44). Mice were injected with 10 μL/g of clodronate liposomes i.v. into the tail vein 24 h before induction of inflammation. Control mice received the same dose of PBS liposomes. Multiphoton microscopy of the kidney of Cx3cr1GFP/+ mice demonstrated a >80% reduction in patrolling monocytes in glomerular capillaries after clodronate treatment (control, 6.4 ± 1.8 monocytes per glomerulus per hour; clodronate, 1.1 ± 1.5 monocytes per glomerulus per hour; n = 4 and 3, respectively, P < 0.01).
Detection of Reactive Oxygen Species.
Generation of ROS in leukocytes in the glomerulus was investigated using the oxidant-sensitive fluorochrome DHE in imaging experiments, as previously described (20). DHE allows detection of the intracellular superoxide anion (45). DHE stock was prepared as 15.7 mg in 1.5 mL of DMSO and stored at −20 °C. For experiments, 2 mg/kg (multiphoton microscopy) or 4 mg/kg (spinning disk microscopy) was used. DHE was diluted in 150 μL of saline warmed up to 60 °C and immediately injected i.v. into mice prepared for renal intravital microscopy. ROS production was examined 20 min after DHE injection and 1–2 h after anti-GBM Ab or NSG administration. In some experiments, mice were pretreated with clodronate liposomes or anti-TNF blocking Ab before anti-GBM Ab and DHE administration.
Image Analysis.
Image sequences captured by multiphoton (maximum projections) or spinning disk confocal microscopy were analyzed offline using IMARIS (Bitplane). The number of monocytes and neutrophils recruited to glomerular capillaries, as well as their duration of retention in the glomerulus (referred to as “dwell time”), were assessed as described previously (20). Briefly, monocytes and neutrophils were defined as adherent if they remained arrested in the glomerulus for at least two consecutive frames (30 s). Leukocyte dwell time was determined based on the number of frames leukocytes persisted in the glomerulus. Leukocytes were also characterized as either static or crawling, according to their behavior within the glomerulus. Neutrophils or monocytes were defined as interacting if they were in contact with the other cell type for at least two consecutive frames (30 s). In addition, the number and percentage of adherent neutrophils also positive for DHE staining were determined. Three to five glomeruli per mouse were analyzed and averaged, and data were plotted as mean per mouse (unless stated otherwise).
Assessment of Renal Injury.
Renal function was assessed by measuring albumin and creatinine levels in urine collections using a mouse albumin ELISA kit (Bethyl Laboratories) and an alkaline picric acid method and autoanalyzer, respectively. Urine was collected overnight commencing 2 h after anti-GBM Ab administration. To measure albuminuria/creatinine in monocyte-depleted mice, anti-GBM Ab was given 5 h after clodronate or control liposome injection. Data were expressed as albumin:creatinine ratio (µg/µmol).
Renal Leukocyte Isolation and Flow Cytometry.
For ex vivo analysis of renal cytokine/chemokine production, kidneys of Cx3cr1GFP/+ mice were removed 2 h after anti-GBM or NSG treatment (15 mg i.v.). Tissues were finely minced with a scalpel and digested for 25 min in collagenase D (1 mg/mL) and DNase I (100 μg/mL) in RPMI medium containing Brefeldin A (10 μg/mL) at 37 °C under agitation. Kidneys were further disrupted mechanically. Suspensions were then filtered through 100-μm nylon mesh, and red blood cells were lysed (46). For ex vivo restimulation, isolated cells were cultured at 1 × 106 cells per milliliter in the presence of Brefeldin A (10 μg/mL) in RPMI supplemented with 10% (vol/vol) FCS, penicillin, streptomycin, l-glutamine, and 50 mM β-mercaptoethanol at 37 °C (47). Three hours after restimulation, cells were washed and treated with mouse BD Fc Block (BD Biosciences) before being stained for the surface markers CD45, CD11c, and Gr-1. Subsequently, cells were fixed and permeabilized with a BD Cytofix/Cytoperm kit (BD Biosciences) and stained for intracellular TNF and CXCL1, or using the appropriate isotype controls rat IgG1 and rat IgG2a, respectively.
Flow cytometric data were acquired on a FACSFortessa (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc.). Patrolling monocytes were defined as CD45+ CD11b+ CD11c− Gr-1− CX3CR1+ cells, and neutrophils as CD45+ CD11b+ CD11c− Gr-1+ CX3CR1− cells.
Statistics.
Data are presented as mean ± SEM of n observations, where n represents the number of animals studied. Statistical differences between groups were analyzed using Student’s t test or ANOVA followed by Dunnett or Bonferroni multiple comparison tests using GraphPad Prism 6.0 (GraphPad Software). P values of <0.05 were considered significant.
Data in this paper will be made available on request after contacting the senior author via email.
Supplementary Material
Acknowledgments
We thank Dr. Camden Lo (Monash Micro Imaging) for assistance with imaging and image analysis and Dr. Sarah Snelgrove (Monash University) for training in multiphoton imaging of the intact kidney. This study was supported by National Health and Medical Research Council (NHMRC), Australia Grants 1045165 and 1064112 (to M.J.H. and A.R.K.), the Rebecca L. Cooper Medical Research Foundation, and ANZ Trustees. M.J.H. is an NHMRC Senior Research Fellow (Grant 1042775). M.F. was supported by an Erwin Schroedinger Fellowship from the Austrian Science Fund (Project J 3752-B28).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606253113/-/DCSupplemental.
References
- 1.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3-4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 3.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89(3):359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 6.Carlin LM, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grainger JR, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19(6):713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreisel D, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA. 2010;107(42):18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, et al. In vivo imaging implicates CCR2(+) monocytes as regulators of neutrophil recruitment during arthritis. Cell Immunol. 2012;278(1-2):103–112. doi: 10.1016/j.cellimm.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maus UA, et al. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: Role of the CCL2-CCR2 axis. J Immunol. 2003;170(6):3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler-Heitbrock L, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 12.Sunderkötter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Palframan RT, et al. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194(9):1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlmark KR, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 17.Hamers AA, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110(3):428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 18.Hanna RN, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110(3):416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi S, et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19(1):107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 21.Li W, et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J Clin Invest. 2012;122(7):2499–2508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol. 2010;185(11):7057–7066. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: A critical role for platelet-derived P-selectin in the absence of rolling. J Immunol. 2006;176(11):6991–6999. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- 24.Kitching AR, Holdsworth SR, Hickey MJ. Targeting leukocytes in immune glomerular diseases. Curr Med Chem. 2008;15(5):448–458. doi: 10.2174/092986708783503230. [DOI] [PubMed] [Google Scholar]
- 25.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landsman L, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113(4):963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 27.Auffray C, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206(3):595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misharin AV, et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Reports. 2014;9(2):591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152(10):5000–5008. [PubMed] [Google Scholar]
- 30.Schiwon M, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156(3):456–468. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkar AM, et al. Modulation of antibody-mediated glomerular injury in vivo by IL-1ra, soluble IL-1 receptor, and soluble TNF receptor. Kidney Int. 1995;48(6):1738–1746. doi: 10.1038/ki.1995.472. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan MS, et al. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993;91(2):577–587. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiner GF, Cotran RS, Pardo V, Unanue ER. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holdsworth SR, Neale TJ, Wilson CB. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit: Use of an antimacrophage serum. J Clin Invest. 1981;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thaiss F, et al. Mediator systems in a passive model of in situ immune complex glomerulonephritis: Role for complement, polymorphonuclear granulocytes and monocytes. Lab Invest. 1986;54(6):624–635. [PubMed] [Google Scholar]
- 36.Tang WW, Qi M, Warren JS. Monocyte chemoattractant protein 1 mediates glomerular macrophage infiltration in anti-GBM Ab GN. Kidney Int. 1996;50(2):665–671. doi: 10.1038/ki.1996.363. [DOI] [PubMed] [Google Scholar]
- 37.Huang XR, Kitching AR, Tipping PG, Holdsworth SR. Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol. 2000;11(2):262–269. doi: 10.1681/ASN.V112262. [DOI] [PubMed] [Google Scholar]
- 38.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63(1):83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 39.Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18(3):343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 40.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76(3):262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 41.Hickey MJ, Westhorpe CL. Imaging inflammatory leukocyte recruitment in kidney, lung and liver: Challenges to the multi-step paradigm. Immunol Cell Biol. 2013;91(4):281–289. doi: 10.1038/icb.2012.83. [DOI] [PubMed] [Google Scholar]
- 42.Devi S, et al. Platelet recruitment to the inflamed glomerulus occurs via an alphaIIbbeta3/GPVI-dependent pathway. Am J Pathol. 2010;177(3):1131–1142. doi: 10.2353/ajpath.2010.091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abeynaike LD, et al. Regulatory T cells dynamically regulate selectin ligand function during multiple challenge contact hypersensitivity. J Immunol. 2014;193(10):4934–4944. doi: 10.4049/jimmunol.1400641. [DOI] [PubMed] [Google Scholar]
- 44.Tacke F, et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203(3):583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao H, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102(16):5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan AJ, et al. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol. 2014;184(5):1411–1418. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Honda T, et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40(2):235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.