Significance
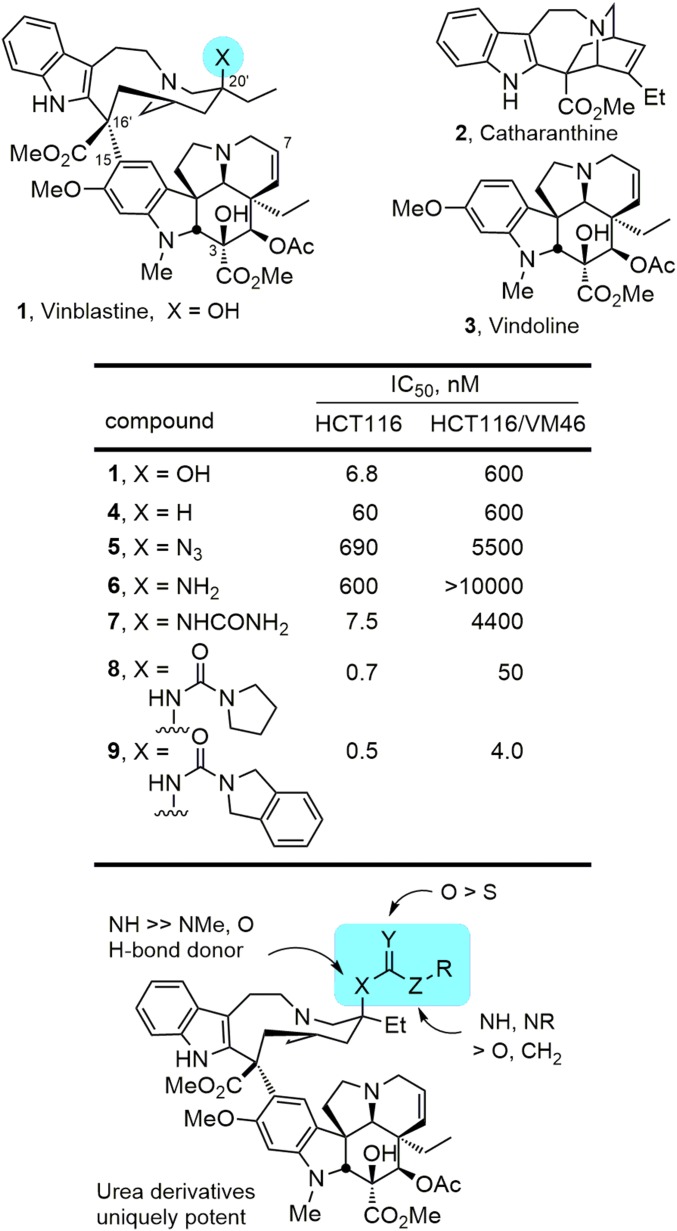
Vinblastine is a clinical drug used in frontline combination therapies for treatment of cancer. It acts by inhibition of mitosis through binding tubulin and disrupting microtubule formation. Because of advances in its total synthesis, we report previously inaccessible and unusual modifications to vinblastine that improve potency a remarkable 100-fold. These ultrapotent vinblastines display much higher tubulin binding affinities and likely further disrupt the tubulin head-to-tail dimer–dimer interaction by strategic placement of an added rigid, extended group along the adjacent continuing protein–protein interface. Significantly, the ultrapotent vinblastines are accessible by chemical synthesis in three steps from commercially available materials (catharanthine, $16/g; vindoline, $36/g) based on newly introduced synthetic methodology and are inaccessible by natural product derivatization, late-stage functionalization, or biosynthetic methods.
Keywords: vinblastine, drug design, chemical synthesis, tubulin, protein–protein interaction
Abstract
Approaches to improving the biological properties of natural products typically strive to modify their structures to identify the essential pharmacophore, or make functional group changes to improve biological target affinity or functional activity, change physical properties, enhance stability, or introduce conformational constraints. Aside from accessible semisynthetic modifications of existing functional groups, rarely does one consider using chemical synthesis to add molecular complexity to the natural product. In part, this may be attributed to the added challenge intrinsic in the synthesis of an even more complex compound. Herein, we report synthetically derived, structurally more complex vinblastines inaccessible from the natural product itself that are a stunning 100-fold more active (IC50 values, 50–75 pM vs. 7 nM; HCT116), and that are now accessible because of advances in the total synthesis of the natural product. The newly discovered ultrapotent vinblastines, which may look highly unusual upon first inspection, bind tubulin with much higher affinity and likely further disrupt the tubulin head-to-tail α/β dimer–dimer interaction by virtue of the strategic placement of an added conformationally well-defined, rigid, and extended C20′ urea along the adjacent continuing protein–protein interface. In this case, the added molecular complexity was used to markedly enhance target binding and functional biological activity (100-fold), and likely represents a general approach to improving the properties of other natural products targeting a protein–protein interaction.
Vinblastine and vincristine are superb clinical drugs successfully used in combination therapies for treatment of cancer (Fig. 1) (1–4). Vinblastine is used in frontline therapies for treatment of Hodgkin's disease, testicular cancer, ovarian cancer, breast cancer, head and neck cancer, and non-Hodgkin's lymphoma, whereas vincristine is used in the curative treatment regimens for childhood lymphocytic leukemia and Hodgkin's disease. Originally isolated from the leaves of Catharanthus roseus (L) G. Don (periwinkle) (5–8), vinblastine and vincristine were among the initial small molecules shown to bind tubulin and to inhibit microtubule formation and mitosis, defining an oncology drug target central to one of the most successful mechanisms of action still pursued today (9). As a result, they continue to be extensively studied due to interest in their complex dimeric alkaloid structures, their role in the discovery of tubulin as an effective oncology drug target, and their clinical importance (10–13).
Fig. 1.
Natural product structures and earlier results.
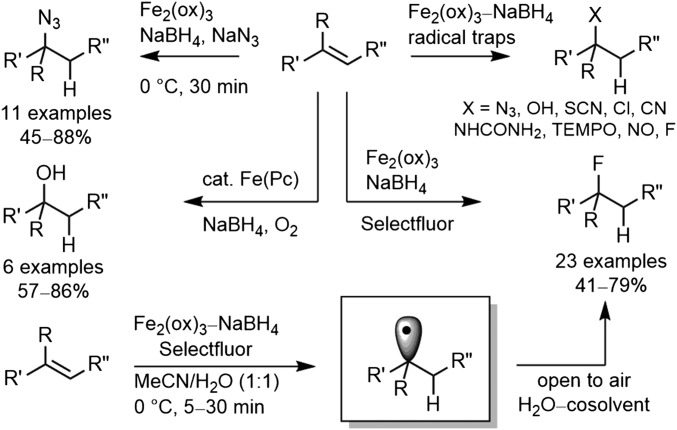
In the development of a total synthesis of vinblastine and vincristine, we introduced an Fe(III)/NaBH4-mediated free-radical oxidation of the anhydrovinblastine trisubstituted alkene for penultimate installation of the C20′ tertiary alcohol found in the natural products (14–16). This now-powerful hydrogen atom transfer (HAT)-initiated free-radical reaction was subsequently developed to provide a general method for functionalization of alkenes through use of a wider range of free-radical traps (17, 18) beyond O2 (air) and was explored specifically for the purpose of providing the late-stage, divergent (19) preparation of vinblastine analogs that bear alternative C20′ functionality at a site previously inaccessible for systematic exploration (Fig. 2) (17). In addition to the alternative free-radical traps that were introduced, the broad alkene substrate scope was defined, the addition regioselectivity was established, the outstanding functional group tolerance was demonstrated, a range of Fe(III) salts and initiating hydride sources were shown to support the reaction, its underlying free-radical reaction mechanism was refined, and mild reaction conditions (0–25 °C, 5–30 min, H2O/cosolvent) were developed that are remarkably forgiving of the reaction parameters (17, 18).
Fig. 2.
Hydrogen atom transfer (HAT) free-radical functionalization of unactivated alkenes.
Although the vinblastine C20′ site and its hydroxyl substituent were known to be important, the prior exploration of C20′ substituent effects had been limited to a handful of alcohol acylation reactions, the removal of the C20′ hydroxyl group, and a specialized set of superacid-mediated functionalizations (3). Our studies permitted systematic changes at C20′ where we initially demonstrated that incorporation of a C20′ azide (5) or its reduced amine (6) provided compounds 100-fold less potent than vinblastine, but that the conversion of the amine 6 to a C20′ urea (7) provided a compound with cell growth inhibition activity equal to vinblastine (Fig. 1) (17). In subsequent studies, we identified the key structural features of such ureas that contribute to their activity, including the importance of the H-bond donor site on the C20′ nitrogen substituent (20). We additionally defined a trend in activity where substitution of the urea terminal nitrogen improves the differential in activity of the derivatives against matched sensitive and resistant tumor cell lines (NR2 > NHR > NH2), discovered a series of potent disubstituted C20′ ureas (e.g., 8 and 9) that displayed further improved activity against resistant tumor cell lines, and established that sterically demanding C20′ ureas were surprisingly well tolerated (20, 21).
The target of vinblastine is the tubulin α/β dimer–dimer interface where its binding destabilizes microtubulin assembly derived from the repetitive head-to-tail tubulin binding (9, 22). This disruption of a protein–protein interaction by vinblastine is often overlooked in discussions of such targets as candidate, but challenging, biological targets to address with small molecules perhaps because the target identification preceded the contemporary interest (23–27). Herein, we report the discovery of compounds modified at C20′ that are now a stunning 100-fold more potent than vinblastine and that may initially look unusual in their structure. We also show that this increase in potency correlates directly with enhanced target tubulin binding affinity. Significantly, the remarkable potency of the compounds (IC50 values as low as 50–75 pM) suggest that it is not likely, or even possible, that their cellular functional activity is derived from stoichiometric occupancy of the relatively large number of intracellular tubulin binding sites, but rather implicates effective substoichiometric or catalytic occupancy of candidate binding sites sufficient to disrupt tubulin dynamics or assembly during mitosis. We suggest that the newly added linear and rigid C20′ urea substituents on vinblastine extend into and create a narrow channel adjacent to the vinblastine binding site, bind across this adjacent region of the protein–protein interaction defined by the tubulin dimer–dimer interface, further disrupt the tubulin α/β head-to-tail dimer–dimer interaction, and extend across the full protein–protein interface contacting solvent on the distal side of the binding site. This further disruption of the target protein–protein interaction by an added structural element to an already complex natural product (added molecular complexity) represents a unique but rational approach to substantially improve the functional properties of such molecules and likely represents a general approach to improving the properties of other natural products that target protein–protein interactions.
Results
Synthesis and Activity of Vinblastine C20′ Ureas.
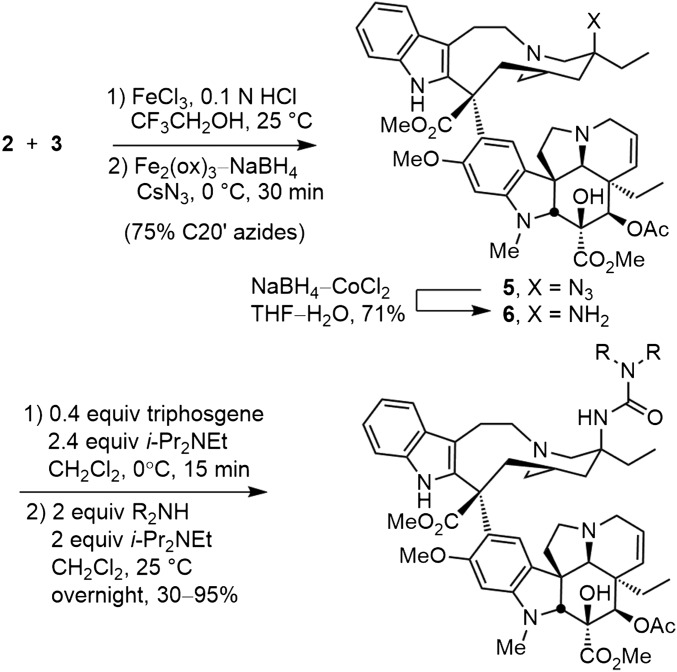
Two C20′ ureas 8 and 9 were prepared in our earlier studies that served as our starting points. The first of these is the pyrrolidine urea 8, which exhibited improved cell growth inhibition relative to vinblastine. Thus, a series of functionalized or substituted pyrrolidines were incorporated into the C20′ urea through reaction of 20′-aminovinblastine (6), derived from reduction of the hydroazidation product 5, with either p-nitrophenyl chloroformate (1.5 equiv 4-NO2PhOCOCl, 10 equiv Et3N, THF, 23 °C, 4 h) or triphosgene (0.4 equiv, 2.4 equiv i-Pr2NEt, CH2Cl2, 0 °C, 15 min) followed by treatment with a secondary amine to afford the product C20′ ureas in good yields (Fig. 3). The latter procedure that uses triphosgene represents an improvement in our original conditions (20), avoiding the purification challenges of removing residual p-nitrophenol from the reaction products. Both set of conditions generate the same intermediate C20′ isocyanate that in turn reacts with the added secondary amine to provide the product ureas.
Fig. 3.
Three-step synthesis of vinblastine C20′ ureas.
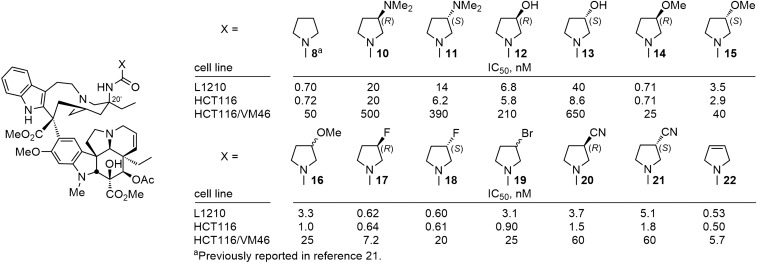
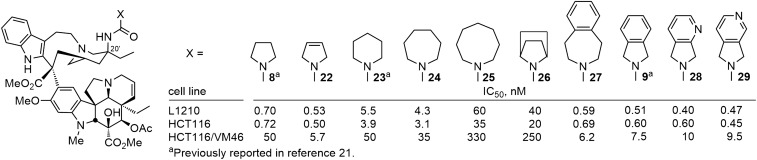
For the comparisons reported herein, the compounds were examined for cell growth inhibition against L1210 (mouse leukemia), HCT116 (human colon cancer), and HCT116/VM46 (a matched resistant human colon cancer) tumor cell lines, the latter of which exhibits resistance (100-fold) to vinblastine through the clinically observed overexpression of P-glycoprotein (Pgp). In initial studies (20), we had established that the electron-rich nature of the urea was responsible in part for their enhanced activity relative to their corresponding carbamate or amide counterparts. As a consequence, we systematically probed the pyrrolidine urea 8 to establish whether β-substituents on the pyrrolidine (10–21) or systematic changes in its structure (22–27) might impact its binding to tubulin and cell growth inhibition properties much like their impact on the pyrrolidine basicity itself. Nearly all such substituted pyrrolidine ureas were less potent than 8 itself, most showed little preference for the substituent stereochemistry (R vs. S), and there did not appear to be a direct correlation of the substituent electronic properties with its impact on the cell growth inhibition activity (Fig. 4). The exceptions to these generalizations are the behavior of 14, bearing the (R)-3-methoxypyrrolidine, and the two isomers of the 3-fluoropyrrolidine urea (17 and 18). As interesting as these latter observations are, these compounds only approach (14) or slightly surpass (17 and 18) the activity of 8, which bears no substituent. As a consequence, and rather than ascribing productive roles to the substituents, it appears that most detract from the properties of the parent compound 8 and a few (14, 17, and 18) constitute benign substitutions.
Fig. 4.
Substituted pyrrolidine ureas.
More significant was the impact of adding unsaturation (compound 22). Whereas incremental decreases in cell growth activity were seen with increasing ring size of the urea terminal cyclic amine (8 vs. 23–25, 5 < 6 or 7 < 8) or with the incorporation of the pyrrolidine in an azabicyclo[2,2,1]heptane (26), the introduction of π-unsaturation in either the form of a 3,4-double bond (22) or a 3,4-fused phenyl ring (27 and 9) provided compounds that enhanced cell growth inhibition and improved the differential in activity between the sensitive and resistant HCT116 cell lines (Fig. 5). It is possible that this improved activity is derived in part from alterations in the basicity of the pyrrolidine, reductions in destabilizing steric interactions surrounding the pyrrolidine 3,4 position, or through acquisition of additional stabilizing interactions with tubulin, and it is most likely the result of a different combination of such features for 22/9 vs. 27. Finally, replacement of the fused benzene in isoindoline 9 with fused pyridines (28 and 29) was not only permitted, but the change provided a further small enhancement in the cell growth inhibition potency. It is especially notable that 22, 9, 28, and 29 all exhibit cell growth inhibition activity in the vinblastine-sensitive tumor cell lines with IC50 values of 400–600 pM and that their activity against the vinblastine-resistant tumor cell line (HCT116/VM46) now matches the activity that vinblastine displays against the matched sensitive HCT116 cell line (IC50 value, 6.8 nM).
Fig. 5.
Pyrrolidine replacements.
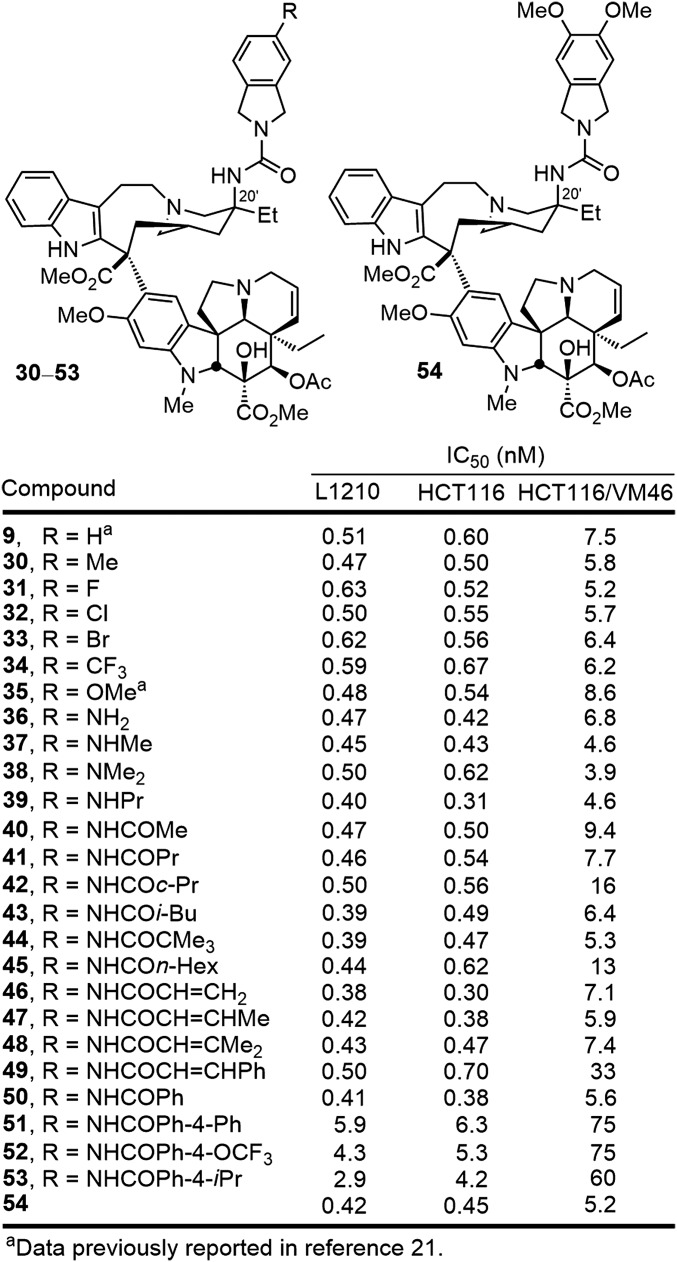
Of these, further modifications of the isoindoline urea 9 were pursued for systematic examination, recognizing the potential for further disruption of the tubulin dimer–dimer interface (Fig. 6). Substitution of the isoindoline was well tolerated and the series displayed a trend where electron-donating substituents (30, 35–39) proved slightly more potent than compounds bearing electron-withdrawing substituents (31–34). Within the former series, further alkylation (37–39) or acylation (40–53) of the aniline 36 was well tolerated. Simple acetylation to provide the acetamide 40 provided a derivative with IC50 values of 470–500 pM in the vinblastine-sensitive tumor cell lines and N-benzoylation maintained this activity with 50 displaying IC50 values of 400 pM. The site proved remarkably tolerant of steric bulk with even the pivoloyl amide 44 displaying potent activity, and it proved capable of accommodating extended rigid acyl groups (e.g., 49). Only bulky or rigid extended substitution on the para position of an added N-benzoyl group (51–53) proved to diminish the activity of the now potent series. Even appending a 4-biphenyl acyl group to the isoindoline aniline provided a compound (51) that, although less potent than either 40 or 50, was still slightly more potent than vinblastine despite being buried in a region of the tubulin binding site thought to be sensitive to steric interactions before our studies. Finally, disubstitution of the isoindoline was found to be well tolerated and, in the case examined (54), provided a compound equipotent with 35 and slightly more potent than the parent isoindoline 9.
Fig. 6.
Substituted isoindoline ureas.
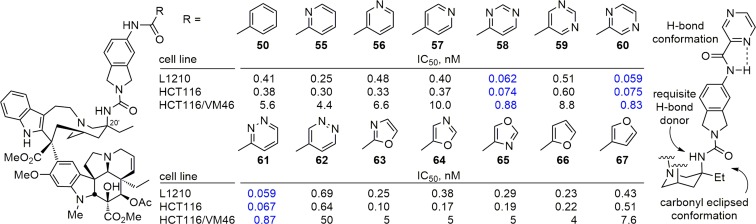
Even more impressive levels of activity and further improvements in the cell growth inhibition were observed when the benzoyl amide of 50 was replaced with heteroaromatic amides (Fig. 7). A clear trend in activity emerged in the series examined (55–67) to confidently indicate the functionality responsible for and needed to provide the additional enhancements in activity. Although all such compounds were exceptionally potent, those with six-membered heteroaromatic groups with nitrogen in the 2-position relative to the acyl carbonyl routinely displayed the more potent activity (e.g., 55 vs. 50, 56, and 57; 58, 60, and 61 vs. 59 and 62), and those with two such nitrogens where at least one of which is in the 2-position displayed stunning potency (58, 60, and 61). A similar trend was observed with the five-membered heteroaromatic substituents. It is likely this reflects an internal hydrogen bond between the heteroaromatic nitrogen at the 2-position and the adjacent amide NH that is observed in solution in the 1H NMR spectra of the compounds. Complementary to this conformational constraint, the C20′ urea also preferentially adopts the carbonyl eclipsed conformation that fixes a preferred orientation of the C20′ urea (Fig. 7). Compounds 58, 60, and 61 exhibited IC50 values of 50–75 pM against the vinblastine-sensitive tumor cell lines (L1210 and HCT116), representing increases of 100-fold relative to vinblastine. They also displayed subnanomolar activity against the vinblastine-resistant tumor cell line (HCT116/VM46; IC50 values, 830–880 pM), representing increases of 700-fold relative to vinblastine. They are also 10-fold more potent than the C20′ urea 9 that lacks a substituent.
Fig. 7.
Ultrapotent heterocyclic amides of the C20′ isoindoline urea.
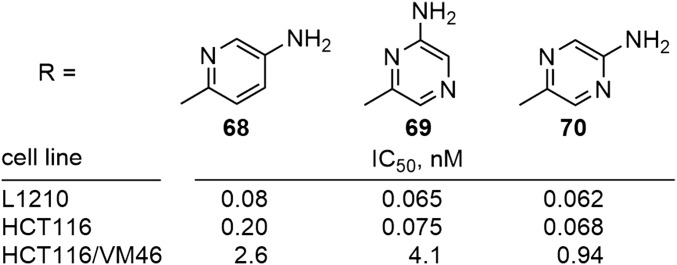
Because of their extraordinary potency, two heterocyclic amides (55 and 60) were further functionalized with an aryl amine, which would permit covalent conjugation with targeting modalities that require this ultrapotent activity (e.g., antibodies, folate) for selective delivery to tumors (Fig. 8). In each case, the aniline derivative maintained the activity of the parent analog (69 and 70 vs. 60) or even further improved its potency (68 vs. 55). Clearly, varied opportunities with subtle impacts on activity are available for both the site and appropriate functionalization of such derivatives for the preparation of tumor-targeting conjugates for which the released free drug payload remains extraordinarily potent.
Fig. 8.
Further functionalized derivatives.
Tubulin Binding.
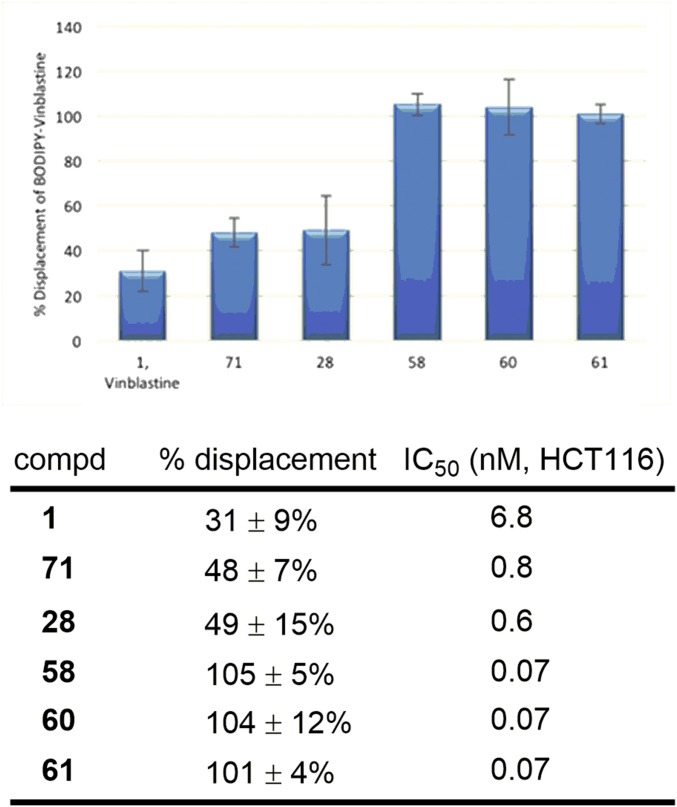
With compounds displaying remarkable functional cellular potency, we sought to determine whether the improvements in activity correlated with enhanced tubulin binding affinity. The most common method for assessing tubulin binding entails inhibition of tubulin polymerization upon exposure to the candidate compounds. However, and as noted by others, this method is insensitive, rarely correlates with the functional activity, and lacks the resolution to discriminate among a series of closely related compounds. Methods that measure competitive binding at the vinblastine tubulin binding site are more predictive, and these typically involve measurement of the competitive displacement of radiolabeled vinblastine or fluorescently labeled vinblastine probes. We have previously used the competitive displacement of 3H-vinblastine to assess the tubulin binding of selected members of the vinblastine C20′ ureas (20, 21). This assay involves filtering coincubation solutions through DE-81 filter disks, which sequester tubulin and its bound ligands from solution (28, 29). However, the assay is not ideal due to both the cost associated with using 3H-vinblastine and the technical challenges of quantitatively reproducing the results of the assay, which uses repeated rinses of the sequestered proteins containing reversibly bound compounds. This is further complicated by the fact that the manufacture of DE-81 filter paper has been discontinued. As a consequence, we elected to examine a commercially available boron-dipyrromethene (BODIPY)–vinblastine fluorescent probe to assess tubulin binding and to develop and generalize its use in a direct competitive binding assay (30–34). Although introduced and used principally to visualize protein driven efflux from cells, BODIPY–vinblastine exhibits a significant increase in fluorescence intensity (FI) upon binding to tubulin. This behavior of BODIPY–vinblastine allows fluorescent measurements of coincubation solutions to establish the percentage displacement of probe by a competitive binding site ligand. Tubulin was incubated with BODIPY–vinblastine and a representative range of competitive ligands including vinblastine, 10′-fluorovinblastine [71, a previously reported vinblastine analog with 10-fold improved functional activity (35)], the potent compound 28, and three ultrapotent C20′ urea analogs (58, 60, and 61) reported here. At the concentration tested, vinblastine displaced 31% of the BODIPY–vinblastine bound to tubulin (Fig. 9). 10′-Fluorovinblastine and 28, which are roughly 10-fold more potent than vinblastine, displayed a higher tubulin affinity, displacing 48–49% of the BODIPY–vinblastine. Remarkably, the three ultrapotent vinblastines (58, 60, and 61), each 100-fold more potent than vinblastine, displaced 100% of the BODIPY–-vinblastine, indicating that these ultrapotent C20′ ureas possess a much stronger binding affinity for tubulin than vinblastine, 10′-fluorovinblastine, and 28. Although it is not possible to rule out the impact of other features or even introduction of an unrecognized second mechanism of action, this direct correlation of functional cell growth inhibition activity with target tubulin binding affinity and the relative magnitude of the effects suggest that the properties of the potent and ultrapotent C20′ ureas are derived predominately, if not exclusively, from on-target effects on tubulin.
Fig. 9.
Tubulin (0.1 mg/mL, 0.91 µM) was incubated with BODIPY–vinblastine (BODIPY–VBL) (1.8 µM) and a vinblastine analog (18 µM) at 37 °C in PEM (80 mM PIPES-K pH 6.8, 2 mM MgSO4, 0.5 mM EGTA pH 6.8) buffer containing 850 µM GTP. The BODIPY–VBL fluorescence intensity (FI) [excitation (ex), 480 nm; emission (em), 514 nm] of 100-µL aliquots from each incubation were measured in a fluorescence microplate reader at 37 °C. Control experiments were performed with BODIPY–VBL (control 1) in the absence of a competitive ligand (maximum FI enhancement due to tubulin binding) and (control 2) in the absence of tubulin (no FI enhancement due to tubulin binding). Percentage BODIPY–VBL displacement was calculated by the formula (control 1 FI – experiment FI)/(control 1 FI – control 2 FI) × 100. Reported values are the average of four measurements ± SD.
Binding Model.
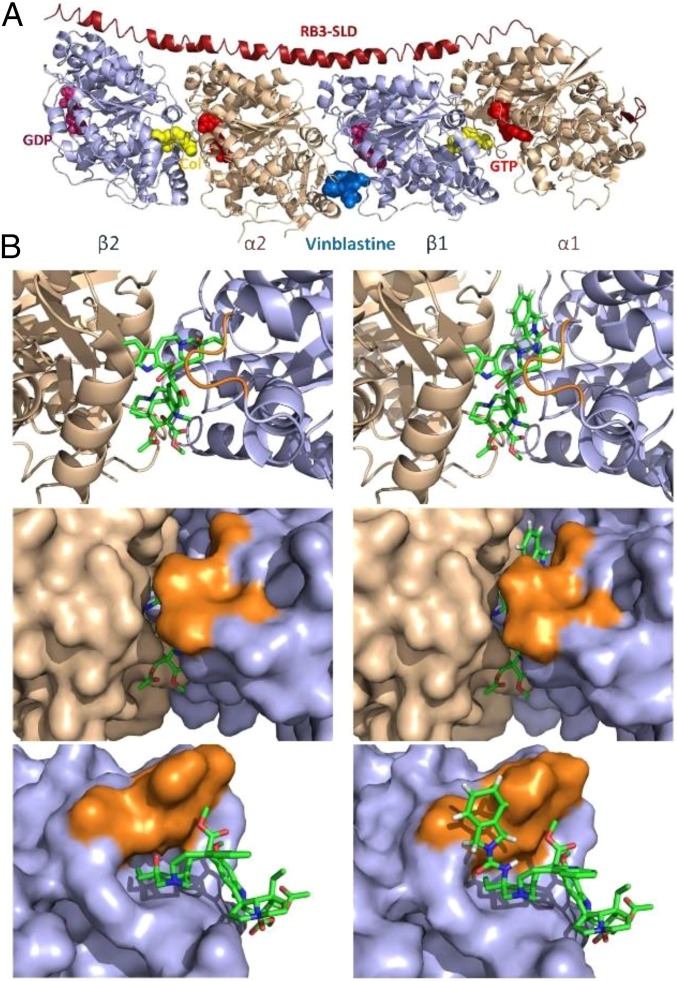
In contrast to expectations based on the steric constraints of the tubulin binding site surrounding the vinblastine C20′ center depicted in the X-ray cocrystal structure of a tubulin-bound complex (22), large C20′ urea derivatives such as those detailed herein are accommodated, with some exhibiting ultrapotent functional activity in cell-based proliferation assays and remarkable tubulin binding affinity. This raises several key questions that include whether there is room for such substituents in the purported vinblastine binding site. The binding site for vinblastine is created by and lies at the head-to-tail tubulin α/β dimer–dimer interface (Fig. 10). Vinblastine is essentially completely buried in the protein binding site, adopting a T-shaped bound conformation with C3/C4 (bottom of T) lying at the solvent interface and the C20′ site (top corner of T) extending deepest into the binding pocket lying at one corner. This binding of vinblastine destabilizes the protein–protein interaction integral to microtubulin assembly. Inspection of the vinblastine–tubulin X-ray structure reveals that the C20′ alcohol extends toward a narrow channel that leads from the buried C20′ site to the opposite face of the protein, representing the continuation of the protein–protein interaction defined by the tubulin dimer–dimer interface. Even without adjusting the proteins found in the vinblastine-bound X-ray structure, the modeled C20′ isoindoline group of 9 extends behind a key peptide loop in β-tubulin that constitutes the top of the vinblastine binding site into this narrow channel, continuing along the tubulin protein–protein interface and presumably is responsible for further disruption of the dimer tubulin–tubulin interaction (Fig. 10).
Fig. 10.
X-ray crystal structure of tubulin-bound vinblastine (PDB ID code 1Z2B) (22). (A) Binding site at the tubulin α/β head-to-tail dimer–dimer interface. (B) Binding site comparison of vinblastine (left column, X-ray) and modeled vinblastine C20′ urea 9 (right column); ribbon structure of binding site (Top), corresponding space-filling model (Middle), and side view with the adjacent α-tubulin protein (green) at the dimer–dimer interface removed. A peptide loop (highlighted in orange) on the β-tubulin protein (light blue) covers the top side of the vinblastine velbanamine subunit including C20′, creating a deep and largely hydrophobic pocket for ligand binding. Without adjusting the proteins found in the vinblastine-bound X-ray structure, the isoindoline group of modeled 9 extends behind the loop peptide into a channel continuing along the tubulin protein–protein interface. Aryl amide substituents on the isoindoline such as those found in 50 and 55–67 continue to extend along the tubulin dimer protein–protein interface (Fig. 11).
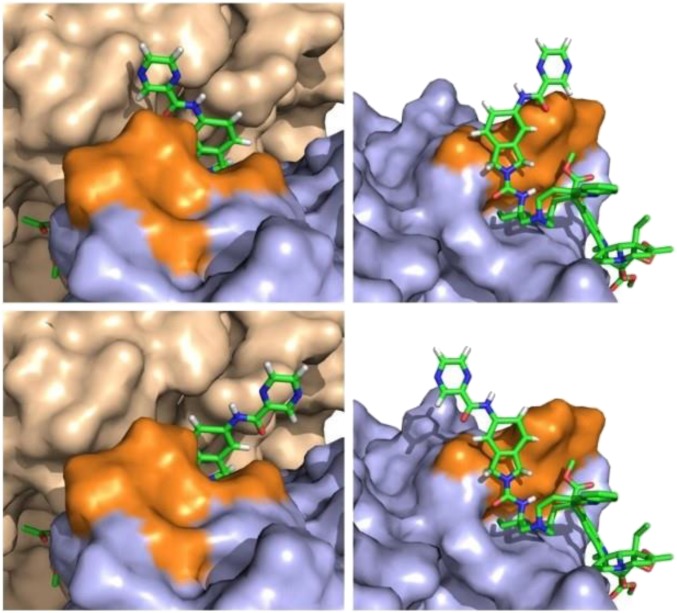
It is likely that the rigid linear C20′ urea of the ultrapotent vinblastines extends into and expands this narrow channel, even further disrupts the tubulin α/β head-to-tail dimer–dimer interface, and extends across the full protein–protein interface, contacting solvent on the distal side (Fig. 11). Thus, and although the structure of the potent C20′ ureas and their optimization may appear unusual on a first inspection, they represent rational structural additions to a specific site on the natural product with appropriate conformational constraints needed to further enhance disruption of the target protein–protein interaction.
Fig. 11.
Models of the ultrapotent vinblastine 60 in the vinblastine binding site of tubulin constructed without altering the proteins found the X-ray crystal structure of tubulin-bound vinblastine (PDB ID code 1Z2B) (22). Two possible binding conformations are shown representing a 180° rotation about the C20′ isoindoline nitrogen bond. In one conformation (Top), the pyrazinoyl group extends over the top of the β-tubulin peptide loop (highlighted in orange) into a region where the α-tubulin (tan) and β-tubulin (orange/blue) subunits are in close contact, thereby further disrupting the protein–protein interaction. In the second conformation (Bottom), the pyrazinoyl group continues to bind along the extended dimer–dimer interface, establishing additional new contacts with α-tubulin.
More provocatively, the activity of the ultrapotent analogs (IC50 values, 50–75 pM) suggest that it is not likely or even possible that their cellular functional activity is derived from stoichiometric occupancy of the relatively large number of intracellular tubulin binding sites. Rather, their potency suggests substoichiometric or catalytic occupancy of candidate binding sites is sufficient to disrupt tubulin dynamics or assembly during mitosis. The question these ultrapotent vinblastines pose is whether substoichiometric binding site occupancy is sufficient to either trap/cap polymerizing microtubulin in nonproductive conformations or disrupt microtubulin dynamics, or whether such compounds serve catalytically to actively disassemble microtubulin. Although we cannot yet answer this question, it is a focus of current efforts.
Discussion
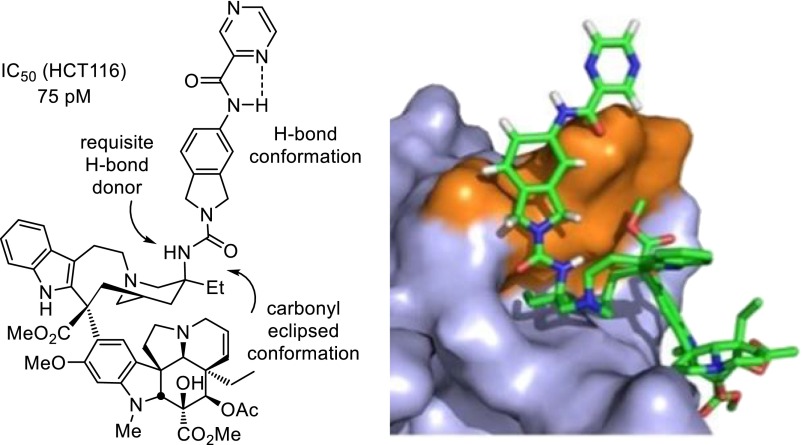
In recent studies, we reported concise 12-step total syntheses of vinblastine and related natural products (14–16) based in part on introduction of a powerful single-pot two-step diastereoselective Fe(III)-promoted vindoline/catharanthine coupling and subsequent Fe(III)-NaBH4/air–mediated free-radical C20′ oxidation that was extended for use with alternative free-radical traps (17, 18). This methodology, inspired by the structure of vinblastine, has permitted systematic studies of the effects of deep-seated structural changes within either the lower vindoline or upper velbanamine subunits (13). One general observation made in these studies is that, although modifications to the key structural features of vinblastine typically result in reductions in biological activity, addition of structural features can substantially improve them (20, 21, 35, 36). This includes not only our demonstration of the impact of the addition of an indole C10′ fluorine substituent (35) and the incorporation of the C20′ ethyl group into a benign more complex cis-fused C15′-C20′ six-membered ring (36), but also the discovery of the improved activity of C20′ urea replacements for the tertiary alcohol (20, 21). Herein, we reported the discovery of compounds in this latter series that are now a stunning 100-fold more potent than vinblastine. Such compounds including 60 exhibited IC50 values of 50–75 pM against the vinblastine sensitive tumor cell lines (e.g., HCT116), representing increases of 100-fold relative to vinblastine, and even subnanomolar activity against a resistant tumor cell line (HCT116/VM46; IC50 values, 830–880 pM) insensitive to vinblastine by virtue of the clinically observed Pgp overexpression, representing increases of 700-fold relative to vinblastine (Fig. 12).
Fig. 12.
Structure of 60, the contributing conformational features of the added molecular complexity, and modeled interaction with tubulin.
Approaches to improving the biological properties of natural products typically involve efforts to simplify their structures or to modify their structures through semisynthetic transformations, diverted total synthesis (37), or late-stage functionalizations (38, 39). Rarely does one consider adding molecular complexity to the underlying structure in a way that requires chemical synthesis of a modified natural product. In part, this may be attributed to the added challenge that might accompany the more complex compound synthesis. The synthetically derived ultrapotent vinblastines that we detailed, which are 100-fold more active than the natural product, represent vinblastine analogs accessible by chemical synthesis in three steps from commercially available and relatively inexpensive materials (catharanthine, $16/g; and vindoline, $36/g) based on methodology we introduced and are presently inaccessible by classical natural product derivatization, late-stage functionalization, or biosynthetic methods. The newly discovered ultrapotent vinblastines, which display much higher tubulin binding affinities, likely further disrupt the target tubulin head-to-tail α/β dimer–dimer interaction by virtue of the strategic placement of the added rigid and extended C20′ urea along the adjacent continuing protein–protein interface (Fig. 12). The stunning success with vinblastine suggests the approach of adding molecular complexity to enhance target binding and functional biological activity for other natural products targeting a protein–protein interaction may represent an appealing and general approach to achieving such objectives.
Materials and Methods
Synthesis of Vinblastine C20′ Ureas.
Full details of the synthesis, purification, and characterization of all compounds reported herein, including copies of the 1H NMR of all tested compounds, are provided in SI Appendix. All reagents were obtained from commercial sources unless noted otherwise.
Cell Growth Inhibition Assays.
Full details of the cell growth inhibition assays (L1210, HCT116, and HCT116/VM46) are provided in SI Appendix. Compounds were tested in duplicate (n = 2−18 times) at six concentrations between 0–1,000 nM, or 0 and 10,000 nM. The IC50 values reported represent of the average of 4−36 determinations (SD ± 10%).
Tubulin Binding Assay.
Full details of the tubulin binding assay are provided in SI Appendix. Reported values are the average of four measurements ± SD.
Supplementary Information.
Full experimental details and copies of 1H NMR spectra are provided. The supplementary data associated with this article can be found in SI Appendix.
Supplementary Material
Acknowledgments
We especially thank Katharine K. Duncan for the biological assessments of early samples in the series reported in refs. 20 and 21. We gratefully acknowledge the financial support of National Institutes of Health Grants CA115526 and CA042056.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611405113/-/DCSupplemental.
References
- 1.Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol 37. Academic; San Diego: 1990. pp. 229–240. [Google Scholar]
- 2.Kuehne ME, Marko I. Syntheses of vinblastine-type alkaloids. In: Brossi A, Suffness M, editors. The Alkaloids. Vol 37. Academic; San Diego: 1990. pp. 77–131. [Google Scholar]
- 3.Borman LS, Kuehne ME. Functional hot spot at the C20′ position of vinblastine. In: Brossi A, Suffness M, editors. The Alkaloids. Vol 37. Academic; San Diego: 1990. pp. 133–144. [Google Scholar]
- 4.Pearce HL. Medicinal chemistry of bisindole alkaloids from catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol 37. Academic; San Diego: 1990. pp. 145–204. [Google Scholar]
- 5.Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann N Y Acad Sci. 1958;76(3):882–894. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]
- 6.Noble RL. Catharanthus roseus (Vinca rosea)—importance and value of a chance observation. Lloydia. 1964;27(4):280–281. [Google Scholar]
- 7.Noble RL. The discovery of the vinca alkaloids—chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68(12):1344–1351. [PubMed] [Google Scholar]
- 8.Svoboda GH, Neuss N, Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J Am Pharm Assoc (Sci Ed) 1959;48(11):659–666. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 10.Potier P. Synthesis of the antitumor dimeric indole alkaloids from Catharanthus species (vinblastine group) J Nat Prod. 1980;43(1):72–86. [Google Scholar]
- 11.Kutney JP. Plant cell culture combined with chemistry: A powerful route to complex natural products. Acc Chem Res. 1993;26(10):559–566. [Google Scholar]
- 12.Fahy J. Modifications in the upper velbenamine part of the Vinca alkaloids have major implications for tubulin interacting activities. Curr Pharm Des. 2001;7(13):1181–1197. doi: 10.2174/1381612013397483. [DOI] [PubMed] [Google Scholar]
- 13.Sears JE, Boger DL. Total synthesis of vinblastine, related natural products, key analogues and development of inspired methodology suitable for the systematic study of their structure–function properties. Acc Chem Res. 2015;48(3):653–662. doi: 10.1021/ar500400w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa H, Colby DA, Boger DL. Direct coupling of catharanthine and vindoline to provide vinblastine: Total synthesis of (+)- and ent-(–)-vinblastine. J Am Chem Soc. 2008;130(2):420–421. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa H, et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J Am Chem Soc. 2009;131(13):4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoh H, Sears JE, Eschenmoser A, Boger DL. New insights into the mechanism and an expanded scope of the Fe(III)-mediated vinblastine coupling reaction. J Am Chem Soc. 2012;134(32):13240–13243. doi: 10.1021/ja306229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leggans EK, Barker TJ, Duncan KK, Boger DL. Iron(III)/NaBH4-mediated additions to unactivated alkenes: Synthesis of novel 20′-vinblastine analogues. Org Lett. 2012;14(6):1428–1431. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker TJ, Boger DL. Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J Am Chem Soc. 2012;134(33):13588–13591. doi: 10.1021/ja3063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boger DL, Brotherton CE. Total synthesis of azafluoranthine alkaloids: Rufescine and imelutine. J Org Chem. 1984;49(21):4050–4055. [Google Scholar]
- 20.Leggans EK, Duncan KK, Barker TJ, Schleicher KD, Boger DL. A remarkable series of vinblastine analogues displaying enhanced activity and an unprecedented tubulin binding steric tolerance: C20′ urea derivatives. J Med Chem. 2013;56(3):628–639. doi: 10.1021/jm3015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker TJ, Duncan KK, Otrubova K, Boger DL. Potent vinblastine C20′ ureas displaying additionally improved activity against a vinblastine-resistant cancer cell line. ACS Med Chem Lett. 2013;4(10):985–988. doi: 10.1021/ml400281w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigant B, et al. Structural basis for the regulation of tublin by vinblastine. Nature. 2005;435(7041):519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 23.Arkin MR, Wells JA. Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nat Rev Drug Discov. 2004;3(4):301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 24.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature. 2007;450(7172):1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 25.Yin H, Hamilton AD. Strategies for targeting protein–protein interactions with synthetic agents. Angew Chem Int Ed. 2005;44(27):4130–4163. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]
- 26.Meireles LMC, Mustata G. Discovery of modulators of protein–protein interactions: Current approaches and limitations. Curr Top Med Chem. 2011;11(3):248–257. doi: 10.2174/156802611794072632. [DOI] [PubMed] [Google Scholar]
- 27.Boger DL, Desharnais J, Capps K. Solution-phase combinatorial libraries: Modulating cellular signaling by targeting protein–protein or protein–DNA interactions. Angew Chem Int Ed. 2003;42(35):4138–4176. doi: 10.1002/anie.200300574. [DOI] [PubMed] [Google Scholar]
- 28.Owellen R, Donigian D, Hartke C, Hains F. Correlation of biologic data with physico-chemical properties among the Vinca alkaloids and their congeners. Biochem Pharmacol. 1977;26(13):1213–1219. doi: 10.1016/0006-2952(77)90108-3. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya B, Wolff J. Tubulin aggregation and disaggregation: Mediation by two distinct vinblastine-binding sites. Proc Natl Acad Sci USA. 1976;73(7):2375–2378. doi: 10.1073/pnas.73.7.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang JD, et al. 3-(Iodoacetamido)-benzoylurea: A novel cancericidal tubulin ligand that inhibits microtubule polymerization, phosphorylates bcl-2, and induces apoptosis in tumor cells. Cancer Res. 1998;58(23):5389–5395. [PubMed] [Google Scholar]
- 31.Zhang C, et al. S9, a novel anticancer agent, exerts its anti-proliferative activity by interfering with both PI3K-Akt-mTOR signaling and microtubule cytoskeleton. PLoS One. 2009;4(3):e4881. doi: 10.1371/journal.pone.0004881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai A, Surolia A, Panda D. An antitubulin agent BCFMT inhibits proliferation of cancer cells and induces cell death by inhibiting microtubule dynamics. PLoS One. 2012;7(8):e44311. doi: 10.1371/journal.pone.0044311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibbeson BM, et al. Diversity-oriented synthesis as a tool for identifying new modulators of mitosis. Nat Commun. 2014;5:3155. doi: 10.1038/ncomms4155. [DOI] [PubMed] [Google Scholar]
- 34.Rashid A, Kuppa A, Kunwar A, Panda D. Thalidomide (5HPP-33) suppresses microtubule dynamics and depolymerizes the microtubule network by binding at the vinblastine binding site on tubulin. Biochemistry. 2015;54(12):2149–2159. doi: 10.1021/bi501429j. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh H, Duncan KK, Robertson WM, Boger DL. 10′-Fluorovinblastine and 10′-fluorovincristine: Synthesis of a key series of modified Vinca alkaloids. ACS Med Chem Lett. 2011;2(12):948–952. doi: 10.1021/ml200236a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allemann O, Brutsch M, Lukesh JC, III, Brody DM, Boger DL. Synthesis of a potent vinblastine: Rationally designed added benign complexity. J Am Chem Soc. 2016;138(27):8376–8379. doi: 10.1021/jacs.6b04330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson RM, Danishefsky SJ. Small molecule natural products in the discovery of therapeutic agents: The synthesis connection. J Org Chem. 2006;71(22):8329–8351. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]
- 38.Szpilman AM, Carreira EM. Probing the biology of natural products: Molecular editing by diverted total synthesis. Angew Chem Int Ed. 2010;49(50):9592–9628. doi: 10.1002/anie.200904761. [DOI] [PubMed] [Google Scholar]
- 39.Hong J. Natural products at the interface of chemistry and biology. Chemistry. 2014;20(33):10204–10212. doi: 10.1002/chem.201402804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.