Significance
Angiogenesis is essential for the health of all vertebrates, but the outgrowth of new blood vessels also plays a role in disease by facilitating tumorigenesis, boosting inflammation, and causing blindness. Despite intensive investigation, pathological angiogenesis remains a formidable clinical challenge. Here we adopted an experimental strategy focusing on the bioelectric impact of neovascularization. Surprisingly, although the transmembrane voltage of vascular cells is known to regulate blood flow, no previous electrophysiological analysis of pathological angiogenesis has been reported. Using animal models and human specimens of retinal neovascularization, we discovered that neovascular complexes generate an extremely high voltage, whose transmission into the retinovascular network exerts a function-altering impact. Uncovering bioelectric mechanisms in the pathogenesis of neovascularization is likely to reveal new therapeutic targets.
Keywords: neovascularization, proliferative retinopathy, retinopathy of prematurity, proliferative diabetic retinopathy, retina
Abstract
Pathological angiogenesis, as seen in many inflammatory, immune, malignant, and ischemic disorders, remains an immense health burden despite new molecular therapies. It is likely that further therapeutic progress requires a better understanding of neovascular pathophysiology. Surprisingly, even though transmembrane voltage is well known to regulate vascular function, no previous bioelectric analysis of pathological angiogenesis has been reported. Using the perforated-patch technique to measure vascular voltages in human retinal neovascular specimens and rodent models of retinal neovascularization, we discovered that pathological neovessels generate extraordinarily high voltage. Electrophysiological experiments demonstrated that voltage from aberrantly located preretinal neovascular complexes is transmitted into the intraretinal vascular network. With extensive neovascularization, this voltage input is substantial and boosts the membrane potential of intraretinal blood vessels to a suprahyperpolarized level. Coincident with this suprahyperpolarization, the vasomotor response to hypoxia is fundamentally altered. Instead of the compensatory dilation observed in the normal retina, arterioles constrict in response to an oxygen deficiency. This anomalous vasoconstriction, which would potentiate hypoxia, raises the possibility that the bioelectric impact of neovascularization on vascular function is a previously unappreciated pathophysiological mechanism to sustain hypoxia-driven angiogenesis.
The abnormal growth of blood vessels is a key pathophysiological feature of numerous disorders, including tumorigenesis, arthritis, endometriosis, and retinopathies. Despite substantial progress from studies of patients and animal models, abnormal neovascularization remains a common threat to health and well-being. To help address this challenge, we devised a unique experimental approach to better understand neovascular pathophysiology. Because almost nothing is known about the electrogenic profile of neovascular complexes and how these complexes functionally interact with their parent vessels, we focused on the bioelectric features of neovascularization. These gaps in knowledge are surprising, considering that it is well established that the transmembrane voltage of vascular cells (1), as well as electrotonic cell–cell interactions within a vascular network (2–4), play vital roles in regulating blood flow.
In this electrophysiological analysis of pathological angiogenesis, we focused on abnormal vasoproliferation in rodent and human retinas. For multiple reasons, the retina is an ideal tissue for this undertaking. First is its clinical importance. Retinal vasoproliferation is the major cause of blindness in prematurely born infants (retinopathy of prematurity) and persons with diabetes (proliferative diabetic retinopathy) and sickle cell disease (sickle cell retinopathy). The hallmark of these disorders is abnormal growth of new blood vessels (neovessels) triggered by failure of the retinal vasculature to adequately supply oxygen and nutrients. Unfortunately, rather than improving the metabolic status, neovessels sprouting from the retinal vasculature extend aberrantly onto the surface of the retina, where they form preretinal neovascular complexes, which have a propensity to bleed and to detach the underlying retina, profoundly interfering with visual function.
A second reason for studying pathological angiogenesis in the retina is the availability of well-characterized rat and mouse models of retinal neovascularization (5–7). In the most commonly used rodent models, experimental alteration of the ambient oxygen level in early postnatal life disrupts retinal vascular development, resulting in the growth of neovessels onto the retinal surface, where blood vessels are never found under physiological conditions. The clinical relevance of these models is supported by the similarity of their preretinal neovascular complexes with those observed in infants with retinopathy of prematurity (ROP) (8). Of practical importance, the preretinal location of pathological neovascular complexes makes them relatively easy targets for electrophysiological recording.
A third experimental advantage is the ability to maintain rodent retinas ex vivo for many hours. Finally, the retina is an excellent tissue for beginning an exploration of the bioelectric impact of pathological angiogenesis because we can obtain electrophysiological recordings not only from rodent models, but also from human neovascular specimens excised during surgery for sight-threatening complications of proliferative diabetic retinopathy.
In this study, our perforated-patch recordings revealed that aberrant preretinal neovascular complexes generate extraordinarily high voltage. Owing to the bioelectric interactions between tufts of neovessels and the intraretinal parent vasculature, hyperpolarizing voltage is transmitted into the retinovascular network. When the number of neovascular complexes is abundant, this voltage input is substantial and boosts the membrane potential of retinal blood vessels to a suprahyperpolarized level. Associated with suprahyperpolarization, the vasomotor response to hypoxia is fundamentally altered. Instead of the compensatory vasodilation observed in normal retina, hypoxia triggers arterioles to constrict. Because this anomalous vasoconstriction would delimit oxygen delivery to the hypoxic sites of vasoproliferation, the bioelectric impact of pathological angiogenesis on vascular function may serve to sustain hypoxia-driven neovascularization.
Results
Vascular Suprahyperpolarization in Pathological Angiogenesis.
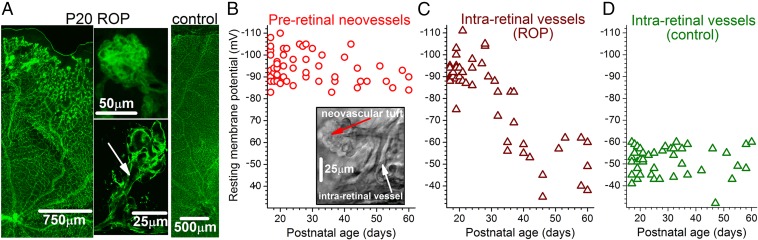
In initial experiments using the rat ROP model of Penn and coworkers (5), we measured vascular voltages in ex vivo retinas of postnatal day (P) 17–P20 pups. At this age, there was robust vasoproliferation in the context of an experimentally-induced retardation of retinal vascularization (Fig. 1A and Fig. S1 A and B). Perforated-patch recordings revealed that pathological neovascular complexes aberrantly located on the retinal surface had an extraordinarily high mean resting membrane potential of −95 8 mV (range, −83 to −110 mV; median, −94 mV; n = 23) (Fig. 1B).
Fig. 1.
Vascular voltages in ex vivo retinas of P17 to P60 ROP and control rats. (A) Ex vivo retinas stained with the endothelial marker isolectin GS-IB4. (Left) A P20 ROP retina with preretinal neovascular complexes in the periphery. (Center) Preretinal neovascular complexes shown at higher magnification. The lower panel is a confocal image with an arrow showing the lumen linking the neovascular tuft with its parent vessel. (Right) Control rat retina. (B) Resting membrane potentials of preretinal neovascular complexes in rat ROP retinas. (Inset) Image of an ex vivo P19 ROP retina in which a perforated-patch pipette was sealed onto the preretinal neovascular tuft. (C) Voltages of arterioles located in the superficial vascular layer beneath preretinal neovascular complexes of ROP retinas. (D) Arteriolar voltages in the superficial vascular layer of control retinas.
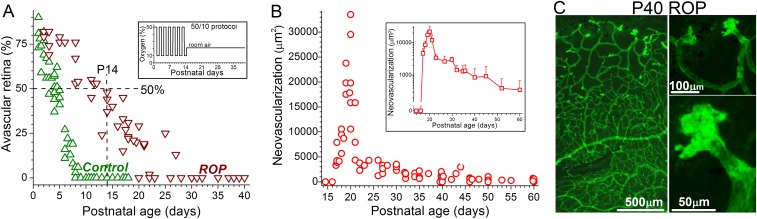
Fig. S1.
Physiological and pathological retinal vascularization in Long–Evans rats. (A) Percentage of avascular peripheral retina vs. postnatal age for control and ROP Long–Evans rats. Because the time course for retinal vascularization appears to vary in different strains of rats, we measured the percent of avascular retina during the development of the strain used in this study. Green triangles indicate control retinas; brown triangles, ROP retinas. This panel shows that 50/10 protocol delays retinal vascularization. (Inset) The 50/10 variable oxygen protocol. (B) Amount of preretinal neovascularization vs. postnatal age of ROP rats. (Inset) Averaged values plotted on a log scale. After physiological intraretinal angiogenesis results in vascularization of the peripheral retina, preretinal neovascular complexes start to regress dramatically, although some complexes persist for weeks. (C, Left) Isolectin GS-IB4–stained P40 ROP retina with residual neovascular complexes in the far periphery. (C, Right) Higher-magnification images of the neovascular complexes.
In addition, we found that intraretinal blood vessels located beneath clusters of preretinal neovascular complexes were also extremely hyperpolarized in P17–P20 ROP retinas. Using pipettes sealed onto abluminal mural cells located on the outer walls of arterioles with diameters of 10–20 µm, we measured a mean membrane potential of −92 6 mV (range, −75 to −103 mV; median, −92 mV; n = 16) (Fig. 1C). This suprahyperpolarization contrasts sharply with the mean membrane potential of −50 ± 6 mV (range, −41 to −60 mV; median, −51 mV; n = 14; P < 0.0001) recorded in arterioles located within ex vivo retinas of age-matched control rats (Fig. 1D).
To bolster the experimental support for suprahyperpolarization being a bioelectric feature of pathological retinal angiogenesis, we measured vascular voltages in another animal model, oxygen-induced retinopathy (OIR) in mice (6). In ex vivo retinas at P16−P18 (the period of abundant neovascularization in this model), the mean membrane potentials of preretinal neovascular complexes and intraretinal vessels were −87 ± 5 mV (range, −80 to −95 mV; median, −86 mV; n = 9) and −84 ± 8 mV (range, −68 to −92 mV; median, −83 mV; n = 8), respectively. In contrast, the mean resting membrane potential of blood vessels within age-matched ex vivo control mouse retinas was −62 ± 15 mV (range, −40 to −84 mV; median, −57 mV; n = 13; P ≤ 0.0012).
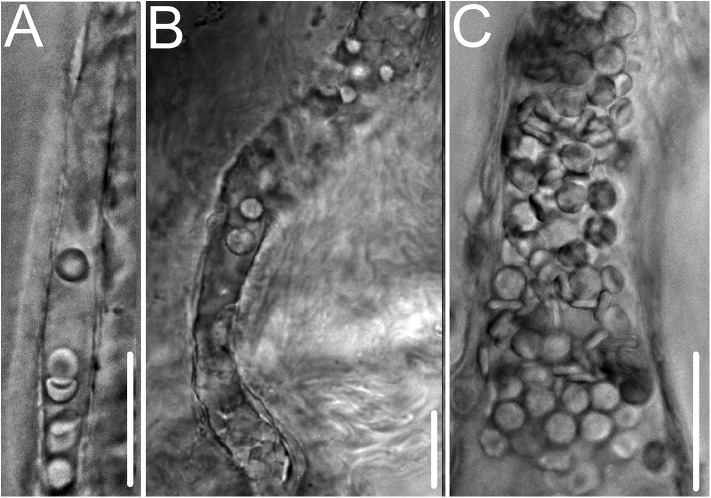
Having found extremely high voltages in the two most commonly studied animal models of retinal neovascularization, we then asked whether suprahyperpolarization is also a feature of pathological retinal angiogenesis in humans. To address this question, we obtained perforated-patch recordings from blood vessels within preretinal complexes freshly excised from adult patients undergoing surgery for complications of proliferative diabetic retinopathy (Fig. 2). In these surgical specimens, the mean vascular voltage was −100 ± 7 mV (range, −89 to −108 mV; median, −101 mV; n = 5 recordings from 4 specimens). Although limited by the uncommon availability of appropriate surgical tissue, these recordings support the pathophysiological concept that suprahyperpolarization is a bioelectric characteristic of pathological retinal angiogenesis in humans and rodents. Furthermore, suprahyperpolarization is a feature of neovascular complexes in mature as well as developing retinas.
Fig. 2.
Images of preretinal neovascular complexes excised from adult diabetic patients. Voltages were −104 mV in A, −89 mV in B, and −101 mV in C. Unlike the neovascular complexes of the rodent models, the surgical specimens had extensive fibrosis and had been in eyes that had received laser-induced retinal ablation and, in most cases, anti-vascular endothelial growth factor molecules. Despite these differences, suprahyperpolarization is a bioelectric feature of human, as well as rodent, retinal neovascularization. (Scale bars: 25 µm.)
Impact of Neovascular Regression on Suprahyperpolarization.
In additional experiments using the rat ROP model, we extended the age range studied to ascertain whether suprahyperpolarization persists as neovascular complexes regress spontaneously. Neovascular regression is common in human ROP and commences in the rat model during the fourth postnatal week when the peripheral retina becomes vascularized (Fig. S1 A and B). As shown in Fig. 1B, residual preretinal neovascular complexes remain suprahyperpolarized through at least P60 despite extensive neovascular regression (Fig. S1 B and C). In contrast to the persistent suprahyperpolarization of neovascular complexes, the membrane potential of blood vessels within ROP retinas decreases markedly after ∼P30 (Fig. 1C) to voltages similar to those of control retinas (Fig. 1D).
The observation that after P30, preretinal neovascular complexes remain suprahyperpolarized while the intraretinal vasculature depolarizes (Fig. 1 B and C) indicates that neovascular suprahyperpolarization is not dependent on voltage generated by intraretinal vessels. Additional strong evidence that neovascular suprahyperpolarization is not dependent on voltage derived from the retinovasculature is the previously noted finding that pathological neovascular complexes excised from the surface of human retinas exhibit an extremely high membrane potential of −100 mV. Thus, we conclude that suprahyperpolarization is an intrinsic bioelectric feature of pathological preretinal neovessels.
Neovascular-Driven Suprahyperpolarization.
To guide further experimentation, we formulated a bioelectric model based on the working hypothesis that intrinsically suprahyperpolarized preretinal neovessels electrotonically transmit voltage to underlying parent intraretinal vessels. This model predicts that when neovascular complexes are relatively abundant, the input of neovessel-generated voltage can boost the retinovasculature’s membrane potential to a suprahyperpolarized level. Conversely, when neovessels are sparse, their impact on the voltage of intraretinal vessels is minimal.
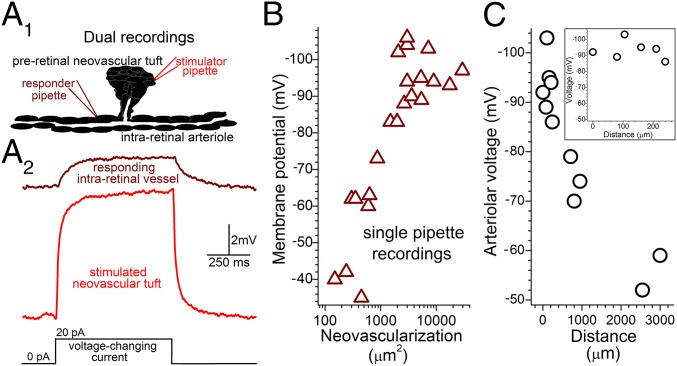
An essential feature of our interactive bioelectric model for pathological angiogenesis is that preretinal and intraretinal vessels are electrotonically coupled. To definitively demonstrate this coupling, we obtained simultaneous dual perforated-patch recordings from preretinal neovascular complexes and underlying parent vessels in ex vivo ROP retinas (Fig. 3A). In each of four successful dual recordings, the injection of a voltage-changing current via one of the recording pipettes resulted in a change in the membrane potential at the passively monitored site. In this series, electrotonic transmission was demonstrated in P17, P24, P42, and P53 ROP retinas; for these ages, the ratio of the voltage change detected at the responding site vs. the voltage change induced at the stimulated site was 0.15, 0.24, 0.40, and 0.15, respectively. Thus, preretinal and intraretinal vessels are electrotonically coupled. Furthermore, even though 98% of the neovascularization regressed by the sixth postnatal week (Fig. S1B), we detected electrotonic transmission between residual neovascular complexes and the retinovasculature.
Fig. 3.
Bioelectric interactions between preretinal neovascular complexes and intraretinal vessels in ex vivo ROP retinas. (A, 1) Schematic of simultaneous dual perforated-patch recordings. (A, 2) Voltage traces from a passively monitored intraretinal vessel (Upper) and a nearby preretinal neovascular complex (Lower), whose current-induced 8.4-mV depolarization caused a 2-mV voltage decrease at the passive site. (B) Intraretinal arteriolar voltages versus amount of preretinal neovascularization ≤100 µm from the recording site. (C) Voltages of intraretinal arterioles recorded in ex vivo P17–P22 ROP retinas at locations distant from preretinal neovascular complexes. 0-µm data are from Fig. 1C. (Inset) Enlarged distance scale showing the low decay rate of voltage spreading from sites of preretinal neovascularization.
In addition to dual recording experiments, data from single pipette recordings from ex vivo ROP retinas also lend support for a bioelectric model in which preretinal neovascular complexes drive the hyperpolarization of intraretinal vessels. As shown in Fig. 3B, the membrane potential of a sampled arteriole is correlated with the amount of pathological neovascularization in the locale (i.e., within 100 µm) of the recording site. When the total area of overlying neovascularization within a 100-µm radius is >10%, the underlying intraretinal vasculature is suprahyperpolarized; conversely, when the neovascular area regresses to <10%, as observed after ∼P30 (Fig. S1B), the voltage of intraretinal vessels drops. Taken together, data from dual and single recordings suggest that the decline in retinovascular voltage observed after P30 is not due to cessation of electrotonic transmission between preretinal and intraretinal vessels. Rather, it appears likely that retinovascular depolarization is a consequence of neovascular regression causing a decrease in the number of preretinal neovessels available to transmit hyperpolarizing voltage into the intraretinal vascular network.
Retinovascular Spread of Neovessel-Generated Voltage.
Although this bioelectric analysis of pathological angiogenesis focused chiefly on preretinal neovascular complexes and the intraretinal vessels located beneath these complexes, a critical question is whether the hyperpolarizing neovascular input spreads more widely through the retinovasculature. Using P17–P22 ROP retinas, we addressed this question by recording arteriolar voltages at sites distant from where the sampled vessel passed below preretinal neovascular complexes. As shown in Fig. 3C, suprahyperpolarization extends well beyond the location of neovascularization. Based on these data, it appears that as neovessel-generated voltage spreads efficiently along an intraretinal arteriole with decay rate of only ∼5% per 100 µm, which is strikingly similar to the highly interactive electrotonic architecture of the retinovasculature in normal adult rats (4). Thus, the bioelectric impact of pathological neovascularization extends well beyond its physical location in the retina.
Electrogenic Basis for Neovessel Suprahyperpolarization.
What electrogenic mechanisms account for neovascular suprahyperpolarization? To help address this question, we devised an experimental preparation consisting of microvessels (i.e., outer diameter <20 µm) freshly isolated from ROP retinas. Similar to the neovascular complexes in intact ROP retinas, isolated ROP vessels contain suprahyperpolarized (mean, −90 ± 15 mV; range, −60 to −118 mV; median, −92 mV; n = 87) complexes of endothelial cells (Fig. S2). This preparation was experimentally advantageous, because possible confounding effects mediated by nonvascular cells were eliminated, and also because the ability to obtain approximately three vessel-containing coverslips per retina allowed a marked reduction in the number of ROP rats required for electropharmacologic assays.
Fig. S2.
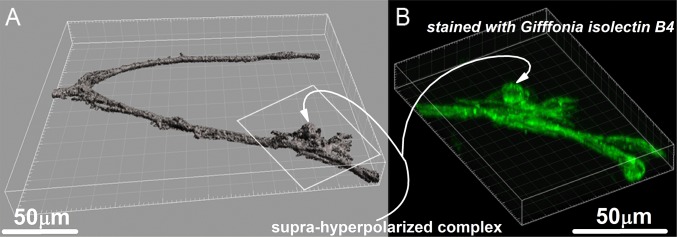
Isolated ROP retinal microvessel with a suprahyperpolarized complex. (A) A 3D surface reconstruction (surpass) of a microvessel freshly isolated from a P35 ROP rat. Before fixation, a perforated-patch pipette sealed onto the complex (outlined by the white box) recorded a resting membrane potential of −99 mV. Subsequently, the vessel was stained with isolectin GS-IB4, which binds to endothelial cells. (B) Reconstruction showing fluorescence at the binding sites of conjugated isolectin GS-IB4. Images were generated with Imaris 8.2 software (Bitplane) using 27 0.77-µm-thick slices.
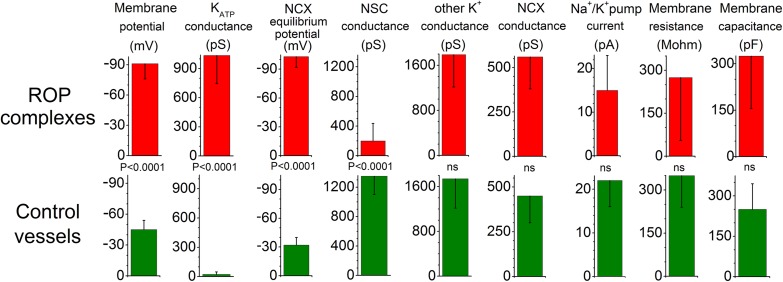
In a series of voltage-clamp experiments, perforated-patch pipettes were sealed onto the suprahyperpolarized complexes and inhibitors of various ion channels and electrogenic transporters/pumps were used to establish a bioelectric profile. For comparison, we also determined the electrogenic features of vessels isolated from control retinas. As summarized in Fig. 4, the major hyperpolarizing influences in the ROP complexes are (i) basally active ATP-sensitive potassium (KATP) channels, (ii) an extremely negative equilibrium potential for the electrogenic Na+/Ca2+ exchangers (ENCX), and (iii) a relatively small nonspecific cation (NSC) conductance. Supporting the validity of this bioelectric profile, a limited number of recordings from neovascular complexes located on the surface of ex vivo ROP retinas also detected a basal KATP conductance (1,350 ± 330 pS; n = 3), a markedly hyperpolarized ENCX (−110 ± 15 mV; n = 3), and a low NSC conductance (270 ± 95 pS; n = 3).
Fig. 4.
Bioelectric profiles. (A) Electrogenic parameters of suprahyperpolarized vascular complexes on freshly isolated ROP vessels. Lower panel, electrogenic features of vessels from control rat retinas. Table S1 provides additional data.
Of note, with ENCX being more negative than the equilibrium potential for K+ (EK = −90 mV), the outward current generated as NCX runs in its “reverse” mode (1 Ca2+ in, 3 Na+ out) is capable of driving the membrane potential beyond EK, which in fact was observed in 36 of 69 neovascular complexes sampled in ex vivo ROP retinas (Fig. 1B). Interestingly, although it is known that the reverse mode of NCX operation (rNCX) is critical for the angiogenic actions of vascular endothelial growth factor (9)—the target for existing medical treatments of vasoproliferative retinal disorders—there is no previous electrophysiological documentation of rNCX function in pathological angiogenesis.
How is the bioelectric profile of neovascular complexes established? Because preretinal neovascular tufts contain high levels of oxidants (10), we hypothesized that oxidation may play a role. To assess this possibility, we exposed isolated ROP vessels to the antioxidant n-acetyl cysteine (NAC; 100 µM). This treatment decreased the KATP conductance by 82 ± 12% (n = 6; P < 0.0001) (Table S1). This effect is consistent with the known redox sensitivity of retinovascular KATP channels, the activity of which in retinal vessels of normal rats is boosted by oxidants (11, 12). In contrast, this NAC treatment did not significantly affect ENCX or the NSC conductance (Table S1). Nevertheless, an impact of oxidation on the function of these electrogenic components cannot be completely excluded; more experiments might reveal a statistical significance of the small differences shown in Table S1. In fact, vascular ENCX appears to be somewhat oxidant-sensitive, with exposure of normal retinal microvessels to the oxidant, H2O2 (30 µM), modestly increasing the mean ENCX value from −32 ± 8 mV (n = 18) to −40 ± 6 mV (n = 6; P = 0.037). On the other hand, our earlier observation that H2O2 activates NSC channels in retinal vessels (13) is contrary to a scenario in which endogenous neovascular oxidants inhibit the NSC conductance. Taken together, our data indicate that oxidation is likely to account for the basal activation of KATP channels in neovascular complexes, although the mechanisms playing the predominant roles in minimizing NSC conductance and establishing an extremely negative ENCX remain uncertain and require future investigation.
Table S1.
Bioelectric parameters of microvessels isolated from control rat retinas and the suprahyperpolarized complexes of isolated ROP vessels in the absence or presence of the antioxidant NAC
| Electrophysiological parameter | Control vessels | ROP complexes | ROP complexes in NAC |
| KATP conductance, pS | 22 ± 26 | 1035 ± 280 | 190 ± 125 |
| n = 19 | n = 8; P < 0.0001 | n = 6: P < 0.0001 | |
| Non-KATP K+ conductance, pS | 1,740 ± 525 | 1,790 ± 575 | 1,590 ± 505 |
| n = 19 | n = 8; NS | n = 6; NS | |
| NCX conductance, pS | 450 ± 150 | 560 ± 180 | 460 ± 280 |
| n = 17 | n = 12: NS | n = 8; NS | |
| Equilibrium potential for NCX, mV | −32 ± 8 | −103 ± 11 | −88 ± 11 |
| n = 17 | n = 12; P < 0.0001 | n = 11; NS | |
| NSC conductance, pS | 1,350 ± 250 | 200 ± 235 | 440 ± 265 |
| n = 11 | n = 9; P < 0.0001 | n = 6; NS | |
| Na+/K+ pump current, pA | 22 ± 6 | 15 ± 8 | 13 ± 10 |
| n = 12 | n = 5; NS | n = 4; NS | |
| Resting membrane potential, mV | −45 ± 9 | −92 ± 15 | −84 ± 11 |
| n = 114 | n = 87; P < 0.0001 | n = 19; NS | |
| Membrane capacitance, pF | 250 ± 95 | 325 ± 170 | 275 ± 105 |
| n = 21 | n = 5; NS | n = 5; NS | |
| Membrane resistance, MΩ | 350 ± 110 | 275 ± 220 | 420 ± 210 |
| n = 114 | n = 87; NS | n = 19; NS |
Glibenclamide (500 nM), ouabain (1 mM), and OMR-10103 (5 μM) were used to inhibit ATP-sensitive potassium (KATP) channels, the sodium/potassium pump, and the sodium/calcium exchangers, respectively. In experiments using n-acetyl cysteine (NAC), vessels were exposed to 100 µM of this antioxidant in the bathing solution at room temperature for 2.5–3.5 h before recordings were obtained in the NAC-supplemented solution. To assess the effect of the various pharmacologic inhibitors on vascular currents, negative-to-positive voltage ramps (50 mV·s−1) were used to generate current-voltage (I-V) plots at 10-s intervals before and during exposure to a pharmacologic inhibitor. To determine the I-V relations of an inhibitor-sensitive current, the I-V plot generated ∼3 min after the onset of exposure to the inhibitor was subtracted from the I-V plot obtained before addition of the inhibitor. KATP conductances were determined between EK and ENSC. The Na+/K+ pump current at −50 mV is listed. The nonspecific cation (NSC) conductance was calculated between ENSC and EK by subtracting the ouabain-sensitive current and the OMR-10103–sensitive current from the total current measured at EK. The non-KATP potassium conductance between ENSC and EK was calculated by measuring the total current at ENSC and subtracting the glibenclamide-, ouabain- and OMR-10103–sensitive currents. Using current records filtered at 10 kHz, the membrane capacitance was determined from the transient charge induced by a 10- or 20-mV hyperpolarization from a holding potential of −58 mV, as described previously (36). Membrane resistance was calculated using the difference in the total currents measured at −100 mV and 0 mV in the absence of inhibitors. P values listed in the ROP complex column are for comparisons with control vessels; P values for the parameters in the NAC-treated group are for comparisons with the ROP complexes not exposed to the antioxidant. NS, not statistically significant. Of note, the relative hyperpolarizing influence of KATP channels, NSC channels, and NCX in the ROP complexes at a voltage of -45 mV were 0.4, 0.4, and 0.2, respectively.
Functional Impact of Suprahyperpolarization.
In our interactive bioelectric model of pathological retinal angiogenesis, we hypothesized that the electrotonic transmission of voltage from suprahyperpolarized neovascular complexes exerts a function-altering impact on the intraretinal vasculature. Specifically, we postulated that vasomotor responses mediated by a K+ channel-induced voltage change could be affected by suprahyperpolarization, because K+ channel activation cannot induce a change in voltage when the membrane potential is near EK, as is the case for <P30 ROP retinovessels (Fig. 1C). To assess this possibility, we monitored the vasomotor response to hypoxia, which triggers a compensatory vasodilation in normal retinas (14). The response to hypoxia was of interest because KATP channels are activated in hypoxic retinal vessels (12) and play a role in vasodilation (15). Of further relevance, pathological angiogenesis occurs in the context of retinal hypoxia.
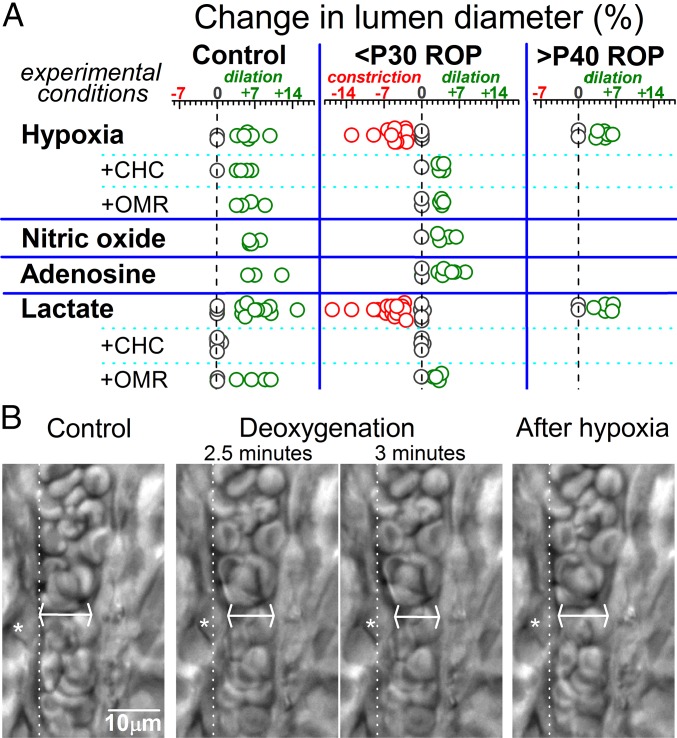
In a series of experiments, we monitored lumens of intraretinal arterioles (8–15 µm inner diameters). In <P30 ROP retinas, the monitored vessels were in areas containing preretinal neovascular complexes. As summarized in Fig. 5, the vasomotor response to hypoxia was fundamentally different in <P30 ROP retinas compared with control retinas. Namely, instead of the compensatory vasodilation observed in control retinas, exposure of <P30 ROP retinas to a deoxygenated bathing solution triggered constriction of arterioles. Consistent with the importance of suprahyperpolarization, hypoxia resulted in the dilation of >P40 ROP retinovessels (Fig. 5), whose resting membrane potentials are no longer suprahyperpolarized (Fig. 1C). These findings suggest that neovascular-driven suprahyperpolarization compromises the ability of the retinovasculature to ameliorate an oxygen deficiency.
Fig. 5.
Vasomotor responses in ex vivo retinas. (A) Arterioles with lumens of 8–15 µm were monitored during exposure to a deoxygenated perfusate (hypoxia), the NO donor sodium nitroprusside (100 µM), 5 µM adenosine, or 40 mM lactate. Responses to hypoxia and lactate were also tested in the presence of an inhibitor of monocarboxylate transporters, CHC (1 mM), and an NCX inhibitor, OMR-10103 (5 µM). (B) Images of an arteriole within an ex vivo P17 ROP retina before, during, and 5 min after exposure to a deoxygenated bathing solution. Asterisks denote a contracting abluminal cell, whose original position before hypoxia is shown by the dotted lines. At the site of maximal narrowing (arrows), the lumen decreased from 10.2 µm to 8.8 µm during hypoxia. In contrast to the compensatory vasodilation observed in control retina, the suprahyperpolarized vasculature of <P30 ROP retinas respond to hypoxia with an anomalous vasoconstriction.
Hypoxia-Induced Vasoconstriction: Role of Lactate.
What mechanism accounts for the vasoconstriction observed in hypoxic <P30 ROP retinas? We hypothesized that vasoactive molecules, such as adenosine, nitric oxide (NO), and lactate, which are released by hypoxic cells and have putative roles in angiogenesis (16–18), may play a role. As summarized in Fig. 5, neither adenosine nor the NO donor, sodium nitroprusside, mimicked the vasoconstrictive effect of hypoxia in ex vivo <P30 ROP retinas; instead, they caused arterioles to dilate. However, similar to hypoxia, exposure to lactate triggered arterioles in <P30 ROP retinas to constrict (Fig. 5). Also similar to hypoxia and consistent with lactate’s role as a vasodilator in the normal retinas (15, 19–21), arterioles within both control and >P40 ROP retinas—whose vessels were not suprahyperpolarized (Fig. 1C)—dilated in response to lactate (Fig. 5).
Indicative that the vasomotor effects of lactate are dependent on its uptake via monocarboxylate transporters (MCTs), lactate did not elicit a detectable change in arteriolar lumens in retinas pretreated with the MCT inhibitor 2-cyano-3-(4-hydroxyphenyl)-2-propenoic acid (CHC) (22) (Fig. 5). Furthermore, CHC’s prevention of hypoxia-induced vasoconstriction in <P30 ROP retinas (Fig. 5) indicates that this anomalous response is triggered by the uptake of endogenously produced lactate.
How does the uptake of lactate in <P30 ROP retinas result in vasoconstriction? Based on our earlier study of retinovessels (20), we considered a role for a cascade of events involving lactate, MCT, and NCX. Previously, we found that under certain conditions, the activation of this cascade can cause retinovessels to contract (20), although this vasoconstricting influence is usually vitiated by the potent vasorelaxing impact of lactate on precapillary arterioles (21), as well as on larger retinal arterioles, in which the activation of a voltage-changing KATP current plays a key role (15). However, we posited that the relative impact of the lactate/MCT/NCX pathway would be boosted when suprahyperpolarization to a voltage near EK, as observed in <P30 ROP retinas (Fig. 1C), precludes a K+ channel-induced change in voltage. Consistent with this scenario, we did not detect vasoconstrictive responses to lactate or hypoxia in <P30 retinas that had been pretreated with the NCX inhibitor OMR-10103 (23) (Fig. 4). Taken together, our pharmacologic experiments point to roles for endogenously produced lactate, monocarboxylate transporters, and Na+/Ca2+ exchangers in mediating the anomalous vasoconstrictive response to hypoxia observed in <P30 ROP retinas.
Discussion
This bioelectric analysis of pathological angiogenesis reveals that preretinal tufts of neovessels aberrantly sprouting from blood vessels within rodent and human retinas generate extraordinarily high resting membrane potentials. Electrophysiological recordings further demonstrated that neovessels and parent vessels interact bioelectrically. As a consequence of this electrotonic coupling, hyperpolarizing voltage is transmitted from neovascular complexes into the retinovascular network. When pathological neovascularization is extensive, the membrane potential of retinal vessels is boosted to a suprahyperpolarized level. We found that with this suprahyperpolarization, the vasomotor response to hypoxia is fundamentally altered, so that hypoxia triggers arteriolar constriction rather than the compensatory vasodilation observed in normal retinas. By delimiting the delivery of oxygen to hypoxic sites of abnormal vasoproliferation, this response of the vasculature to hypoxia may play a previous unappreciated role in the pathogenesis of neovascularization.
In this study, we used ex vivo rodent retinas to obtain electrophysiological recordings from vessels of an intact retina. A caveat is that the retinal vasculature was not internally perfused, because it is not currently feasible to obtain patch-clamp recordings from pressurized arterioles within ex vivo or in vivo retinas. Given that luminal pressurization is known to cause vascular depolarization (24, 25), it is likely that the membrane potentials measured in our study are higher than those in vivo. However, the difference in voltage is likely modest, because the small retinal arterioles (<20 µm outer diameter) sampled in our study have a reported internal pressure of ∼30 mm Hg in vivo (26), which causes a depolarization of only ∼10 mV as indicated in analysis of nonocular vessels (25).
Consistent with this modest effect of pressurization, the voltage of blood vessels recorded in nonperfused ex vivo control retinas (Fig. 1C) was <10 mV more negative than the vascular voltages measured at experimentally advantageous in vivo sites, such as the rat cremaster muscle (−42 mV) and the hamster cheek pouch (−46 mV) (27, 28). Thus, a lack of internal pressurization does not account for the suprahyperpolarized membrane potentials of ∼-90 mV that we recorded in arterioles of nonperfused ex vivo <P30 ROP retinas (Fig. 1C). Furthermore, because blood flow in preretinal neovascular complexes in vivo is sluggish (29), it is unlikely that the suprahyperpolarized membrane potentials recorded in neovascular complexes (Fig. 1B) is due to a lack of internal perfusion. Thus, although it would be ideal to obtain electrophysiological recordings from vessels in internally perfused ex vivo retinas or in retinas in vivo, the foregoing considerations support the pathophysiological concept that suprahyperpolarization is a bioelectric characteristic of pathological retinal angiogenesis.
Because no previous electrophysiological analyses of pathological angiogenesis have been reported, it remains to be established whether suprahyperpolarization is a bioelectric feature of neovascularization at nonretinal sites. Nonetheless, because the fundamental mechanisms for hypoxia-driven neovascularization almost certainly evolved to meet the needs of nonretinal tissues, we posit that this may be the case. With retinal neovascularization only recently impacting health owing to increased life expectancies of premature infants and patients with diabetes or sickle cell disease, there has been little time for evolutionary pressure to optimize neovascular mechanisms to meet the unique anatomic and physiological challenges of the retina (30). In nonretinal tissues, neovascularization is well adapted to enhance functional recovery; for example, in stark contrast to pathological retinal angiogenesis, vasoproliferation in hypoxic muscle is a highly effective adaptive response, even though the neovessels grow aberrantly and fail to replicate the pattern of the original vascular network (31). In tissues in which hypoxia-driven vasoproliferation is beneficial, we propose that a mechanism in which neovascular-induced suprahyperpolarization causes vessels to constrict in response to a local oxygen deficiency is likely to sustain the hypoxic microenvironment needed to drive adaptive neovascularization. Unfortunately, in the retina, sustained neovascularization is never beneficial and causes blindness. Our hope is that elucidation of the bioelectric impact of pathological angiogenesis on vascular function will provide new targets for therapeutic intervention in vasoproliferative disorders of the retina. It will also be important to analyze neovascularization in nonretinal tissues and tumors from a bioelectric perspective, because this new approach is likely to lead to novel strategies for therapeutic interventions at these sites.
Materials and Methods
Experimental protocols for rodent and human tissue were approved by the University of Michigan’s Institutional Animal Care and Use Committee and Institutional Review Board. The University of Michigan’s Institutional Review Board approved the study without the need for patient consent, based on the experimental use of excised tissue that normally would be discarded during an intraocular procedure. Detailed information on the materials and experimental procedures used in this study is provided in SI Materials and Methods.
Rodent Models of Proliferative Retinopathy.
The 50/10 variable oxygen protocol of Penn and coworkers (5) was used to generate a pathological retinal neovascularization that closely resembles ROP in infants. In mice, the protocol of Smith et al. (6) was followed to create a model of OIR in which aberrant preretinal neovascularization is most abundant at ∼P17.
Experimental Preparations.
Numerous experiments were performed using intact ex vivo retinas of ROP rats, OIR mice, and age-matched controls. Another experimental preparation consisted of preretinal neovascular tissue excised from adult patients with diabetes undergoing surgery for sight-threatening complications of proliferative retinopathy. A third preparation consisted of microvessels (i.e., <20-µm diameter) freshly isolated from the retinas of normal and ROP rats aged P30–P60 (30). The vessels isolated from ROP retinas often contained ∼15- to 25-µm-diameter multicellular complexes with a positivity for the endothelial cell marker isolectin GS-IB4 (Fig. S2) and a suprahyperpolarization (mean, −90 ± 15 mV; range, −40 to −118 mV; median, −92 mV; n = 87) strikingly similar to the neovascular complexes observed in intact ROP retinas (Fig. 1A and Fig. S1C).
Electrophysiology.
Voltages and currents were monitored via perforated-patch pipettes. Ex vivo retinas and surgical specimens were viewed using differential interference contrast (DIC)/infrared (IR) optics at 400× using a 40× water immersion objective. Isolated microvessels were viewed with phase-contrast optics at 400× . In recordings from ex vivo retinas, pipettes were sealed onto preretinal neovascular complexes and/or abluminal mural cells on intraretinal arterioles (10–20-µm diameter) located within the superficial vascular layer underneath clusters of preretinal neovascular complexes. In surgical specimens, recordings were obtained from erythrocyte-containing vessels (Fig. 2). In the case of retinal microvessels isolated from control rats, recording pipettes were sealed onto abluminal cells. For microvessels from ROP retinas, their suprahyperpolarized endothelial complexes (Fig. S2) were the recording targets.
Isolectin Griffonia simplicifolia-IB4 Staining.
Fixed specimens were incubated with 5.7 µg/mL Alexa Fluor 488-conjugated isolectin GS-IB4 from G. simplicifolia (Molecular Probes and Life Technologies).
Quantification of Neovascularization.
Quantification was performed in living retinas positioned in the recording chamber and viewed with DIC/IR optics at 400× with a 40× water immersion objective. Measurements were from the 200-µm-diameter retinal region (i.e., a high-power field), in which the area covered by preretinal neovascular complexes was maximal. NIS Elements software (Nikon) aided this quantification.
Vasomotor Responses.
Images of arterioles (inner diameter, 8–15 µm; outer diameter, 10–20 µm) located in the superficial vascular layer of ex vivo retinas viewed with DIC/IR optics at 400× with a 40× water immersion objective were captured at ∼30-s intervals with a digital camera (Photometrics Cool Snap) using 50- to 90-ms exposures. In ROP retinas, monitored arterioles were in the locale of preretinal neovascular complexes. Offline, a software package (NIS Elements version 4.0) facilitated the lumen measurements used to calculate the change in mean diameter during exposure to experimental solutions. Of note, similar to in vivo observations (32), changes in lumen diameter of arterioles in ex vivo retinas were not uniform along a vessel; this study used the maximal change detected within the well-focused portion (∼25-µm long) of a monitored vessel.
Chemicals.
All chemicals were obtained from Sigma-Aldrich unless noted otherwise.
Statistical Analyses.
Values are presented as mean ± SD. Student’s t test was used to evaluate probability.
SI Materials and Methods
Animals.
Long–Evans rats (Charles River Laboratories) and C57bl/6 mice (Jackson Laboratory) were used to establish in-house breeding colonies. At all times, animals were maintained on a 12-h alternating light/dark cycle and received food and water ad libitum. Pups ≤P20 were euthanized by decapitation; older rodents were euthanized by a rising concentration of CO2.
The 50/10 variable oxygen protocol of Penn and coworkers (5) was used to generate pathological retinal neovascularization. Wthin 8 h of birth, a litter of 10–15 Long–Evans rat pups along with the mother was housed for 14 d in a custom-built chamber in which the oxygen level was cycled between 50% and 10% every 24 h by a gas controller (OxyCycler; Biospherix). Subsequently, the rats were maintained in room air.
As shown for Long–Evans rats in Fig. S1, the 50/10 protocol slows physiological intraretinal angiogenesis, and on a return to room air, there is robust physiological angiogenesis within the retina but also extensive pathological angiogenesis, in which neovessels aberrantly extend from intraretinal parent vessels and form preretinal neovascular complexes (Fig. S1 A and B). When intraretinal vessels reach the retinal periphery early in the fourth postnatal week, pathological neovascular complexes undergo regression, although some preretinal complexes persist for weeks (Fig. S1 B and C).
Following the protocol of Smith et al. (6) for generating an OIR model in mice, a litter of C57bl/6 pups was placed at P7 with their mother in chamber in which the oxygen level was maintained at 75% for 5 d. After a return to room air, there was robust intraretinal angiogenesis as well as aberrant preretinal neovascularization, most abundant at ∼P17.
Experimental Preparations.
Ex Vivo Rodent Retinas.
After enucleation, eyes were placed in a 60-mm Petri dish containing 5 mL of Hibernate A (Life Technologies), and the retinas were removed, vitreous was debulked, and four relaxing incisions were created. To facilitate the complete removal of adherent vitreous (the presence of which precludes formation of a successful seal between the tip of a recording pipette and a vessel), each retina was incubated at 30 °C for 11 min in a 1.5-mL microcentrifuge tube containing 500 µL of Hibernate A supplemented with 12 U of collagenase (Worthington Biochemical) and 50 U of hyaluronidase (Worthington Biochemical), as described by Schmidt and Kofuji (33). Gentle bubbling of 100% oxygen kept the retina suspended in the incubation solution. To facilitate removal of adherent vitreous, the retina was placed in Hibernate A and gently sucked into and out of a large-bore (5 mm) pipette 11 times. This process did not fragment the retina. The retina was then transferred to a custom-made glass-bottom recording chamber (1 mL volume), which was initially dry. This transfer was facilitated by positioning a ∼4 × 8-mm piece of lens paper (catalog no. 110996; Thermo Fisher Scientific) underneath the retina with the inner vitreal surface facing up. Once in the chamber, a #3 paint brush moistened with Hibernate A was used to gently flatten the retina. Subsequently, a ∼4 × 8-mm piece of lens paper with a circular hole sized to touch the extreme peripheral edge of the retina was put in place.
The vitreous was completely removed using a fine forceps (Dumont #5; Fine Science Tools) to gently apply a continuous curvilinear tractional force at the peripheral edge of the posterior hyaloid, the membrane-like structure separating the vitreous from the retina. Once removal of adherent vitreous was complete, a harp-shaped tissue anchor (SHD26GH/10; Warner Instruments) was positioned to gently touch the retina and provide stabilization. Hibernate A was placed into the recording chamber. One retina was then positioned on the stage of an upright microscope, and the other retina was kept in a 100% humidity container until use in the experiment.
Human Surgical Specimens.
As is routine in surgical procedures performed in diabetic patients with tractional retinal detachment, preretinal neovascular complexes were carefully excised from the retinal surface and removed from the eye. These specimens were then placed on a Weck-Cel sponge (Beaver Visitec), which was promptly immersed in 1.5 mL of Hibernate A and transported to the laboratory. Of note, these preretinal specimens would normally be discarded during the intraocular procedure; the patients’ retinas remained in oculo. The selected excised specimens contained erythrocyte-containing vessels (Fig. 2) and ranged in size from ∼140 µm × ∼300 µm to ∼2 mm × ∼2 mm.
In the laboratory, a specimen was incubated for 30 min in the same collagenase and hyaluronidase solution as described above. After this incubation, the specimen was placed in Hibernate A and gently sucked into and out of a 5-mm bore pipette ∼25 times. This trituration step aided in the dislodgement of adherent vitreous, but did not cause tissue fragmentation. Subsequently, the specimen was placed in a Hibernate A-containing 35-mm Petri dish, in which fine forceps (Dumont #5; Fine Science Tools) and Vannas scissors (Fine Science Tools) were used to assiduously remove residual adherent vitreous. After vitreous removal, the specimen was positioned onto a piece of lens paper (catalog no. 110996; Thermo Fisher Scientific) and transferred to the same type of recording chamber as used for ex vivo retinas. An anchoring harp (Warner Instruments) or lens paper covering the outer ∼20 µm of one specimen edge was used to provide stability.
Freshly Isolated Retinal Vessels.
Our previously described tissue print procedure (30) was used to isolate microvessels from the retinas of normal and ROP rats aged P30–P70. For unknown reasons, this technique fails to yield viable retinal microvessels from <P30 rats. In brief, after euthanasia was induced by a rising concentration of CO2, retinas were quickly removed and incubated for ∼24 min at 30 °C in Earle’s balanced salt solution supplemented with 0.5 mM EDTA, 6 U of papain (Worthington Biochemical), and 2 mM cysteine. Subsequently, each retina was positioned vitreal surface up in a glass-bottom chamber containing a solution consisting of 140 mM NaCl, 3 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM Na-Hepes, 15 mM mannitol, and 5 mM glucose, pH 7.4, with osmolarity adjusted to 310 mosmol·L−1.
After adherent vitreous was removed, each retina was quadrasected, and each quadrant was gently compressed by a glass coverslip (Warner Instrument) onto which retinal microvessels adhered. This process was repeated several times with new coverslips. Typically, between four and eight microvessel-containing coverslips were obtained from a pair of retinas. Because retinovessels isolated by this technique have an outer diameter <20 µm, they are commonly referred to as microvessels (30). Vessels isolated from ROP retinas often contained ∼15- to 25-µm-diameter multicellular complexes whose positivity for the endothelial cell marker isolectin GS-IB4 (Fig. S2), as well as their suprahyperpolarization (mean, −90 ± 15 mV; range, −40 to −118 mV; median, −92 mV; n = 87), are strikingly similar to the neovascular complexes observed in intact ROP retinas (Fig. 1A and Fig. S1C). Of note, we have never observed similar complexes in any of many thousands of retinal microvessels isolated from normal Long–Evans rats.
Electrophysiology.
Voltage and current recordings were obtained via perforated-patch pipettes, which were sealed onto preretinal neovascular complexes or mural cells located on arteriole walls (diameters of 10–20 µm) located in the superficial vascular layer. Unless noted otherwise, sampled arterioles in ROP retinas were underneath clusters of neovascular complexes. Recordings were performed within 4 h after tissue removal from an eye. Vascular voltages and currents were monitored at 22–23 °C via 5- to 10-MΩ perforated-patch pipettes fabricated from thin-walled inner filament-containing borosilicate glass (TW150F-4; World Precision Instruments), filled with a solution consisting of 50 mM KCl, 65 mM K2SO4, 6 mM MgCl2, 10 mM K-Hepes, 60 μg·mL−1 amphotericin B, and 60 μg·mL−1 nystatin, at pH 7.35 and 280 mosmol·L−1, and mounted in the holder of the patch-clamp amplifier (Axopatch 200B; Molecular Devices).
Ex vivo retinas, isolated vessels, and surgical specimens were positioned in custom-built 1-mL chambers that could be perfused (∼2 mL/min) with solutions from a gravity-fed system using multiple reservoirs. A remotely controlled micromanipulator (MP-225; Sutter Instruments) facilitated the positioning of a recording pipette tip onto the targeted vascular site. A ≥10-GΩ seal was obtained by applying gentle suction to the back end of the recording pipette. For recordings in this study, access resistances were <35 MΩ, and voltage and/or current-voltage (I-V) relations were stable for ≥8 min. Voltages and currents were filtered with a four-pole Bessel filter, sampled digitally using a Digidata 1440A acquisition system (Molecular Devices), and stored by a computer equipped with pClamp version 10 (Molecular Devices), which, along with other software (Origin version 8; OriginLab), aided data analysis. Reported membrane potentials were from current-clamp recordings; adjustment for the junction potential of −8 mV was made offline.
A chamber containing an intact freshly isolated retina bathed in Hibernate A (Life Technologies) was placed vitreal side up on the stage of the upright microscope (Nikon Eclipse FN1) and viewed using DIC/IR optics at 400× using a 40× water immersion objective. In ROP retinas, recordings were obtained at sites of greatest preretinal neovascular complexes abundance. In ex vivo retinas ROP retinas, it was relatively straightforward to obtain perforated-patch recordings from preretinal neovascular complexes, which are easily seen, not covered by nonvascular tissue, and tend to easily form gigaohm seals with recording pipettes.
It was more challenging to obtain recordings from arterioles located within ex vivo retinas, which are located beneath the inner limiting membrane (ILM), a continuous, rather rigid sheath created at the retinal surface by the endfeet of Mȕller glial cells. To provide the recording pipette with unobstructed access to a targeted intraretinal vessel, this ILM barrier had to be disrupted. As described previously (33), ILM disruption was initiated by first poking a ∼3-μm-diameter tip of a bath-filled pipette through this barrier and then using pressure applied to the pipette’s backend to generate a puff of fluid that induced an observable 1,000- to 1,500-μm2 detachment of the ILM from the underlying retinal tissue. In surgically excised neovascular complexes, vessels were typically covered by a fibrous covering, which we disrupted using a technique similar to that described above for opening the ILM of ex vivo retinas. To avoid localized vessel trauma, we made no effort to remove abluminal cells to create direct access to the vascular endothelium.
In most cases, intraretinal recordings were from arteriolar sites located in the locale of clusters of preretinal neovascular complexes; however, in Fig. 3C, recording sites were distant from sites of neovascularization. For recordings in Fig. 3C at sites located ≤240 µm from a cluster of neovascular complexes, the sampled arterioles were observed to pass under a cluster of neovascular complexes. At greater distances, this was not ascertained, although recordings were within the same retinal sector, i.e., between the same primary arteriole and venule, as neovascular complexes.
In simultaneous dual perforated-patch recordings, we followed a protocol similar to our previous dual recording studies (4). Voltages were monitored in current-clamp mode while a 750-ms current step was injected via one of the pipettes at 4-s intervals for ∼2 min. The amplitude of the injected current was adjusted to cause a 7- to 10-mV voltage change at the stimulation site. Traces in which the voltages at each monitored site did not vary by ± 0.3 mV for the 200-ms period immediately before the onset of the current injection were averaged. Of note, conduction through the bathing solution did not contribute to the voltage change detected by the nonstimulated pipette, because pipette-to-pipette transmission was not detected when one of the pipettes was positioned close to, but not sealed onto, a vessel. Electrotonic transmission between preretinal and intraretinal vessels was detected with both depolarizing and hyperpolarizing voltage changes and from neovascular complex to abluminal cell or vice versa.
In recordings from isolated microvessels, voltage and currents were monitored via perforated-patch pipettes sealed onto vessels isolated from ROP and age-matched control rats. Vessels adhering to coverslips positioned in a recording chamber were viewed at 400× magnification with an inverted microscope equipped with phase-contrast optics. Unless noted otherwise, the bathing solution consisted of 140 mM NaCl, 3 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM Na-Hepes, 15 mM mannitol, and 5 mM glucose, at pH 7.4 and 310 mosmol·L−1. Recordings of retinal microvessels isolated from control rats were made via pipettes sealed onto abluminal cells. In the case of microvessels isolated from ROP retinas, pipettes were sealed onto the suprahyperpolarized endothelial complexes described above and illustrated in Fig. S2. Methodologic details of the electropharmacologic experiments used to generate the data for Fig. 4 and Table S1 are provided in the table legend.
Isolectin GS-IB4 Staining.
Tissue was fixed for 15 min in 4% paraformaldehyde at room temperature, washed in PBS, and then stored at 4 °C in PBS or at −20° C in 70% methanol. After additional PBS washes, the tissue was exposed to 5.7 µg/mL of Alexa Fluor 488-conjugated isolectin GS-IB4 from G. simplicifolia (Molecular Probes and Life Technologies) for 1 d at 4° C. Subsequently, the tissue was mounted in Prolong Gold antifade reagent (Molecular Probes). Images were captured with a Leica DM6000B fluorescence microscope and in some cases also with a Leica TCS SP5 confocal microscope using 0.77-µm-thick optical Z sections. Vessels did not show autofluorescence.
Area of Avascular Retina.
In isolectin GS-IB4–stained control and ROP retinas from Long–Evans rats at P0–P40, the peripheral edge of a retina and the region of avascularity were outlined and their areas computed using MetaMorph (Molecular Devices) and Leica Application Suite X, software. The percentage of retinal area lacking blood vessels was then computed (Fig. S1A).
Quantification of Neovascularization.
NIS Elements (Nikon) was used to demarcate each neovascular complex located within a 200-µm-diameter field (3.1 × 104 µm2). Because isolated retinas were not perfectly flat, careful focusing was required to ensure that each complex was in precise focus while being outlined. The software program calculated the total area of retina covered by preretinal neovascular complexes in the 200-µm-diameter locale. Of note, 3,000 µm2 is ∼10% of the locale.
It was advantageous to quantify neovascularization while the living retina was in the recording chamber, because we found that retinas are easily fragmented as the anchoring harp and adhering lens paper are removed after an electrophysiological recording session. This fragmentation almost always prevented relocation of the exact recording site and thus precluded a correlation of the amount of local neovascularization with the resting membrane potential of the sampled intraretinal vessel after fixation. In cases in which the recording site could be relocated in fixed retinas stained with isolectin GS-IB4 (because DIC optics cannot be used to visualize preretinal neovascular complexes after fixation), quantification of neovascularization closely matched the value obtained in the unstained viable retina.
Vasomotor Responses.
The lumen diameters of arterioles within ex vivo retinas were assayed at 22–23 °C within 4 h after their retinal isolation. Retinal vessels were not internally perfused. Monitored arterioles had inner diameters of 8–15 µm (outer diameters of 10–20 µm), were located in the superficial vasculature, and lacked pathological signs such as swollen endothelial cells or a speculated/compressed erythrocytes. In ROP retinas, sampled arterioles were in the locale of preretinal neovascular complexes. Because monitored arterioles were within a few microns of the retinal surface, superfused bathing solutions did not receive supplemental oxygen. Vessels were visualized with DIC/IR optics at 400× with a 40× water immersion objective, and images were captured at ∼30-s intervals by a digital camera (Photometrics Cool Snap) using 50- to 90-ms exposures.
The experimental protocol consisted of an initial 3 min in the control perfusate, then a 3-min exposure to the experimental solution, followed by a ≥3-min washout period. In most experiments, an ex vivo retina was exposed to the control perfusate for >10 min before initiation of the experimental protocol. In the case of control solutions containing the monocarboxylate transporter inhibitor CHC (1 mM) or the NCX inhibitor OMR-10103 (5 µM), exposure was for 3–4 min before initiation of the experimental protocol. The experimental bathing solutions used to assess the vasomotor effects of adenosine and NO consisted of Hibernate A supplemented with 5 µM adenosine or 100 µM of the NO donor, sodium nitroprusside. Control and washout perfusates were Hibernate A.
For experiments assessing vasomotor responses to lactate, perfusates were designed to have essentially the same sodium and chloride concentrations, as well as the same pH and osmolarity. Namely, the control and washout perfusates consisted of 10 mM lactate, 92 mM NaCl, 12 mM NaOH, 36 mM Na-gluconate, 3 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM Na-Hepes, 15 mM mannitol, and 5 mM glucose, at pH 7.4, with osmolarity adjusted to 310 mosmol·L−1. The experimental perfusate differed by containing 40 mM lactate and 48 mM NaOH and lacking Na-gluconate; its pH was identical to that of the control solution. In experiments assessing the vasoresponse to hypoxia, the experimental bathing solution was vigorously bubbled with 100% N2 for ∼15 min immediately before its entry into the recording chamber. The N2 bubbling had no effect on the pH of the perfusate, which consisted of Hibernate A supplemented with 10 mM lactate with pH adjusted to 7.4. Of note, both the control and the deoxygenated perfusates contained 10 mM lactate, which is the reported concentration of lactate in the normal rat retina in vivo (34), and which our preliminary studies indicated is required to elicit a detectable lumen change during a 3-min exposure to the deoxygenated bathing solution.
To help optimize the stability of focus throughout a protocol, an image of the vessel of interest was printed before initiation of the experimental protocol and was subsequently used to help determine whether refocusing between acquisitions was needed. A plane of focus was chosen approximately midway between the top and bottom of the selected vessel. An attempt was made to have one or more abluminal mural cells in the focus plane. Each ex vivo retina was exposed to only one experimental solution, and this was done only once.
Offline, NIS Elements version 4.0 (Nikon) facilitated the manual measurement of lumen diameters, which were made without knowledge of the composition of the experimental perfusate. As noted by Mishra et al. (35), DIC imaging most likely provides the most accurate method of lumen measurement, but must be done manually because algorithms for automated measurement are designed for the much higher contrast present when vessel walls are fluorescently stained. A vessel was not included for analysis if its lumen diameter fluctuated by >2% during the 3-min control period. In control experiments using vessels showing this level of stability (n = 5), lumen diameters did not change by >2% during an additional 5-min perfusion with the control solution. The percentage change in lumen diameter was calculated by comparing the mean lumen diameter in the final minute of the control period with the maximum 1-min average during exposure to the experimental solution. As noted in rat retinas in vivo (32), changes in lumen diameter are not uniform along a vessel. This study used the maximal change in lumen diameter measured within the well-focused portion of the monitored vessel, which is typically ∼25 µm long. This report includes data from all runs meeting the aforementioned criteria, including those in which no lumen change was detected.
Of note, the amplitude of the luminal responses observed in this study (i.e., ∼3–15%) appears to be within a physiological range, because flickering light evokes similar lumen changes in the small arterioles of the rat retina in vivo (32). However, in contrast to the responses of the small distal arterioles of rat retinas, large primary arterioles (diameters >65 µm) of the porcine retina respond to vasoactive signals, such as lactate, with a >20% increase in lumen diameter (15, 19). This larger response may reflect differing vasomotor mechanisms at different sites within the retinal vascular tree.
Acknowledgments
We thank B. Berkowitz for lending equipment and sharing information about the rat model, A. Barajas-Espinosa for sharing knowledge about the mouse model, A. Liepa for generating the rodent models, S. Lentz for assisting with confocal microscopy, D. Murrel for providing graphics expertise, and B. Hughes for reviewing drafts of the manuscript. This work was supported by National Institutes of Health Grants EY012507, EY20582, EY007003, and DK020572; the Alliance for Vision Research; and the A. Alfred Taubman Medical Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604757113/-/DCSupplemental.
References
- 1.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35(1 Pt 2):173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal SS, Neild TO. Conducted depolarization in arteriole networks of the guinea pig small intestine: Effect of branching of signal dissipation. J Physiol. 1996;496(Pt 1):229–244. doi: 10.1113/jphysiol.1996.sp021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: Modulation by angiotensin II. J Physiol. 2011;589(Pt 9):2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Doc Ophthalmol. 2010;120(1):3–12. doi: 10.1007/s10633-009-9180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 7.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DK, Kylstra JA, Greenman DB, Freedman SF. Significance of isolated neovascular tufts (“popcorn”) in retinopathy of prematurity. J AAPOS. 1998;2(1):52–56. doi: 10.1016/s1091-8531(98)90111-2. [DOI] [PubMed] [Google Scholar]
- 9.Andrikopoulos P, et al. Ca2+ influx through reverse-mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem. 2011;286(44):37919–37931. doi: 10.1074/jbc.M111.251777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuno Y, Nakamura-Ishizu A, Otsu K, Suda T, Kubota Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med. 2012;18(8):1208–1216. doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaki E, Fukumoto M, Puro DG. Functional KATP channels in the rat retinal microvasculature: Topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol. 2009;587(Pt 10):2233–2253. doi: 10.1113/jphysiol.2009.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakaizumi A, Puro DG. Vulnerability of the retinal microvasculature to hypoxia: Role of polyamine-regulated KATP channels. Invest Ophthalmol Vis Sci. 2011;52(13):9345–9352. doi: 10.1167/iovs.11-8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumoto M, et al. Vulnerability of the retinal microvasculature to oxidative stress: ion channel-dependent mechanisms. Am J Physiol Cell Physiol. 2012;302(9):C1413–C1420. doi: 10.1152/ajpcell.00426.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen L, Bek T. Diameter changes of retinal arterioles during acute hypoxia in vivo are modified by the inhibition of nitric oxide and prostaglandin synthesis. Curr Eye Res. 2015;40(7):737–743. doi: 10.3109/02713683.2014.954676. [DOI] [PubMed] [Google Scholar]
- 15.Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: Role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47(2):693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- 16.Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: A role for adenosine. Prog Retin Eye Res. 2003;22(1):95–111. doi: 10.1016/s1350-9462(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 17.Brooks SE, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42(1):222–228. [PubMed] [Google Scholar]
- 18.Sonveaux P, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3):e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazitikos PD, Pournaras CJ, Munoz JL, Tsacopoulos M. Microinjection of L-lactate in the preretinal vitreous induces segmental vasodilation in the inner retina of miniature pigs. Invest Ophthalmol Vis Sci. 1993;34(5):1744–1752. [PubMed] [Google Scholar]
- 20.Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(3):H925–H934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- 21.Jensen PS, Pedersen SMM, Aalkjaer C, Bek T. The vasodilating effects of insulin and lactate are increased in precapillary arterioles in the porcine retina ex vivo. Acta Ophthalmol. 2016 doi: 10.1111/aos.13025. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 23.Jost N, et al. ORM-10103, a novel specific inhibitor of the Na+/Ca2+ exchanger, decreases early and delayed afterdepolarizations in the canine heart. Br J Pharmacol. 2013;170(4):768–778. doi: 10.1111/bph.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: Actions of angiotensin II. Am J Physiol. 1997;273(2 Pt 2):F307–F314. doi: 10.1152/ajprenal.1997.273.2.F307. [DOI] [PubMed] [Google Scholar]
- 25.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glucksberg MR, Dunn R. Direct measurement of retinal microvascular pressures in the live, anesthetized cat. Microvasc Res. 1993;45(2):158–165. doi: 10.1006/mvre.1993.1015. [DOI] [PubMed] [Google Scholar]
- 27.Segal SS, Bény JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992;263(1 Pt 2):H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 28.Stekiel WJ, Contney SJ, Rusch NJ. Altered beta-receptor control of in situ membrane potential in hypertensive rats. Hypertension. 1993;21(6 Pt 2):1005–1009. doi: 10.1161/01.hyp.21.6.1005. [DOI] [PubMed] [Google Scholar]
- 29.Gass JD. Stereoscopic Atlas of Macular Diseases. 4th Ed. Mosby; St. Louis: 1997. Macular dysfunction caused by retinal vascular disease; pp. 437–599. [Google Scholar]
- 30.Puro DG. Retinovascular physiology and pathophysiology: New experimental approach/new insights. Prog Retin Eye Res. 2012;31(3):258–270. doi: 10.1016/j.preteyeres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dor Y, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21(8):1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci. 2014;34(34):11504–11513. doi: 10.1523/JNEUROSCI.1971-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt TM, Kofuji P. An isolated retinal preparation to record light response from genetically labeled retinal ganglion cells. J Vis Exp. 2011;(47):e2367. doi: 10.3791/2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkowitz BA, Bansal N, Wilson CA. Non-invasive measurement of steady-state vitreous lactate concentration. NMR Biomed. 1994;7(6):263–268. doi: 10.1002/nbm.1940070603. [DOI] [PubMed] [Google Scholar]
- 35.Mishra A, et al. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat Protoc. 2014;9(2):323–336. doi: 10.1038/nprot.2014.019. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K, Puro DG. Topographical heterogeneity of KIR currents in pericyte-containing microvessels of the rat retina: Effect of diabetes. J Physiol. 2006;573(Pt 2):483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]