Abstract
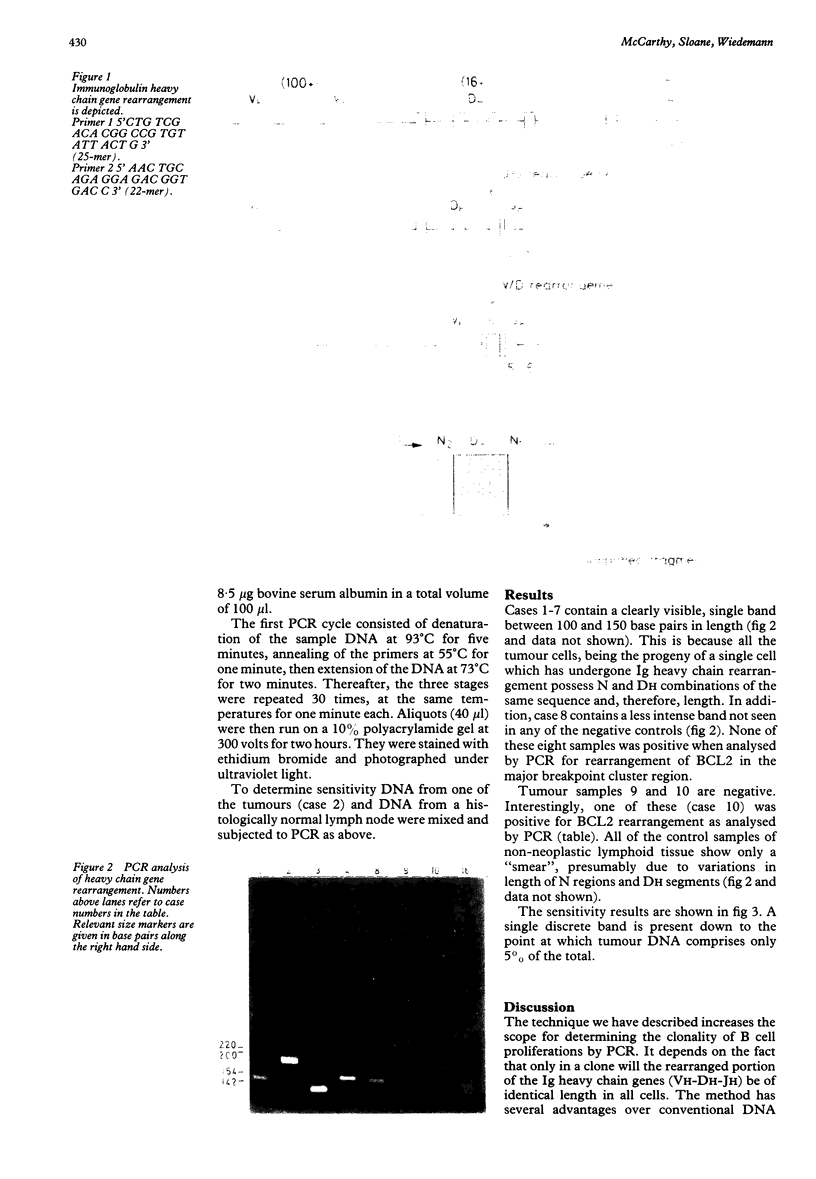
The polymerase chain reaction (PCR) was used to detect clonal rearrangements of the immunological heavy chain gene in frozen samples of human lymphoid tissue. DNA sequences in rearranged genes were amplified using oligomeric primers predicted from conserved sequences in the variable (VH) and joining (JH) regions. On polyacrylamide gel electrophoresis, polyclonal B cell proliferations showed a "smear", probably due to the variable lengths of the diversity (DH) region genes and the N regions separating the VH and DH and JH regions. In contrast, DNA from B cell lymphomas showed a clear single band in eight out of 10 cases. PCR undertaken on germ line DNA from non-lymphoid tumours showed no detectable bands or smears. The method can be completed within one day of biopsy, compared with several days in the case of conventional DNA blot analysis. Furthermore, it is cheaper, simpler, avoids the need for radioactive materials and requires very small amounts of DNA (about 1 micrograms).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Wright J. J., Graninger W., Seto M., Owens J., Cossman J., Jensen J. P., Goldman P., Korsmeyer S. J. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2396–2400. doi: 10.1073/pnas.84.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Ford A. M., Wiedemann L. M., Chan L. C., Furley A. J., Greaves M. F., Molgaard H. V. Rearrangement of immunoglobulin heavy chain genes in human T leukaemic cells shows preferential utilization of the D segment (DQ52) nearest to the J region. EMBO J. 1986 Dec 20;5(13):3467–3473. doi: 10.1002/j.1460-2075.1986.tb04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. J., Hughes T., Janssen J. W., Gow J., Guo A. P., Goldman J. M., Wiedemann L. M., Bartram C. R. Polymerase chain reaction for detection of residual leukaemia. Lancet. 1989 Apr 29;1(8644):928–929. doi: 10.1016/s0140-6736(89)92508-7. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Chang C. A., Levenson C. H., Warren T. C., Boehm C. D., Kazazian H. H., Jr, Erlich H. A. Diagnosis of sickle cell anemia and beta-thalassemia with enzymatically amplified DNA and nonradioactive allele-specific oligonucleotide probes. N Engl J Med. 1988 Sep 1;319(9):537–541. doi: 10.1056/NEJM198809013190903. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Bashir M. M., Givol I., Cossman J., Jaffe E., Croce C. M. DNA rearrangements in human follicular lymphoma can involve the 5' or the 3' region of the bcl-2 gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1329–1331. doi: 10.1073/pnas.84.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The arrangement of immunoglobulin and T cell receptor genes in human lymphoproliferative disorders. Adv Immunol. 1987;40:247–321. doi: 10.1016/s0065-2776(08)60241-2. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Yamada M., Hudson S., Tournay O., Bittenbender S., Shane S. S., Lange B., Tsujimoto Y., Caton A. J., Rovera G. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5123–5127. doi: 10.1073/pnas.86.13.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D., Voetdijk B. M., Van Ommen G. J., Kluin-Nelemans J. C., Beverstock G. C., Kluin P. M. Translocation t(14;18) in B cell lymphomas as a cause for defective immunoglobulin production. J Exp Med. 1989 Mar 1;169(3):613–624. doi: 10.1084/jem.169.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]