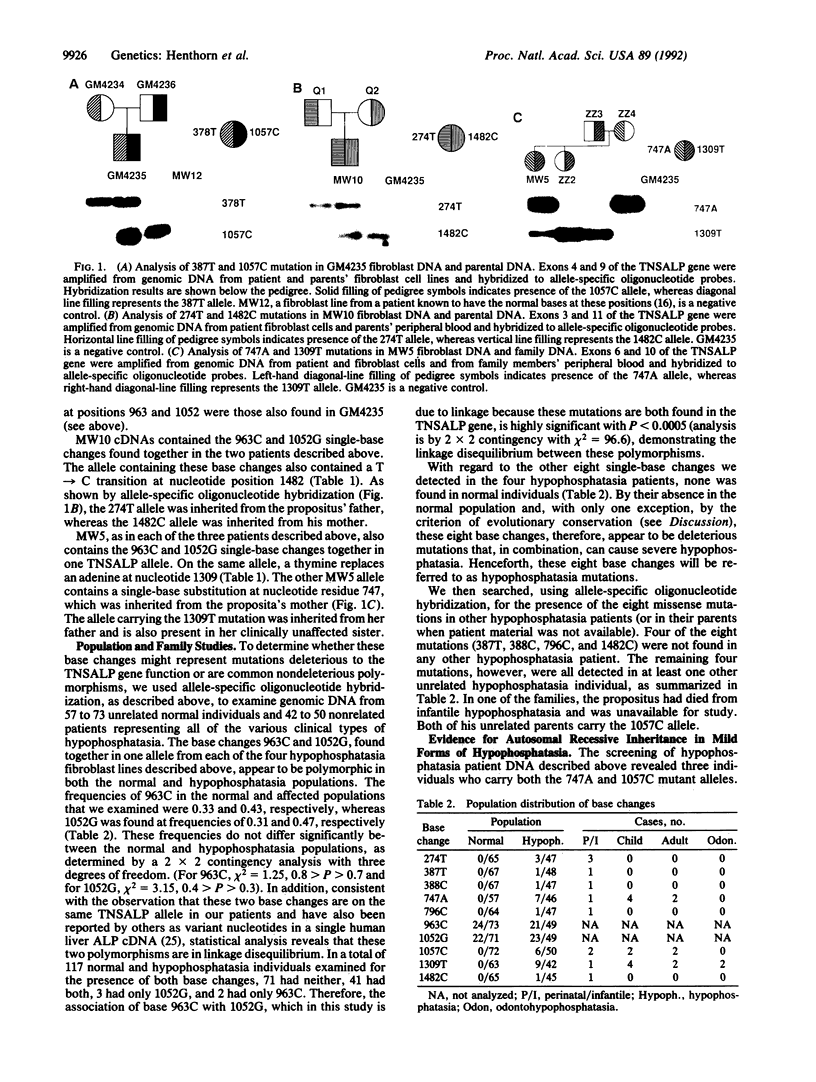
Abstract
Hypophosphatasia is a heritable form of rickets/osteomalacia with extremely variable clinical expression. Severe forms are inherited in an autosomal recessive fashion; the mode of transmission of mild forms is uncertain. The biochemical hallmark of hypophosphatasia is deficient activity of the tissue-nonspecific isozyme of alkaline phosphatase (TNSALP). Previously, we demonstrated in one inbred infant that an identical missense mutation in both alleles of the gene encoding TNSALP caused lethal disease. We have now examined TNSALP cDNAs from four unrelated patients with the severe perinatal or infantile forms of hypophosphatasia. Each of the eight TNSALP alleles from these four individuals contains a different point mutation that causes an amino acid substitution. These base changes were not detected in at least 63 normal individuals and, thus, appear to be causes of hypophosphatasia in the four patients. (Two additional base substitutions, found in one allele from each of the four patients, are linked polymorphisms.) Twenty-three unrelated patients (of 50 screened), who reflect the entire clinical spectrum of hypophosphatasia, possess one of our of the above eight mutations. In two of these additional patients, mild forms of the disease are also inherited in an autosomal recessive fashion. Our findings indicate that hypophosphatasia can be caused by a number of different missense mutations and that the specific interactions of different TNSALP mutant alleles are probably important for determining clinical expression. Severe forms, perinatal and infantile disease, are largely the result of compound heterozygosity for different hypophosphatasia alleles. At least some cases of childhood and adult hypophosphatasia are inherited as autosomal recessive traits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J., Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):695–698. doi: 10.1073/pnas.84.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. M., Whyte M. P., Russell R. G. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 1991;28(3):175–232. doi: 10.3109/10408369109106862. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Kuang W. J., Chen E. Y. Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene. 1986;44(1):121–125. doi: 10.1016/0378-1119(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Chodirker B. N., Evans J. A., Lewis M., Coghlan G., Belcher E., Philipps S., Seargeant L. E., Sus C., Greenberg C. R. Infantile hypophosphatasia--linkage with the RH locus. Genomics. 1987 Nov;1(3):280–282. doi: 10.1016/0888-7543(87)90056-5. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D. Hypophosphatasia. Am J Med. 1957 May;22(5):730–746. doi: 10.1016/0002-9343(57)90124-9. [DOI] [PubMed] [Google Scholar]
- Fedde K. N., Lane C. C., Whyte M. P. Alkaline phosphatase is an ectoenzyme that acts on micromolar concentrations of natural substrates at physiologic pH in human osteosarcoma (SAOS-2) cells. Arch Biochem Biophys. 1988 Aug 1;264(2):400–409. doi: 10.1016/0003-9861(88)90305-0. [DOI] [PubMed] [Google Scholar]
- Fedde K. N., Whyte M. P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-5'-phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. Am J Hum Genet. 1990 Nov;47(5):767–775. [PMC free article] [PubMed] [Google Scholar]
- Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of bovine kidney alkaline phosphatase cDNA. Gene. 1987;59(1):41–46. doi: 10.1016/0378-1119(87)90264-2. [DOI] [PubMed] [Google Scholar]
- HARRIS H., ROBSON E. B. A genetical study of ethanolamine phosphate excretion in hypophosphatasia. Ann Hum Genet. 1959 Dec;23:421–441. doi: 10.1111/j.1469-1809.1959.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Knoll B. J., Raducha M., Rothblum K. N., Slaughter C., Weiss M., Lafferty M. A., Fischer T., Harris H. Products of two common alleles at the locus for human placental alkaline phosphatase differ by seven amino acids. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5597–5601. doi: 10.1073/pnas.83.15.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Edwards Y. H., Weiss M. J., Slaughter C., Lafferty M. A., Harris H. Nucleotide and amino acid sequences of human intestinal alkaline phosphatase: close homology to placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1234–1238. doi: 10.1073/pnas.84.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Kadesch T., Weiss M. J., Harris H. Sequence and characterization of the human intestinal alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12011–12019. [PubMed] [Google Scholar]
- Hulett F. M., Kim E. E., Bookstein C., Kapp N. V., Edwards C. W., Wyckoff H. W. Bacillus subtilis alkaline phosphatases III and IV. Cloning, sequencing, and comparisons of deduced amino acid sequence with Escherichia coli alkaline phosphatase three-dimensional structure. J Biol Chem. 1991 Jan 15;266(2):1077–1084. [PubMed] [Google Scholar]
- Kam W., Clauser E., Kim Y. S., Kan Y. W., Rutter W. J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Hayashi N., Toh-e A., Banno I., Oshima Y. Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase in Saccharomyces cerevisiae. Gene. 1987;58(1):137–148. doi: 10.1016/0378-1119(87)90036-9. [DOI] [PubMed] [Google Scholar]
- Kim E. E., Wyckoff H. W. Structure of alkaline phosphatases. Clin Chim Acta. 1990 Jan 15;186(2):175–187. doi: 10.1016/0009-8981(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Kishi F., Matsuura S., Kajii T. Nucleotide sequence of the human liver-type alkaline phosphatase cDNA. Nucleic Acids Res. 1989 Mar 11;17(5):2129–2129. doi: 10.1093/nar/17.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B. J., Rothblum K. N., Longley M. Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5' flanking region by deletion/substitution. J Biol Chem. 1988 Aug 25;263(24):12020–12027. [PubMed] [Google Scholar]
- Lowe M., Strauss A. W., Alpers R., Seetharam S., Alpers D. H. Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochim Biophys Acta. 1990 Feb 9;1037(2):170–177. doi: 10.1016/0167-4838(90)90164-b. [DOI] [PubMed] [Google Scholar]
- Manes T., Glade K., Ziomek C. A., Millán J. L. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics. 1990 Nov;8(3):541–554. doi: 10.1016/0888-7543(90)90042-s. [DOI] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Harpel B., Mory Y., Schwartz E., Surrey S. Construction of human gene libraries from small amounts of peripheral blood: analysis of beta-like globin genes. Hemoglobin. 1982;6(1):27–36. doi: 10.3109/03630268208996930. [DOI] [PubMed] [Google Scholar]
- Robison R. The Possible Significance of Hexosephosphoric Esters in Ossification. Biochem J. 1923;17(2):286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sowadski J. M., Handschumacher M. D., Murthy H. M., Foster B. A., Wyckoff H. W. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985 Nov 20;186(2):417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- Terao M., Mintz B. Cloning and characterization of a cDNA coding for mouse placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7051–7055. doi: 10.1073/pnas.84.20.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede M. A., Yoon K., Golub E. E., Noda M., Rodan G. A. Structure and expression of rat osteosarcoma (ROS 17/2.8) alkaline phosphatase: product of a single copy gene. Proc Natl Acad Sci U S A. 1988 Jan;85(2):319–323. doi: 10.1073/pnas.85.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Cole D. E., Ray K., Whyte M. P., Lafferty M. A., Mulivor R. A., Harris H. A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a lethal form of hypophosphatasia. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7666–7669. doi: 10.1073/pnas.85.20.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Henthorn P. S., Lafferty M. A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Fallon M. D., Whyte M. P., Fedde K. N., Lafferty M. A., Mulivor R. A., Harris H. Analysis of liver/bone/kidney alkaline phosphatase mRNA, DNA, and enzymatic activity in cultured skin fibroblasts from 14 unrelated patients with severe hypophosphatasia. Am J Hum Genet. 1989 May;44(5):686–694. [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]
- Whyte M. P., Teitelbaum S. L., Murphy W. A., Bergfeld M. A., Avioli L. V. Adult hypophosphatasia. Clinical, laboratory, and genetic investigation of a large kindred with review of the literature. Medicine (Baltimore) 1979 Sep;58(5):329–347. [PubMed] [Google Scholar]
- Whyte M. P., Valdes R., Jr, Ryan L. M., McAlister W. H. Infantile hypophosphatasia: enzyme replacement therapy by intravenous infusion of alkaline phosphatase-rich plasma from patients with Paget bone disease. J Pediatr. 1982 Sep;101(3):379–386. doi: 10.1016/s0022-3476(82)80061-9. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Vrabel L. A., Schwartz T. D. Alkaline phosphatase deficiency in cultured skin fibroblasts from patients with hypophosphatasia: comparison of the infantile, childhood, and adult forms. J Clin Endocrinol Metab. 1983 Oct;57(4):831–837. doi: 10.1210/jcem-57-4-831. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]