Significance
The human ether-à-go-go–related gene (hERG) potassium channel has an important role in controlling heartbeat. Genetic alterations or drug side effects can reduce the amount of current passing through hERG, prolonging action potential duration and causing irregular heartbeat and sudden death. We uncovered a family of hERG modulators, antibody fragments that bind to a cytoplasmic region [Per-Arnt-Sim (PAS) domain], increasing the magnitude of the repolarizing current IKr and reducing duration of the ventricular action potential. Using the antibodies as tools, we established that it is possible to modulate hERG channel function through the PAS domain and showed a way to increase hERG channel function with the potential to treat not only perturbations of the channel itself but also arrhythmias arising from heart failure.
Keywords: potassium channel, hERG, KCNH, PAS domain, scFv
Abstract
The human human ether-à-go-go–related gene (hERG) potassium channel plays a critical role in the repolarization of the cardiac action potential. Changes in hERG channel function underlie long QT syndrome (LQTS) and are associated with cardiac arrhythmias and sudden death. A striking feature of this channel and KCNH channels in general is the presence of an N-terminal Per-Arnt-Sim (PAS) domain. In other proteins, PAS domains bind ligands and modulate effector domains. However, the PAS domains of KCNH channels are orphan receptors. We have uncovered a family of positive modulators of hERG that specifically bind to the PAS domain. We generated two single-chain variable fragments (scFvs) that recognize different epitopes on the PAS domain. Both antibodies increase the rate of deactivation but have different effects on channel activation and inactivation. Importantly, we show that both antibodies, on binding to the PAS domain, increase the total amount of current that permeates the channel during a ventricular action potential and significantly reduce the action potential duration recorded in human cardiomyocytes. Overall, these molecules constitute a previously unidentified class of positive modulators and establish that allosteric modulation of hERG channel function through ligand binding to the PAS domain can be attained.
The human ether-à-go-go–related gene (hERG; or KCNH2) encodes the voltage-gated potassium channel that conducts IKr (delayed rectifier potassium current), a critical cardiac repolarizing current (1, 2). Mutations in hERG (3) or channel block can cause long QT syndrome (LQTS) and catastrophic ventricular arrhythmias (reviewed in ref. 4). Because most drugs causing acquired LQTS block IKr channels (5), lead compounds in development are counterscreened using a hERG cell-based safety test (6) to reduce the incidence of sudden cardiac death caused by off-target drug effects (7).
A defining feature of hERG and its relatives in the potassium channel family, KCNH (8), is a large cytoplasmic region with an N-terminal Per-Arnt-Sim (PAS) domain (9). Within the hERG PAS domain, there are two functionally and structurally distinct regions: the PAS-Cap (residues 1–25) and the globular region (residues 26–135). NMR and crystallographic structures of the isolated PAS domain show that the PAS-Cap region is partially unstructured (10–12). The globular region interacts with a C-terminal domain, which, because of sequence homology with cyclic nucleotide binding domains, is termed the cyclic nucleotide binding homology (CNBh) domain (13, 14). Truncations and mutations of the PAS-Cap and PAS clearly affect the gating mechanisms of the channel, which is particularly apparent in the deactivation kinetics (9, 15–18). PAS domains within the tetrameric hERG channels exert a suppressive effect by slowing channel activation and recovery from inactivation, the two parameters that predominantly determine current amplitude during an action potential (AP) (19). These properties are genetically tuned in native IKr produced by the heteromeric association of hERG1a (an isoform containing the PAS domain) with hERG1b (an isoform lacking the PAS domain) (19–22). In addition, evolutionary tuning of the properties of these channels by the presence or absence of the PAS domain has been described (23). The functional importance of the PAS domain is reinforced by genetic alterations in the domain that have been linked to LQTS (15, 24, 25).
Because of the physiological role of hERG and its link to LQTS, there has been great interest in understanding the modulatory mechanisms of the channel and the functional role played by its cytosolic domains. From this perspective, the PAS domain is particularly interesting because, in other proteins, these domains bind small molecules and modulate protein activity (26). However, no such ligands have been identified for the PAS domain in hERG or for any member of the KCNH superfamily. In fact, we have recently reported that two different small molecule screening campaigns failed to yield PAS domain-specific binders (27). Importantly, despite extensive work showing that mutations and truncations of hERG PAS cause functional changes, it remains unclear if this domain mediates allosteric regulation in the channel (28).
In search of novel tools to interrogate the functional role of the hERG PAS domain, we generated single-chain variable fragment (scFv) antibodies that recognize this domain and found that these protein molecules change the functional properties of both heterologously expressed and native hERG channels. scFv antibodies consist only of the variable light (VL) and variable heavy (VH) antibody domains and therefore are relatively small (27 kDa) but retain their high antibody specificity. Two of the scFv molecules exhibit differential effects reflecting their interaction with different regions of the PAS domain, allowing us to explore the properties of the PAS domain within the context of the full-length channel.
Results
Creation of scFv Molecules Against the PAS Domain.
To obtain antibody molecules against the PAS domain from hERG, we first immunized chickens with pure protein (hERG residues 1–135) expressed in Escherichia coli. Total mRNA was isolated from bone marrow and spleen to generate cDNA, and the VL and VH regions of IgY were amplified. An scFv phage-display library was generated where the VL IgY regions were fused to the VH IgY regions through a linker, forming a single polypeptide, which could be displayed on the surface of phage particles (details are in SI Materials and Methods) (29, 30). These scFv molecules were screened for their ability to bind PAS domain using two different strategies. For both strategies, four rounds of selection and amplification were carried out in duplicate or triplicate, yielding between 2,000- and 3,000-fold enrichment in the number of eluted phage (Table S1). In total, 136 individual colonies were randomly picked from the fourth round of screening from both strategies and subjected to DNA sequencing. In parallel, DNA was transformed into the nonsuppressor E. coli strain BL21(DE3) for the expression of scFv proteins in the periplasmic space. Crude lysates were generated and used in an ELISA to measure binding specificity to PAS protein over a GST protein control or a Western blot overlay assay to assess their ability to recognize denatured PAS protein. As shown in Fig. 1A, a variety of responses was seen in ELISA, and positive hits were defined as showing a greater than or equal to fivefold increase in binding to PAS protein compared with GST alone. Interestingly, Western blot overlay experiments of the same samples revealed that one-half of the scFv antibodies (R2, R12, R15, 2.10, EC2, and EC33) recognize denatured PAS protein, whereas one-half did not (R14, 2.4, H18, 2.12, EC24, and EC30) (Fig. 1B). Comparison of the results from the Western blot overlay assay (unfolded PAS protein) and ELISA (folded PAS protein) shows that some scFv molecules only recognize epitopes in the folded state of the protein. For example, we did not detect a Western blot signal for scFv 2.12; however, in ELISA, scFv 2.12 gives rise to a clear signal.
Table S1.
Panning data for the selection of scFv antibodies against hERG PAS domain
| Panning cycle no. | Solution-phase selection | Solid-phase selection: PAS | |||
| GSTPAS_1 | GST_1 | GSTPAS_2 | GST_2 | ||
| 1 | |||||
| Eluted phage (% of input) | 0.333000 | 0.181000 | 0.000034 | ||
| Enrichment* | n.d. | n.d. | 1 | ||
| 2 | |||||
| Eluted phage (% of input) | 0.000094 | 0.000128 | 0.004400 | ||
| Enrichment* | 1 | 1 | 129 | ||
| 3 | |||||
| Eluted phage (% of input) | 0.028 | 0.044 | 0.115 | ||
| Enrichment* | 297.9 | 343.8 | 3,382 | ||
| 4 | |||||
| Eluted phage (% of input) | 0.19300 | 0.00083 | 0.35600 | 0.00131 | 0.09600 |
| Enrichment* | 2,053 | 0.03 | 2,781 | 0.03 | 2,823 |
GSTPAS_1 and GSTPAS_2 were purified as fusion proteins and coupled to magnetic beads for screening. GST_1 and GST_2 were purified as control. PAS protein was purified and coated onto Nunc Maxisorp plates for screening. n.d., not determined.
Enrichment was calculated as the ratio between the eluted phage in a certain cycle and the eluted phage in the first cycle.
Fig. 1.
Characterization of clones by ELISA and Western blot overlay. (A) ELISA response for scFv binding to PAS protein over GST control protein expressed as fold increase in OD at 450 nm. All samples were run in duplicate. (B) Western blot showing scFv lysate recognition of unfolded PAS. Blot was overexposed to determine if scFv clones recognize blotted PAS protein.
Functional scFv Screen.
A group of three scFv molecules (R15, 2.10, and 2.12) was expressed in E. coli and purified, and their functional impact on the hERG channel was evaluated at room temperature (22 °C) in HEK293 cells expressing hERG 1a (Fig. 2 and Table S2). Control currents measured using whole-cell patch clamp were compared with those in which the individual antibodies were perfused via the patch pipette into the cytosol in separate experiments. The majority of the scFvs accelerated channel deactivation as if they perturb the PAS domain that maintains slow deactivation (9). The more modest effect of the antibodies compared with PAS deletions suggests the antibodies do not fully disrupt PAS function. Importantly, one of the selected scFv molecules (R15) did not alter the deactivation properties, thus serving as a negative control. As expected, an antibody directed against the unique N terminus of hERG 1b (22) did not affect hERG 1a deactivation (Fig. S1). Fitting a double exponential function to the deactivation time course evoked at −110 mV showed that the fast time constant of current decay (τfast) was significantly reduced (Fig. 2B, Fig. S1, and Table S2). The slow time constant of current decay (τslow) was not significantly affected (Fig. S1 and Table S2).
Fig. 2.
scFv antibodies accelerate deactivation. (A) Whole-cell patch clamp recordings at 22 °C in HEK293 cells expressing hERG 1a. Antibodies were delivered to the cytoplasm via the pipette. (B) Fast time constants of deactivation at −110 mV for control (black), scFv 2.10 (green), scFv 2.12 (blue), and scFv R15 (red). Mean ± SEM (n = 11–17). *Statistical significance between vehicle control and scFv 2.10 at P < 0.05. †Statistical significance between vehicle control and scFv 2.12 at P < 0.05.
Table S2.
Deactivation time constants and amplitudes of currents recorded at −110 mV from HEK293 cells expressing hERG 1a at two different temperatures
| AFast | τFast, ms | ASlow | τSlow, ms | n | |
| HEK (22 °C) | |||||
| Control | 0.91 ± 0.01 | 85.1 ± 9.5 | 0.09 ± 0.01 | 741 ± 79 | 15 |
| scFv 2.10 | 0.92 ± 0.01 | 54.6 ± 3.1* | 0.08 ± 0.01 | 628 ± 77 | 11 |
| scFv 2.12 | 0.93 ± 0.01 | 55.9 ± 3.3* | 0.07 ± 0.01 | 585 ± 35* | 11 |
| HEK (36 °C ± 1 °C) | |||||
| Control | 0.89 ± 0.02 | 6.9 ± 1.2 | 0.11 ± 0.02 | 38.8 ± 12.8 | 5 |
| scFv 2.10 | 0.90 ± 0.02 | 6.2 ± 0.7 | 0.10 ± 0.02 | 42.1 ± 6.5 | 5 |
| scFv 2.12 | 0.82 ± 0.09 | 8.0 ± 0.8 | 0.18 ± 0.09 | 31.6 ± 12.1 | 5 |
All data are means ± SEM; n is the number of replicates.
P < 0.05 vs. control.
Fig. S1.
scFv antibodies accelerate deactivation. (A) Sample traces recorded at 22 °C in HEK293 cells expressing hERG 1a, in which antibodies are delivered to the cytoplasm via the pipette. Accelerated deactivation in the presence of scFv molecules selected against the PAS domain is observed relative to antibody (anti-1b) recognizing the unique N terminus of hERG 1b (protein region not present in hERG 1a). (B) Fast time constants of deactivation recorded at −110 mV for control (black), scFv 2.10 (green), scFv 2.12 (blue), and an anti-hERG 1b antibody (red). (C) Fast time constants plotted as a function of test potential for control (black), scFv 2.10 (green), scFv 2.12 (blue), and R15 (orange). scFv 2.10 and scFv 2.12 significantly accelerated the fast time constant of deactivation across a range of test potentials. (D) Slow time constants of deactivation plotted as a function of test potential for control (black), scFv 2.10 (green), scFv 2.12 (blue), and R15 (orange). All data are means ± SEM (n = 11–17). *Statistical significance between vehicle control and scFv 2.10 at P < 0.05. †Statistical significance between vehicle control and scFv 2.12 at P < 0.05.
Binding Properties of scFv 2.10 and scFv 2.12 Antibodies.
We used biochemical approaches to better understand the modes of action of the scFv 2.10 and scFv 2.12 antibodies on the hERG channel. We determined their binding affinities for the PAS protein using isothermal titration calorimetry (ITC). Titrations of PAS domain into scFv 2.10 antibody revealed an exothermic association with a Kd of 254 nM (Fig. 3A and Table 1). The association of PAS protein with the scFv 2.12 antibody is endothermic and shows a lower affinity, with a Kd of 4.1 µM (Fig. 3B and Table 1). In both cases, stable complexes formed with 1:1 binding stoichiometry as derived from the midpoint of the ITC titrations. The differences in the thermodynamic parameters give an indication of different modes of binding by the two antibodies to the PAS domain.
Fig. 3.
ITC measurements of antibody and PAS domain. Thermograms recorded at 15 °C. (A) hERG PAS protein titrated into scFv 2.10HisHA. (B) hERG PAS protein titrated into scFv 2.12HisHA. (C) mEAG1 PAS protein titrated into scFv 2.10HisHA. (D) mEAG1 PAS protein titrated into scFv 2.12HisHA. Averaged Kd values are shown. Panels show injections of 10 μL titrant to the target (Upper) and binding isotherms (Lower). Titrations were repeated at least twice.
Table 1.
ITC parameters for scFv binding to hERG PAS and mEAG1 PAS
| hERG PAS | mEAG1 PAS scFv 2.10 | ||
| scFv 2.10 | scFv 2.12 | ||
| Kd (µM) | 0.254 ± 0.09 | 3.92 ± 1.11; 4.31 ± 0.81 | 3.11 ± 0.02 |
| ΔH (kcal/mol) | −5.06 ± 0.13 | 1.67 ± 0.12; 1.82 ± 0.11 | −8.98 ± 1.172 |
| −TΔS (kcal/mol) | −11.0 ± 0.2 | 8.79; 8.90 | 1.72 ± 1.10 |
| N | 0.92 ± 0.03 | 0.93 ± 0.05; 0.81 ± 0.03 | 0.84 ± 0.18 |
| n | 3 | 2 | 3 |
Means ± SD are shown, except for scFv 2.12, where values (and fit errors) of individual measurements are shown. N is stoichiometry of binding, and n is number of replicates.
We also determined how specific these antibodies were for the hERG channel. For this purpose, we used the PAS domain of the mouse ether-à-go-go 1 (mEAG1) channel. The mEAG1 PAS domain is ∼38% identical to the PAS domain of the hERG channel and ∼98% identical to the domain in the human EAG1 channel. The binding of scFv 2.10 to the mEAG1 PAS domain was exothermic, which was previously seen for hERG PAS, but the affinity of binding was 10-fold lower, with a Kd ∼ 3 µM (Fig. 3C and Table 1). This effect resulted from a change in binding entropy, which is positive (−TΔS) in the interaction with the hERG domain and negative in the interaction with the mEAG1 domain. No interaction was detected for the titration of mEAG1 PAS with the scFv 2.12 molecule, despite performing titrations at various temperatures (Fig. 3D).
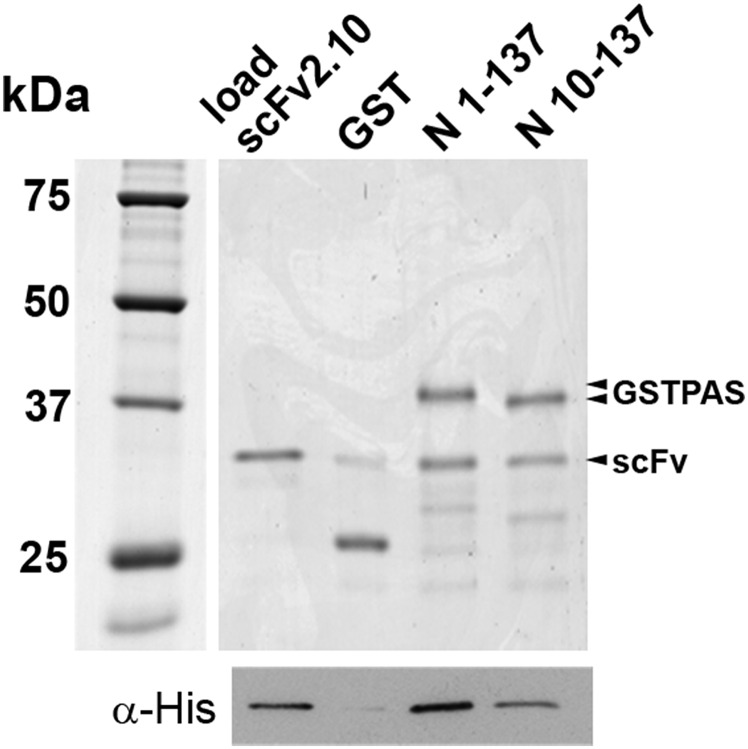
To define the binding epitopes on the PAS domain for the two antibodies, we performed in vitro pulldown experiments using different N-terminal truncations (Fig. 4 A, Upper and B, Upper). Glutathione magnetic beads were saturated with purified N-terminal GST fusions of (i) full-length PAS (N 1–135), (ii) deletion of the first 9 residues of PAS (N 10–135), and (iii) deletion of the first 23 residues of PAS (N 24–135). With these truncations, we removed either the first section of the PAS-Cap, which is disordered in the NMR structure, or the entire PAS-Cap region. Beads saturated with GST alone were used as a control. We incubated the beads with equal amounts of purified His-tagged scFv 2.10 (Fig. 4A) or scFv 2.12 protein (Fig. 4B) and after thorough washing, determined if antibody was bound to the beads. The beads were analyzed by SDS/PAGE and Western blot. As shown by Coomassie staining of the gels (Fig. 4 A, Upper and B, Upper), all GST fusions were present on the beads in equal amounts. The scFv 2.10 antibody bound only when incubated with the full-length PAS protein; even a small deletion of the first nine amino acids was sufficient to interfere with antibody binding (Fig. 4A, Lower). In contrast, scFv 2.12 antibody bound to all of the GST fusion proteins (Fig. 4B, Lower). These results suggest that scFv 2.12 binds to the globular region of the PAS, whereas scFv 2.10 binds to the first nine residues of the PAS-Cap region. We also performed pulldown experiments with the mEAG1 full-length and N-terminally truncated PAS domain and observed similar effects (Fig. S2). To better define the hERG PAS residues involved in the interaction with 2.10, we mutated conserved residues in PAS-Cap, R4, R5, and F14 (Fig. 4C). Pulldown experiments show that the double mutants R4E/R5E or R4A/R5A drastically affect the interaction with scFv 2.10, whereas the F14A mutation does not, showing that the conserved residues in the initial section of the PAS-Cap are part of the 2.10 epitope.
Fig. 4.
Epitope mapping of scFv interaction with PAS domain. Pulldowns of (A and C) scFv 2.10HisHA or (B) scFv 2.12HisHA with GST alone or GST fusions attached to beads. Fusions include full-length hERG PAS domain (residues 1–135:N 1–135 or WT), domain truncations (residues 10–135:N 10–135 and 24–135:N 24–135), and mutants of full-length domain (R4E/R5E, R4A/R5A, or F14A). Upper depicts Coomassie staining of proteins retained in beads. Lower represents Western blots (with an anti-His tag antibody) of scFv retained in beads. Initial load sample of scFv proteins is included. Two independent experiments were performed, each with two replicates.
Fig. S2.
Pulldown of scFv 2.10 antibody with mEAG1 PAS domain. Pulldown experiments of scFv 2.10HisHA with GST alone or GST fusions attached to GMBs. Fusions include full-length mEAG1 PAS domain (residues 1–137:N 1–137) or PAS truncation (residues 10–137:N 10–137). Upper depicts Coomassie-stained SDS/PAGE of proteins retained in the beads. Lower represents Western blot detection (with an anti-His tag antibody) of scFv antibody retained in the beads after washing. The initial load sample of scFv protein is also included. As seen for hERG PAS (Fig. 4), truncation of nine residues reduces the amount of scFv 2.10 that is pulled down. However, the amount of scFv 2.10 that is retained in the beads is clearly larger than background, and the effect is not as severe as with truncated hERG PAS. A possible explanation is that mEAG1 has three extra residues at the N terminus relative to hERG PAS, and a nine-residue truncation in mEAG1 probably leaves part of the scFv 2.10 epitope intact, so that a weakened interaction can still occur.
Gating Effects of scFv Antibodies on hERG Current.
To determine whether the differential targeting of the scFv 2.10 and scFv 2.12 antibodies exerts a corresponding difference in function, we analyzed their effects on hERG currents heterologously expressed in HEK293 cells and recorded at physiological temperature (36 °C ± 1 °C). We applied the antibodies to the cytoplasm via the patch pipette at saturating concentrations of 10 µM and found that scFv 2.10 and scFv 2.12 differentially modulated activation and inactivation kinetics. scFv 2.12 significantly accelerated the time course of activation (Fig. 5 A and B and Table 2) and inactivation recovery (Fig. 5 C and D and Table 2) relative to vehicle controls. scFv 2.10 significantly slowed the time course of inactivation onset (Fig. 5 E and F and Table 2) but had no effect on channel activation or inactivation recovery. Curiously, the antibodies had no effect on deactivation at physiological temperatures (Table S2). Traces showing endogenous currents during key protocols from untransfected HEK293 cells for comparison are provided in Fig. S3. These results define distinct mechanisms by which the scFv 2.10 and scFv 2.12 antibodies modulate hERG channel gating through the PAS domain.
Fig. 5.
scFv antibodies alter hERG 1a kinetics. Whole-cell patch clamp recordings from HEK293 cells expressing hERG 1a at 36 °C ± 1 °C. (A) Envelope of tails activation protocol and traces. (B) Activation time constant for control, 2.10, and 2.12 antibodies derived from peak tails as in A. (C) Inactivation recovery measured over a range of voltages at onset of voltage change from 30 mV. (D) Time constants of inactivation determined from single exponential fits to data as in C vs. test potential. (E) Inactivation onset measured with the three-pulse protocol over a range of potentials. (F) Inactivation time constants determined with single exponential fits to data as in E vs. test potentials. Data are means ± SEM (n = 5–10). *Statistical significance between scFv 2.10 and control at P < 0.05. †Statistical significance between scFv 2.12 and control at P < 0.05.
Table 2.
Time constants of activation, inactivation onset, and inactivation recovery
| Control, ms | scFv 2.10, ms | scFv 2.12, ms | n | |
| Activation (0 mV) | 71.5 ± 2.8 | 75.6 ± 11.5 | 56.3 ± 3.4* | 5 |
| Inactivation onset (10 mV) | 1.6 ± 0.1 | 2.3 ± 0.2* | 1.7 ± 0.1 | 5–8 |
| Inactivation recovery (−50 mV) | 1.8 ± 0.2 | 2.1 ± 0.1 | 1.5 ± 0.1* | 5–10 |
All data are means ± SEM; n is the number of replicates.
P < 0.01 compared with control.
Fig. S3.
Endogenous HEK293 cell currents during key protocols. (Upper) Typical current traces recorded from HEK293 cells stably expressing hERG 1a for each of the protocols as labeled. (Lower) Typical current traces recorded from untransfected HEK293 cells.
hERG-Interacting scFv Antibodies Enhance hERG Current.
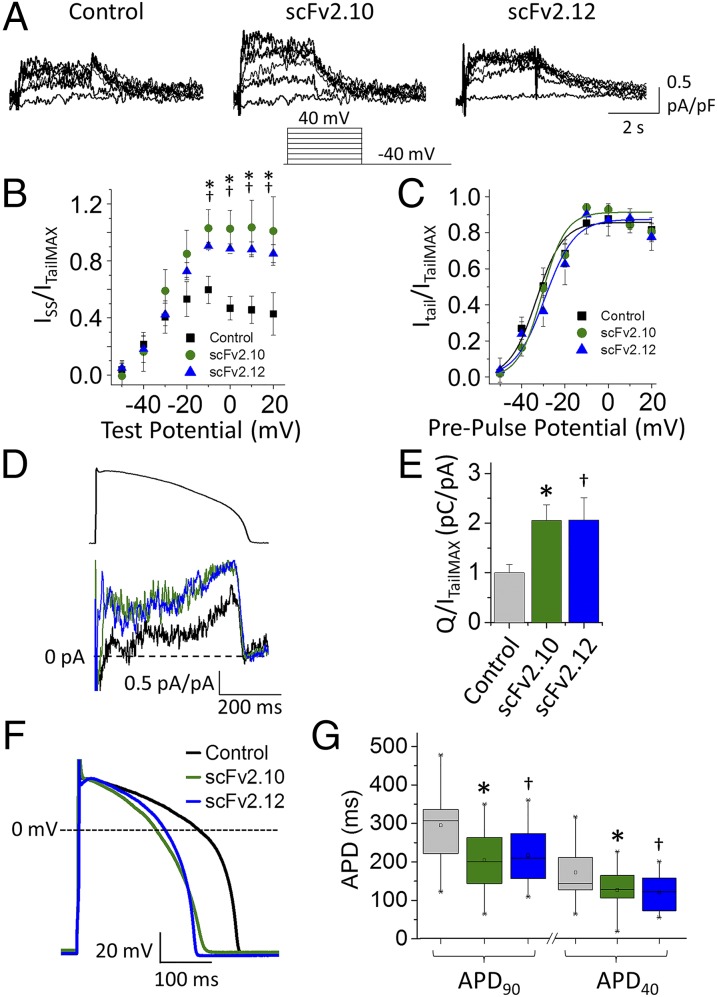
We next determined the consequences of the changes in gating induced by scFv 2.10 and scFv 2.12 antibodies on current amplitude both at steady state and in response to a voltage command mimicking the ventricular AP. It is apparent from the examples in Fig. 6A that both antibodies increased the magnitude of the steady-state current, which is shown normalized to the peak tail current evoked at −50 mV (Fig. 6 A and B). The antibodies had little effect on the conductance–voltage plot for activation (Fig. 6C, SI Materials and Methods, and Table S3), but both shifted the voltage dependence of inactivation by ∼15 mV (Fig. 6D, SI Materials and Methods, Fig. S4, and Table S3).
Fig. 6.
scFv antibodies increase hERG 1a currents. (A) Traces from HEK293 cells expressing hERG 1a for vehicle control, scFv 2.10, and scFv 2.12 at 36 °C ± 1 °C. Pulse protocol is shown. (B) Relative steady-state current (ISS) vs. test potential. (C) Normalized peak tail current vs. prepulse potential fitted with Boltzmann function for control (black), scFv 2.10 (green), and scFv 2.12 (blue). (D) Voltage dependence of inactivation for control (black), scFv 2.10 (green), and scFv 2.12 (blue). After a pulse to +40 mV, currents evoked over a range of potentials from −120 to 30 mV were measured. Normalized conductance vs. test potential fitted with a Boltzmann function (Materials and Methods and Fig. S4). (E) Currents from control (black), scFv 2.10 (green), scFv 2.12 (blue), and untransfected HEK cells (blank; red; Lower) during voltage command mimicking human ventricular AP (Upper). (F) Relative repolarizing charge for vehicle control (gray), scFv 2.10 (green), and scFv 2.12 (blue). Data are means ± SEM (n = 5–10). *Statistical significance between control and scFv 2.10 at P < 0.05. †Statistical significance between control and scFv 2.12 at P < 0.05.
Table S3.
Boltzmann function parameters for activation and inactivation
| Activation | Inactivation | |||||
| V1/2, mV | Slope constant (k), mV | n | V1/2, mV | Slope constant (k), mV | n | |
| Control | −34.9 ± 1.3 | 6.1 ± 0.1 | 6 | −42.1 ± 2.0 | 21.8 ± 1.3 | 8 |
| scFv 2.10 | −30.8 ± 1.5 | 5.1 ± 3.6 | 10 | −26.5 ± 2.9** | 26.1 ± 2.7 | 6 |
| scFv 2.12 | −34.0 ± 1.1 | 6.0 ± 0.4 | 10 | −26.5 ± 4.7* | 21.1 ± 1.3 | 5 |
All data are means ± SEM; n is the number of replicates.
P < 0.01 compared with control.
P < 0.0001 compared with control.
Fig. S4.
scFv 2.10 alters rectification. (A) Sample traces used to measure hERG 1a rectification in HEK293 cells at 36 °C ± 1 °C for control, scFv 2.10, and scFv 2.12. Pulse protocol used is shown in column 4. (B) Peak tail current density plotted as a function of test potential for control (black), scFv 2.10 (green), and scFv 2.12 (blue). ScFv 2.10 but not scFv 2.12 significantly altered rectification compared with controls. All data are means ± SEM (n = 5–9). *Statistical significance at P < 0.05 between control and scFv 2.10.
To predict how these changes would affect current during a ventricular AP, we measured “repolarizing charge” (Q) from currents elicited during a voltage protocol that mimics a human ventricular AP (Fig. 6E). Relative repolarizing charge was calculated as the integral of membrane current elicited during the AP protocol normalized to the maximum peak tail current recorded from the same cell after a saturating voltage step (19). Both antibodies significantly increased the total repolarizing charge compared with control (Fig. 6F). The differential effect on gating kinetics is reflected in the exemplar traces in Fig. 6E. Current recorded from an untransfected HEK293 cell is shown in red in Fig. 6E. These results reflect how the differential binding of scFv 2.10 and scFv 2.12 to either the PAS-Cap or the PAS domain of hERG increases repolarizing charge during a ventricular AP.
scFv Antibodies Enhance IKr and Shorten AP Durations in Human Cardiomyocytes.
We measured the effect of the antibodies on cardiac IKr from cardiomyocytes derived from human induced pluripotent stem cells (iPSC-CMs) at physiological temperature (36 °C ± 1 °C). Similar to the effects on heterologously expressed hERG currents, scFv 2.10 and scFv 2.12 antibodies significantly increased steady-state IKr compared with control (Fig. 7 A and B), without a measurable effect on the conductance–voltage relationship of activation (Fig. 7C). Both antibodies also significantly increased currents evoked by AP voltage clamp (Fig. 7 D and E). Action potential durations (APDs) were correspondingly shortened as shown at 40% and 90% repolarization (Fig. 7 F and G and Table S4). Neither antibody affected the maximum diastolic potential, AP amplitude, or AP rise rate, suggesting the antibodies are specific for IKr (Table S4). Although with this approach we have not ruled out nonspecific effects of the antibody on other channels, the effects on AP morphology are consistent with a targeted increase in IKr as observed using patch clamp analysis in the cardiomyocyte and heterologous expression system. Overall, these data show that the PAS domains in native IKr channels are accessible to scFv 2.10 and scFv 2.12 antibody binding, allowing targeted enhancement of repolarizing IKr magnitude and subsequent shortening of the human cardiac AP.
Fig. 7.
scFv antibodies increase IKr and shorten APD. (A) Traces from iPSC-CMs for control, scFv 2.10, and scFv 2.12 at 36 °C ± 1 °C. Pulse protocol is shown. (B) Relative steady-state current vs. test potential. (C) Normalized peak tail current vs. prepulse potential fitted with Boltzmann function for control (black), scFv 2.10 (green), and scFv 2.12 (blue). (D) Currents evoked during AP voltage command (Upper) from control (black), scFv 2.10 (green), and scFv 2.12 (blue; Lower). (E) Relative repolarizing charge determined by integrating currents as in D. Data are means ± SEM (n = 9–12). (F) AP traces from control (black), scFv 2.10 (green), and scFv 2.12 (blue) Kir2.1-transduced iPSC-CMs. (G) Box plots of APDs measured at 90% and 40% repolarization times from control (black), scFv 2.10 (green), and scFv 2.12 (blue) Kir2.1-transduced iPSC-CMs. Boxes show 25 to 75% confidence intervals; error bars show 10 to 90% confidence intervals. The internal line is the median (n = 13–15). *Statistical significance between control and scFv 2.10 at P < 0.05. †Statistical significance between control and scFv 2.12 at P < 0.05.
Table S4.
Effect of antibodies on APD and morphology
| Control | scFv 2.10 | scFv 2.12 | |
| APDs, ms | |||
| APD90 | 294 ± 25 | 203 ± 22* | 216 ± 19* |
| APD80 | 275 ± 24 | 197 ± 22* | 200 ± 16* |
| APD70 | 256 ± 24 | 188 ± 21* | 184 ± 14* |
| APD40 | 172 ± 20 | 114 ± 17* | 119 ± 12* |
| APD30 | 131 ± 20 | 74 ± 15* | 82 ± 14 |
| n | 13 | 13 | 15 |
| AP morphology | |||
| Ratio (APD40 − APD30)/(APD80 − APD70) | 3.9 ± 0.8 | 4.9 ± 0.8 | 2.5 ± 0.4 |
| AP upstroke (dV/dtMAX) | 214 ± 31 | 261 ± 25 | 244 ± 30 |
| Diastolic potential, mV | −73.6 ± 1.6 | −77.7 ± 1.0 | −74.3 ± 1.5 |
| AP amplitude, mV | 128 ± 4 | 136 ± 4 | 128 ± 6 |
| n | 10 | 13 | 11 |
All data are means ± SEM; n is the number of replicates.
P < 0.05 compared with control.
SI Materials and Methods
Immunization and Serum Characterization.
Two female Shaver chickens were immunized over a period of 84 d as follows: s.c. administration of WT PAS protein (residues N 1–135) at days 0, 16, 30, and 79 with one i.m. boost at day 65 for a total volume of 500 µL. Antigen was prepared fresh for each immunization and administered as a mixture with Freud’s incomplete adjuvant (1:1); 2-mL test bleeds were collected at days 0, 55, and 79, and the polyclonal antibody response to PAS protein was evaluated by standard Western blot analysis using HRP-conjugated anti-chicken IgY antibody (whole molecule; Sigma) as secondary antibody.
Generation of scFv Fragments.
Total RNA was isolated from the spleen and bone marrow of one femur from each chicken following the manufacturer’s instructions (Purelink; Ambion). Total RNA samples were run on agarose gels to check RNA integrity and quantified using the NanoDrop. The total RNA samples from both chickens were pooled, and 20 µg total RNA was used to generate first-strand cDNA synthesis (Superscript; Invitrogen). Amplification of scFv fragments (VL–peptide linker sequence–VH) with a short peptide linker GQSSRSS was carried out using primers and procedures that have been previously described (29, 45).
Construction of the scFv Library.
scFv library construction was generated in the VL–VH orientation as described previously (30) with the following modifications. Both scFv fragments and the pComb3XSS phagemid vector were digested with SfiI (ThermoFisher) and gel purified. Ligations were performed with a 2:1 molar ratio of scFv fragment to vector using T4 ligase overnight at 4 °C. The DNA ligation was extracted using a nucleotide removal column and resuspended in nuclease-free water, and at least 30 electroporations were performed into electrocompetent XL1 Blue Escherichia coli cells (Stratagene). After electroporation, cells were plated on at least 60 (150 × 10 mm) dishes of 2× yeast extract and tryptone (YT) agar containing 20 mM glucose and 100 µg/mL ampicillin (Amp) and incubated overnight at 37 °C. The clones were scraped off the plates into 200 mL superbroth (SB) medium containing 20% (vol/vol) glycerol and subsequently stored at −80 °C. The primary library is estimated to have a size of ∼4 × 107 transformants.
Rescue of scFv Phage.
Fifty milliliters SB medium containing 50 µg/mL Amp was inoculated with 1 mL of bacterial-phage library glycerol stock, incubated for 1 h at 37 °C, supplemented with 50 µg/mL Amp, and grown for another 1 h at 37 °C. Phage–scFv expression was induced by coinfection by the helper phage VCSM13(KanR) and incubation in a total volume of 100 mL SB medium for another 2 h at 37 °C. Kanamycin was added to a final concentration of 70 µg/mL to ensure the efficient replication and packaging of phagemid DNA, and the culture was incubated with shaking overnight at 37 °C. Cells were pelleted, and phage–scFv-containing supernatant was mixed at a 1:5 (vol/vol) ratio with a PEG/NaCl solution [20% (wt/vol) PEG 8000, 2.5 M NaCl] and left on ice for 2 h so that phages would precipitate. Phage library was resuspended in 1% BSA/Tris-buffered saline (TBS), and the titer determined to be ∼3.5 × 1011 pfu/mL.
Selection of Anti-PAS Protein scFvs from Phage Display Library.
Unless otherwise stated, all steps of the phage display selection were done at room temperature (RT). Freshly prepared phage library was used in selection rounds, which were performed either with amino-terminally GST-tagged Per-Arnt-Sim protein (GSTPAS) bound to glutathione magnetic beads (GMBs; solution selection) or untagged PAS protein immobilized directly onto Maxisorp (Nunc) 96-well plates (solid-phase selection).
Solid-phase selection.
For solid-phase selection, 2.5 or 10 µg purified PAS protein was added to triplicate wells of a 96-well Nunc Maxisorp Plate and incubated overnight at 4 °C. Wells were incubated with blocking solution [3% (wt/vol) BSA/TBS] for 1 h before 50 µL library phage was added. After 2 h of incubation, the unbound phages were washed off with 10 × 0.1% TBS-Tween20 and 10× TBS washes. The bound phages were eluted with 50 µL per well 100 mM glycine (pH 4.2) and 150 mM NaCl and incubated for 10 min. The pHs of the eluted phage solutions were neutralized with 3 µL 2 M Tris Base per 50 µL eluate. Four rounds of panning were performed, increasing stringency each round by decreasing the amount of PAS protein coating the plate: (A) 2.5, 1.0, 0.5, and 0.25 µg PAS and (B) 10, 5, 1, and 0.1 µg PAS.
Solution selection.
For solution selection, 5 µg GST or GSTPAS protein was bound to GMBs for 1 h. Washed beads were incubated with blocking solution [3% (wt/vol) BSA/TBS] for 1 h. In the first round of panning, 100 µL phage library was precleared by incubation with GST beads for 1 h before incubation with GSTPAS beads for another 1 h. Unbound phages were washed off twice with 0.1% TBS-Tween20 and once with TBS. The phages from either 200 µL GSTPAS/beads/phage mixture or eluted GSTPAS/phage (using 50 mM reduced glutathione) were amplified as described below. Four rounds of panning were performed, with increasing stringency each round by decreasing protein concentration and increasing the number of washes per round: preclear with 5 µg GST and then, 5 µg GSTPAS and wash two times with 0.1% TBS-Tween and one time with TBS; preclear with 2.5 µg GST and then, 2.5 µg GSTPAS and wash five times with 0.1% TBS-Tween and five times with TBS; 1.25 µg GSTPAS wash 10 times with 0.1% TBS-Tween and 10 times with TBS; and 0.625 µg GSTPAS wash 10 times with 0.1% TBS-Tween and 10 times with TBS.
Phage Amplification.
Eluted phages were amplified by mixing the phage solution with 3 mL fresh midlog-phase E. coli XL1Blue cells. Cells were incubated at RT with shaking for 15 min, after which Amp was added after 2 subsequent hours until the final Amp concentration was 50 µg/mL. The input and output phage titers were determined. The eluted phages were further amplified overnight at 37 °C in 100 mL SB medium containing 70 µg/mL kanamycin and VCSM13 helper phage. Phages were precipitated and used in the next round of panning.
Sequence Analysis.
All positive clones tested by ELISA were analyzed for the presence of scFv sequence inserts. The VL and VH chains were sequenced using the reverse primer 5′-GCCCCCTTATTAGCGTTTGCCATC-3′ and forward primer 5′-AAGACAGCTATCGCGATTGCAG-3′, respectively.
ELISA and Western Blot Overlay Assays.
Individual colonies were picked from the output plate of the fourth round of scFv selection, inoculated into 5-mL cultures of SB medium (50 µg/mL Amp), and grown overnight at 30 °C after induction with 1 mM isopropyl1-thiol-B-d-galactopyranoside (IPTG). Cells were pelleted and resuspended in TBS (50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl) and lysed by temperature shock. Cells were spun at 4 °C for 10 min at 14,550 × g, and the supernatants (cell lysates) were used for ELISA. Duplicate wells of a Nunc Maxisorp 96-well plate were coated with GST or PAS protein (2 µg in 50 µL) overnight at 4 °C. After blocking for 1 h at 37 °C with blocking buffer [5% (wt/vol) nonfat milk/TBS], 50 µL cell lysates (at 1:2 dilution in blocking buffer) were added to each well and incubated for 2 h at RT. After five washes with TBS, scFv binding was detected by adding a 1:1,000 dilution of anti-HA HRP Clone 3F10 (Roche) antibody for 1 h at RT. Peroxidase activity was detected using Sigmafast OPD Detection. The reaction was stopped using 3 M HCl, and the OD was determined at 450 nm.
For the Western blot overlay assay, PAS protein was run on a 17% (wt/vol) SDS/PAGE gel and transferred to nitrocellulose membrane. Individual lanes of protein were blocked with 5% (wt/vol) nonfat milk, TBS, and 0.2% Tween20 for 1 h at RT, and 400 µL crude lysate preparations as prepared above (in blocking solution) were added overnight at 4 °C. After extensive washing with TBS and 0.2% Tween20, nitrocellulose membranes were incubated with anti-His primary antibody (Qiagen) and then, detected using a secondary anti-mouse HRP antibody and ECL (Amersham Biosciences/GE Healthcare).
Antibody Expression and Purification.
A confluent-transformed LB agar plate supplemented with 100 µg/mL Amp was used to inoculate 2× 1 L SB medium supplemented with 20 mM Mg2Cl and 50 µg/mL Amp. Cultures were grown at 37 °C until an OD at 600 nm of ∼0.6 and induced with 1 mM IPTG at 30 °C for 4 h of growth. Bacteria were resuspended in 50 mM Hepes (pH 8.0), 150 mM NaCl, 10 mM imidazole, 1 mM PMSF, 1 µg/mL leupeptin, and 1 µg/mL pepstatin A with a small amount of DNase added and lysed using a cell cracker. Cell lysates were prepared by centrifugation at 43,960 × g for 45 min at 4 °C. Lysate was combined with NiSelect NTA Beads (Sigma) for 1 h at 4 °C to bind scFv protein. Resin was washed by gravity flow consecutively with 10× bead volumes of wash 1 buffer (50 mM Hepes, pH 8.0, 150 mM NaCl, 10 mM imidazole), wash 2 buffer (50 mM Hepes, pH 8.0, 300 mM NaCl, 10 mM imidazole), and wash 3 buffer (50 mM Hepes, pH 8.0, 150 mM NaCl, 20 mM imidazole). Protein was eluted with elution buffer (50 mM Hepes, pH 8.0, 150 mM NaCl, 250 mM imidazole). For ITC analysis, the tagged protein was immediately dialyzed overnight against Hepes-buffered saline (HBS) (50 mM Hepes, pH 8.0, 150 mM NaCl) before purification by size exclusion chromatography (Superdex-75 10/300 GL; GE Healthcare). Alternatively, tagged protein was dialyzed overnight against 10 mM Hepes (pH 7.2; KOH), 10 mM NaCl, 50 mM KCl, 20 mM EGTA, and 60 mM KF for functional analysis using electrophysiology. For untagged protein preparations, eluted protein was dialyzed overnight against TBS (50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl) containing Bovine α-Thrombin (Haematologic Tech. Inc.) at 4 °C. The protein was incubated with NiSelect NTA Beads once more to remove any uncut protein before being subjected to anion exchange chromatography (HiTrap Q HP). Finally, antibody preparations were further purified by size exclusion chromatography (Superdex-75 10/300 GL; GE Healthcare) in 10 mM Hepes (pH 7.2; KOH), 10 mM NaCl, 50 mM KCl, 20 mM EGTA, and 60 mM KF.
ITC Experiments.
Protein samples were concentrated and dialyzed overnight against 50 mM Hepes (pH 8.0) and 150 mM NaCl and then, spun at 14,550 × g for 30 min at 4 °C. The hERG PAS or mEAG1 PAS protein was loaded into the calorimetric syringe as a 200 µM solution and titrated into 20 µM antibody in the sample cell. An initial injection of 2 µL was followed by 28 injections of 10 µL with enough spacing to allow heat recovery back to baseline in between injections. Heat of dilution was measured in control experiments, where hERG PAS or mEAG1 PAS protein was injected into buffer and subtracted from experimental heats before fitting. Data were fitted with a single-site binding model using Origin 7 (MicroCal) software provided with the instrument.
GST Pulldown Experiments.
Aliquots (50 µL) of GMB slurry were washed with TBS buffer (50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl). Purified GST protein or GST fusions were bound at a final saturating concentration of 1 mg/mL in 1× TBS buffer for 1 h at RT. Beads were thoroughly washed five times with TBS buffer to remove unbound protein. Purified scFv antibody was added and incubated for 1 h at RT on a rotator. Unbound protein was washed five times with TBS buffer. Protein was eluted from beads by addition of SDS sample buffer. Bound scFv protein was detected by Western blot using anti-His antibody and ECL detection as described above.
Cell Culture.
iPSC-CMs (iCell Cardiomyocytes; Cellular Dynamics International) were plated and stored in 12-well dishes as per the manufacturer’s instructions. HEK293 cells were cultured in 100-mm dishes in DMEM supplemented with 10% FBS.
Recordings.
Recordings were performed using an Axon 200A Amplifier and Clampex (Molecular Devices). Data were sampled at 10 kHz and low pass-filtered at 1 kHz. Cells were perfused with extracellular solution containing 150 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 15 mM glucose, 10 mM Hepes, and 1 mM Na-pyruvate and titrated to pH 7.4 using NaOH. Recording pipettes had resistances of 2–4.5 MΩ when backfilled with intracellular solution containing 5 mM NaCl, 150 mM KCl, 2 mM CaCl2, 5 mM EGTA, 10 mM Hepes, and 5 mM MgATP and titrated to pH 7.2 using KOH. Intracellular solution aliquots were frozen until the day of recording. Stock antibodies were diluted to 10 μM in intracellular solution, which in the absence of antibodies, served as vehicle control. Antibodies were administered intracellularly via the patch pipette. For AP recordings, iPSC-CMs were transformed with 1 μL/mL adenoviral lysate. Adenoviral DNA encoded Kir2.1 in frame with GFP and was used to stabilize the resting membrane potential as previously described (20, 46).
Voltage Protocols.
The effects of antibody fragments on hERG stably expressed in HEK293 cells were characterized using whole-cell voltage clamp at both RT (22 °C ± 1 °C) and physiological temperature (36 °C ± 1 °C). The voltage dependence of activation was measured by stepping cells from a −80-mV holding potential to a 4-s prepulse between −80 and +50 mV in 10-mV increments to measure steady-state current. Cells were then stepped to a −50-mV, 5-s tail pulse to measure tail current. The time course of deactivation was measured by fitting current decay at a given potential with a double exponential function. The time course of activation, inactivation onset, and inactivation recovery were measured as described (19). The voltage dependence of inactivation was measured by stepping from a −80-mV holding pulse to a +50-mV conditioning pulse. Maximal current was measured during a test pulse between −120 and +30 mV in 10-mV intervals at a time point no less than three times the measured time constant of inactivation recovery for that potential. No correction was made for deactivation at the more negative potentials, which occurred with time constants ∼14-fold greater than recovery from inactivation. Current was then plotted as a function of test potential. The linear portion of the I/V plot was used to calculate the reversal potential for each cell. Normalized conductance was then plotted as a function of test potential and fitted with the following Boltzmann equation:
where A1 and A2 represent the maximum and minimums of the fit, respectively, V is the membrane potential, and V0 is the midpoint.
In iPSC-CMs, voltage and current clamp protocols were completed at 36 °C ± 1 °C. Voltage protocols were run before and after bath perfusion of 2 µM E-4031, an IKr-specific blocker, and the difference in current was taken to represent IKr (Fig. S5) (20). The voltage dependence of IKr in iPSC-CMs was determined by stepping from a −40-mV holding potential to a 3-s prepulse between −50 and +30 mV in 10-mV increments. Cardiomyocytes were then stepped to a −40-mV, 6-s test pulse to measure tail current. iPSC-CMs were held at −40 mV to inactivate voltage-gated sodium channels. APs were recorded using whole-cell current clamp as described (20).
Fig. S5.
E-4031 subtraction in iPSC-CMs. Sample traces showing membrane currents recorded (A) before and (B) after 2 μM E-4031 perfusion. (C, Upper) Traces resulting from the subtraction of traces in B from those in A. Voltage protocol is shown in C, Lower.
Steady-state currents in HEK293 and iPSC-CMs were measured as the 5-ms mean at the end of each prepulse. The relative steady-state current was calculated by dividing the steady-state measurement by the maximal tail current recorded from the same cell. To describe the voltage dependence of channel activation, peak tail current was normalized to cellular capacitance, and current was plotted as a function of prepulse potential and fitted with the Boltzmann equation. Repolarizing charge was measured from the integral of membrane currents recorded during a voltage protocol designed to mimic a human ventricular AP (6). Relative repolarizing charge was calculated by dividing total repolarizing charge by the maximal tail current recorded from the same cell. Leak subtraction was performed offline based on measured current observed at potentials negative to hERG channel activation.
Analysis was completed using Clampfit (Molecular Devices) and Origin (OriginLab). All data are reported as means ± SEM and compared using Student’s t tests. When applicable, ANOVAs and Bonferroni posthoc t tests were used. Statistical significance was taken at P value < 0.05.
Discussion
This work identifies two scFv antibodies (scFv 2.10 and scFv 2.12) that bind to different regions of the PAS domain and cause an increase in the total amount of current passing through the hERG channel in HEK293 cells and IKr in stem cell-derived human cardiomyocytes. At physiological temperatures, the effects of the scFv molecules on inactivation and activation dictate a net increase in current. The antibody that recognizes the globular PAS region (scFv 2.12) increases the rate of activation and accelerates the time course of inactivation recovery, effects that have been previously associated with the role of the PAS domain (19, 31). In contrast, the antibody that recognizes the PAS-Cap region (scFv 2.10) slows the time course of inactivation onset, an effect not previously associated with PAS-Cap function, which may reflect an allosteric effect of the antibody on the contiguous PAS domain.
The majority of characterized hERG channel modulators are small molecules, broadly divided into blockers and activators (or positive modulators). Blockers share a common binding site in the hERG pore domain cavity, blocking the ion permeation pathway of the channel, reducing the ion current associated with hERG, and often, leading to acquired LQTS (32, 33). In contrast, positive modulators of hERG bind at a pore domain site located between adjacent hERG subunits (34). The differences in chemical nature and mode of interaction with the channel (binding to the PAS domain) lead us to conclude that the two scFv molecules described form a different class of modulators of the hERG channel.
Our work with the scFv antibodies also reveals the potential of the cytosolic domains of hERG and, in particular, the PAS domain as targets for modulating hERG channel function. However, we have also recently shown the difficulties in identifying small molecules that bind to PAS domains from KCNH channels (27). From this perspective and despite the difficulties in targeting and delivering these molecules into specific cell cytosols, we must at least consider the therapeutic potential of antibodies targeting cytosolic domains. Although delivery of these molecules is a major obstacle at this time for their use as therapeutic agents, development of technologies for the cytosolic delivery of large protein molecules is an important focus of research (35–38), and it is reasonable to expect that, in the future, solutions to this problem will be found.
Comparison of the properties of the scFv molecules with other small molecule positive modulators shows that, despite binding to different regions, both the small molecules (39) and scFv molecules generally increase the magnitude of current associated with hERG by disrupting the voltage-dependent inactivation process and favoring the open state. However, the maximum effect of our scFv molecules is an approximately twofold increase in hERG or IKr currents, whereas the small molecule hERG-positive modulators have larger maximal effects that can lead to excessive APD shortening and arrhythmia at elevated doses (40). The subtler effect of scFv molecules targeted toward the hERG PAS domain could potentially correct LQTS without the risk of excessive shortening the QT interval and evoking arrhythmias. In this regard, scFv molecules targeting the PAS domain present a potentially safer mechanism of action.
Importantly, the scFv molecules have allowed us to gain insights into the properties of the PAS domain within the context of the full-length hERG channel. In particular, there is limited structural information about the PAS-Cap region, which is disordered in the structures of isolated PAS domains. The PAS-Cap is thought to extend away from the PAS domain and interact with the machinery influencing channel gating (17, 41, 42). Our functional data show that scFv 2.10 alters the properties of the hERG channel. By defining that scFv 2.10 interacts with the PAS-Cap and that the epitope involves residues R4 and R5, thought to be important for the interaction between PAS-Cap and the C linker of the hERG channel (41, 43), we have established that this channel region is not always engaged with the gating machinery. In at least one functional state of the channel, the N-terminal end of PAS-Cap releases from the gating machinery and becomes accessible for interaction with the scFv, a protein of 27 kDa. This finding fits well with the previously proposed model, in which the PAS-Cap domain transiently interacts with the C-terminal domain (41).
The scFv molecules also allowed us to explore the idea that channel PAS domains have a modulatory function. This idea has been supported by the role of PAS domains in other proteins and many studies that showed that mutations or deletions in the hERG PAS domain alter many aspects of channel gating (9, 15–18). However, those results simply indicate that the PAS domain is an important structural feature of KCNH channels but do not show the potential for allosteric modulation (28). With scFv 2.12, which binds to the globular region of the PAS domain, we have shown that interaction with another protein can modify the domain and elicit a functional change in the channel, establishing that it is possible to modulate hERG channel function allosterically through these cytosolic regions.
Strikingly, the functional impact of the scFv 2.12 antibody strongly resembles the functional differences observed between the heteromeric hERG 1a/1b channel and hERG 1a channel (19, 20). In hERG 1a/1b, there are fewer PAS domains present in the channel, resulting in faster deactivation but also, faster activation and recovery from inactivation, which in turn, yield larger currents. The observation of a similar functional phenotype with scFv 2.12 suggests that the antibody binds to PAS and changes the PAS interaction with its binding site in the channel, the CNBh domain. In effect, the antibody replicates the genetic modulation observed in the heteromeric channel as an allosteric modulation.
Materials and Methods
Protein Purification.
hERG and mEAG1 PAS domain versions cloned with N-terminal GST or His tag were expressed and purified as previously described (12, 44).
Antibody Expression and Purification.
scFv antibodies secreted to periplasmic space of E. coli BL21(DE3) from pComb3XSS vector or a modified pComb3XSS that includes a Thrombin cleavage site (30) were purified using His tag affinity and size exclusion chromatography (SI Materials and Methods).
Affinity Measurements.
ITC experiments between scFv antibodies and hERG or mEAG1 PAS proteins were performed in MicroCal VP-ITC at 15 °C. hERG or mEAG1 PAS proteins at 200 μM solution were titrated into 20 μM antibody in the sample cell. Data were fitted using Origin 7 (MicroCal) (SI Materials and Methods).
GST Pulldown Assay.
Purified GST protein or GST fusions of hERG and mEAG1 PAS domains (truncated or mutated) were bound to glutathione magnetic beads and incubated with purified scFv antibodies. After thorough washing, bound protein was eluted with SDS sample buffer. Bound scFv protein was detected by Western blot with anti-His antibody (SI Materials and Methods).
Electrophysiology.
Patch clamp electrophysiology was carried out on HEK293 cells and human iPSC-CMs as described in detail in SI Materials and Methods.
Acknowledgments
We were supported by National Heart, Lung, and Blood Institute Grant 5T32HL007936 Training Program in Translational Cardiovascular Science (to G.S.), a postdoctoral training award from the University of Wisconsin Stem Cell and Regenerative Medicine Center (to D.K.J.), Fundação para a Ciência e a Tecnologia (FCT) Postdoctoral Fellowship SFRH/BPD/105672/2015 (to A.S.F.), and NIH Grants NS081320 (to G.A.R. and J.H.M.-C.) and HL081780 (to G.A.R.). This work was financed by Fundo Europeu de Desenvolvimento Regional funds through COMPETE 2020—Operational Program for Competitiveness and Internationalization, Portugal 2020 and Portuguese funds through FCT/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601116113/-/DCSupplemental.
References
- 1.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269(5220):92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 3.Curran ME, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 4.Vandenberg JI, et al. hERG K(+) channels: Structure, function, and clinical significance. Physiol Rev. 2012;92(3):1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, et al. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74(1):230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Drug Administration, HHS International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist. 2005;70(202):61133–61134. [PubMed] [Google Scholar]
- 8.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91(8):3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morais Cabral JH, et al. Crystal structure and functional analysis of the HERG potassium channel N terminus: A eukaryotic PAS domain. Cell. 1998;95(5):649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, et al. NMR solution structure of the N-terminal domain of hERG and its interaction with the S4-S5 linker. Biochem Biophys Res Commun. 2010;403(1):126–132. doi: 10.1016/j.bbrc.2010.10.132. [DOI] [PubMed] [Google Scholar]
- 11.Muskett FW, et al. Mechanistic insight into human ether-à-go-go-related gene (hERG) K+ channel deactivation gating from the solution structure of the EAG domain. J Biol Chem. 2011;286(8):6184–6191. doi: 10.1074/jbc.M110.199364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adaixo R, Harley CA, Castro-Rodrigues AF, Morais-Cabral JH. Structural properties of PAS domains from the KCNH potassium channels. PLoS One. 2013;8(3):e59265. doi: 10.1371/journal.pone.0059265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haitin Y, Carlson AE, Zagotta WN. The structural mechanism of KCNH-channel regulation by the eag domain. Nature. 2013;501(7467):444–448. doi: 10.1038/nature12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques-Carvalho MJ, et al. Structural, biochemical, and functional characterization of the cyclic nucleotide binding homology domain from the mouse EAG1 potassium channel. J Mol Biol. 2012;423(1):34–46. doi: 10.1016/j.jmb.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zou A, Splawski I, Keating MT, Sanguinetti MC. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem. 1999;274(15):10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- 16.Schönherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol. 1996;493(Pt 3):635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Trudeau MC, Zappia AM, Robertson GA. Regulation of deactivation by an amino terminal domain in human ether-à-go-go-related gene potassium channels. J Gen Physiol. 1998;112(5):637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107(5):611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sale H, et al. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with Long-QT syndrome. Circ Res. 2008;103(7):e81–e95. doi: 10.1161/CIRCRESAHA.108.185249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DK, et al. hERG 1b is critical for human cardiac repolarization. Proc Natl Acad Sci USA. 2014;111(50):18073–18077. doi: 10.1073/pnas.1414945111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004;279(43):44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- 22.London B, et al. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1997;81(5):870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- 23.Martinson AS, et al. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc Natl Acad Sci USA. 2014;111(15):5712–5717. doi: 10.1073/pnas.1321716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson CL, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113(3):365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 25.Rossenbacker T, et al. Novel mutation in the Per-Arnt-Sim domain of KCNH2 causes a malignant form of long-QT syndrome. Circulation. 2005;111(8):961–968. doi: 10.1161/01.CIR.0000156327.35255.D8. [DOI] [PubMed] [Google Scholar]
- 26.Möglich A, Ayers RA, Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure. 2009;17(10):1282–1294. doi: 10.1016/j.str.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes AS, Morais-Cabral JH, Harley CA. Screening for non-pore-binding modulators of EAG K+ channels. J Biomol Screen. 2016;21(7):758–765. doi: 10.1177/1087057116636592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morais-Cabral JH, Robertson GA. The enigmatic cytoplasmic regions of KCNH channels. J Mol Biol. 2015;427(1):67–76. doi: 10.1016/j.jmb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andris-Widhopf J, Rader C, Steinberger P, Fuller R, Barbas CF., 3rd Methods for the generation of chicken monoclonal antibody fragments by phage display. J Immunol Methods. 2000;242(1-2):159–181. doi: 10.1016/s0022-1759(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 30.Barbas CF, III, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Manual. Cold Spring Harbor Lab Press; Plainview, NY: 2001. [Google Scholar]
- 31.Gustina AS, Trudeau MC. The eag domain regulates hERG channel inactivation gating via a direct interaction. J Gen Physiol. 2013;141(2):229–241. doi: 10.1085/jgp.201210870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry M, Sanguinetti M, Mitcheson J. Revealing the structural basis of action of hERG potassium channel activators and blockers. J Physiol. 2010;588(Pt 17):3157–3167. doi: 10.1113/jphysiol.2010.194670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97(22):12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg V, Stary-Weinzinger A, Sachse F, Sanguinetti MC. Molecular determinants for activation of human ether-à-go-go-related gene 1 potassium channels by 3-nitro-n-(4-phenoxyphenyl) benzamide. Mol Pharmacol. 2011;80(4):630–637. doi: 10.1124/mol.111.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mussbach F, Franke M, Zoch A, Schaefer B, Reissmann S. Transduction of peptides and proteins into live cells by cell penetrating peptides. J Cell Biochem. 2011;112(12):3824–3833. doi: 10.1002/jcb.23313. [DOI] [PubMed] [Google Scholar]
- 37.Verdurmen WP, Luginbühl M, Honegger A, Plückthun A. Efficient cell-specific uptake of binding proteins into the cytoplasm through engineered modular transport systems. J Control Release. 2015;200:13–22. doi: 10.1016/j.jconrel.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Lim KJ, et al. A cancer specific cell-penetrating peptide, BR2, for the efficient delivery of an scFv into cancer cells. PLoS One. 2013;8(6):e66084. doi: 10.1371/journal.pone.0066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanguinetti MC. HERG1 channel agonists and cardiac arrhythmia. Curr Opin Pharmacol. 2014;15:22–27. doi: 10.1016/j.coph.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng J, Shi C, Li L, Du Y, Xu Y. Compound ICA-105574 prevents arrhythmias induced by cardiac delayed repolarization. Eur J Pharmacol. 2013;718(1-3):87–97. doi: 10.1016/j.ejphar.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Ng CA, Phan K, Hill AP, Vandenberg JI, Perry MD. Multiple interactions between cytoplasmic domains regulate slow deactivation of Kv11.1 channels. J Biol Chem. 2014;289(37):25822–25832. doi: 10.1074/jbc.M114.558379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Peña P, et al. Demonstration of physical proximity between the N terminus and the S4-S5 linker of the human ether-a-go-go-related gene (hERG) potassium channel. J Biol Chem. 2011;286(21):19065–19075. doi: 10.1074/jbc.M111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng CA, et al. The N-terminal tail of hERG contains an amphipathic α-helix that regulates channel deactivation. PLoS One. 2011;6(1):e16191. doi: 10.1371/journal.pone.0016191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harley CA, Jesus CS, Carvalho R, Brito RM, Morais-Cabral JH. Changes in channel trafficking and protein stability caused by LQT2 mutations in the PAS domain of the HERG channel. PLoS One. 2012;7(3):e32654. doi: 10.1371/journal.pone.0032654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andris-Widhopf J, Steinberger P, Fuller R, Rader C, Barbas CF 3rd. Generation of human scFv antibody libraries: PCR amplification and assembly of light- and heavy-chain coding sequences. Cold Spring Harb Protoc. 2011;2011(9):pdb.prot065573. doi: 10.1101/pdb.prot065573. [DOI] [PubMed] [Google Scholar]
- 46.Vaidyanathan R, et al. IK1-enhanced human induced pluripotent stem cell-derived cardiomyocytes: An improved cardiomyocyte model to investigate inherited arrhythmia syndromes. AmJ Physiol Heart Circ Physiol. 2016;310(11):H1611–H1621. doi: 10.1152/ajpheart.00481.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]