Significance
CsrA is a conserved global regulatory protein that binds to the 5′UTR of target transcripts to control RNA stability or occlude the Shine–Dalgarno sequence and inhibit translation. Here we show that the protein FliW of Bacillus subtilis noncompetitively antagonizes CsrA RNA-binding activity when FliW binds to CsrA at an allosteric site. Noncompetitive inhibition is likely a common mechanism of CsrA antagonism, as FliW is broadly distributed in bacteria. Furthermore, FliW regulation differs dramatically from the small RNA-based competitive sequestration found in γ-proteobacteria, and the two different mechanisms might allow for the potential of combinatorial CsrA regulation in some organisms. CsrA is a potent pleiotropic regulator of virulence transitions in pathogens, and noncompetitive antagonism improves the potential of CsrA as an antimicrobial drug target.
Keywords: motility, flagella, CsrA, FliW, Hag
Abstract
CsrA (carbon storage regulator A) is a widely distributed bacterial RNA binding protein that regulates translation initiation and mRNA stability of target transcripts. In γ-proteobacteria, CsrA activity is competitively antagonized by one or more small RNAs (sRNAs) containing multiple CsrA binding sites, but CsrA in bacteria outside the γ-proteobacteria is antagonized by a protein called FliW. Here we show that FliW of Bacillus subtilis does not bind to the same residues of CsrA required for RNA binding. Instead, CsrA mutants resistant to FliW antagonism (crw) altered residues of CsrA on an allosteric surface of previously unattributed function. Some crw mutants abolished CsrA–FliW binding, but others did not, suggesting that FliW and RNA interaction is not mutually exclusive. We conclude that FliW inhibits CsrA by a noncompetitive mechanism that differs dramatically from the well-established sRNA inhibitors. FliW is highly conserved with CsrA in bacteria, appears to be the ancestral form of CsrA regulation, and represents a widespread noncompetitive mechanism of CsrA control.
CsrA (carbon storage regulator A) is an RNA binding protein that regulates gene expression in bacteria (reviewed in ref. 1). At present, more than 670 putative CsrA homologs are distributed among 527 different bacterial species, and some species encode multiple paralogs in their genomes (2–6). CsrA binds RNA as an interdigitated homodimer with two active sites, in which each active site is a composite of residues contributed by both monomers (7–11). Each CsrA dimer typically binds to two sites containing critical GGA motifs in the 5′UTR of target transcripts, thereby occluding the Shine–Dalgarno sequence. Thus, bound CsrA represses translation by blocking ribosome binding (12–16). Translational regulation in bacteria is less well studied than transcriptional regulation, and CsrA is one of the few proteins known to regulate translation.
CsrA has been extensively studied in the Gram-negative γ-subfamily of proteobacteria. Mutation of CsrA in these organisms has dramatic and highly pleiotropic defects in carbon metabolism, quorum sensing, biofilm formation, and motility (17–20). CsrA-deficient bacterial pathogens are typically attenuated for virulence, which is likely a result of misregulation of target gene expression and the resulting inability to make critical physiological transitions during an infection (1, 21–23). To further regulate these transitions, small RNAs (sRNAs) containing as many as 20 CsrA binding sites competitively inhibit CsrA by sequestering it from its native mRNA targets (24–28). The primary sequence of CsrA is highly conserved in the γ-proteobacteria (Fig. 1A), and the sRNA antagonists are structurally and functionally similar (29). Species-specific differences in regulation appear to be a result of two-component systems that control expression of the sRNAs and RNases that control their stability (26, 27, 30–34). Little is known about the precise environmental signals that control sRNA expression, and therefore CsrA function (35).
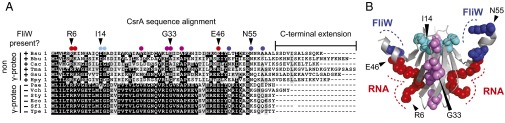
Fig. 1.
hag RNA and FliW interact with separate residues and surfaces of CsrA. (A) A multiple sequence alignment of CsrA primary sequences from γ-proteobacteria (“γ-proteo”) that do not encode FliW (−) and bacteria outside the γ-proteobacteria (“non γ-proteo”) that encode FliW (+). The following organisms were used to generate the multiple sequence alignment: B. subtilis 3610 (Bsu), Borrelia burgdorferi B31 (Bbu), Clostridium acetobutylicum ATCC824 (Cac), Thermotoga maritma MSB8 (Tma), Geobacter sulfurriducens PCA (Gsu), Helicobacter pylori J99 (Hpy), Pseudomonas aeruginosa PAO1 (Pae), Vibrio cholerae O1 El Tor (Vch), Salmonella typhimurium LT2 (Sty), E. coli MG1655 (Eco), Shigella flexneri 5 (Sfl), and Yersinia pestis KIM (Ype). Positions mutated in B. subtilis CsrA are indicated as colored circles above the multiple sequence alignment. Red circles indicate residues making up the RNA binding cleft, and purple circles indicate residues outside the RNA binding cleft that when mutated conferred a lof phenotype (38). Dark blue circles indicate poorly conserved residues with respect to γ-proteobacteria, and light blue circles indicate highly conserved residues that when mutated, conferred a CsrA resistant to FliW antagonism (crw) phenotype. (B) The side chain of each residue mutated by a lof allele (red or purple) or crw allele (dark blue or light blue) is shown and highlighted in the color corresponding to that used in panel 1A on the structure of the CsrA homolog RsmA from Pseudomonas aeruginosa (15). The RNA binding surface is indicated by a red dotted line. The location of residues R6, G33, and E46 required for CsrA function featured in the manuscript are indicated by carets. The predicted FliW binding surface is indicated by a blue dotted line. The location of residues I14 and N55 required for FliW antagonism featured in the manuscript are indicated by carets.
CsrA of the Gram-positive firmicute Bacillus subtilis differs from the paradigm established in the γ-proteobacteria in a number of ways. First, the B. subtilis CsrA primary sequence is divergent and contains a C-terminal extension (Fig. 1A). Second, B. subtilis CsrA seems to be highly specific, perhaps regulating a single RNA target: the hag transcript that encodes the flagellar filament protein, Hag (16). Third, hag inhibition by CsrA is indirectly enhanced by the Hag protein, creating an autoinhibitory loop that homeostatically restricts Hag levels in the cytoplasm but permits high levels of Hag translation concomitant with flagellar assembly (36–38). Finally, CsrA is directly antagonized by the FliW protein. Because Hag is an alternative pairing partner with FliW, this partner-switching mechanism monitors Hag levels and governs Hag homeostatic feedback. FliW appears to be the ancestral form of CsrA control, as FliW homologs are conserved in diverse bacteria that occupy deep branches of the bacterial tree. Furthermore, FliW was recently shown to be a CsrA antagonist in the distantly related epsilon (ε)-proteobacterium Campylobacter jejuni (39). Although FliW is known to antagonize CsrA by direct protein–protein interaction, the mechanism of antagonism and the residues of CsrA required for this interaction are not known.
Here we explore the mechanism by which FliW inhibits CsrA in B. subtilis. We show that FliW does not require the RNA binding residues of CsrA. Instead, residues required for FliW antagonism map to a surface of CsrA for which no function has been identified in the γ-proteobacteria. The location of some of the mutated residues that abolish FliW interaction identified a likely binding surface, and these residues are conserved in genomes that encode FliW. One particular CsrA mutant, however, prevented FliW antagonism, despite retaining the ability to bind FliW protein, and we provide evidence that the mutant protein likely binds both hag mRNA and FliW simultaneously. We conclude that FliW antagonizes CsrA by a noncompetitive mechanism much different from that reported for sRNA antagonism in the γ-proteobacteria. The noncompetitive mechanism of inhibition is consistent with the CsrA target, hag mRNA, being a highly abundant transcript, and is likely applicable to fliW-encoding firmicutes, spirochetes, thermotogales, and ε-proteobacteria.
Results
CsrA Residues Required for RNA Binding Are Not Required for Interaction with FliW.
The RNA-binding activity of B. subtilis CsrA is inhibited by FliW via protein–protein interaction; however, the mechanism of FliW-mediated inhibition is unknown. In vitro protein pulldown assays were conducted to identify amino acid residues of CsrA required for FliW binding. WT and mutant CsrA proteins were purified as GST fusions from Escherichia coli, and 5 µM of each fusion protein was loaded on a glutathione Sepharose (GSH) column. Next, various concentrations of WT FliW protein were mixed with the GST–CsrA bound beads, the beads were centrifuged, and the supernatant and pellet fractions were separately resolved by SDS/PAGE and stained with Coomassie blue. As previously reported (38), FliW was retained in the pellet fraction when FliW was mixed with beads loaded with WT CsrA at roughly equimolar concentrations. Further, FliW accumulated in the supernatant as concentrations increased in molar excess of bound CsrA (Fig. 2A).
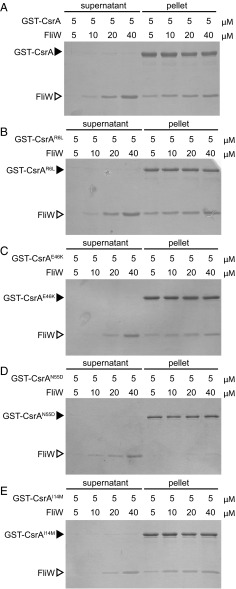
Fig. 2.
Differential binding of FliW to lof and crw alleles of CsrA. (A–E) Protein pull-down assay using the indicated amounts of GST–CsrA (A), GST-CsrAR6L (B), GST-CsrAE46K (C), GST-CsrAN55D (D), and GST-CsrAI14M (E) fusion proteins (black caret) loaded onto a glutathione-Sepharose column with the indicated amounts of FliW protein (open caret) added. Gels were stained with Coomassie Brilliant Blue. “Supernatant” indicates the proteins that failed to bind to the beads, and “pellet” indicates the proteins that remained bound to the beads following a series of washes.
One way in which FliW might inhibit the RNA binding activity of CsrA is by a competitive mechanism, similar to the way the CsrB and CsrC sRNAs inhibit CsrA in γ-proteobacteria. Specifically, FliW might interact with the RNA binding pocket of CsrA or otherwise interfere with residues required for RNA binding. Ten loss-of-function (lof) CsrA alleles were previously isolated that were defective in the ability to inhibit hag translation and suppressed the absence of FliW (Fig. 1A, red and purple dots) (37). Of the original 10 lof alleles, three (CsrAM1T, CsrAV25G, and CsrAL32P) were discarded because they failed to produce stable protein in vivo (Fig. S1). Of the remaining seven alleles, two lof alleles (CsrAR6W and CsrAA36T) were discarded in favor of an alternate missense mutation at the same position. The remaining five unique alleles (CsrAR6L, CsrAK7E, CsrAG33R, CsrAA36V, and CsrAE46K) that encoded stable protein in vivo were cloned as GST fusions, purified from E. coli, and loaded on GSH beads to determine whether they interacted with FliW protein in the pulldown assay. Each of the five lof CsrA proteins bound FliW in a manner indistinguishable from WT (Fig. 2 B and C and Fig. S2). We conclude that residues required for RNA binding, including the highly conserved residues R6 and E46 shown to interact with target mRNAs and sRNA-competitive inhibitors in E. coli, are not required for binding FliW (Fig. 1B) (9, 10). We infer that FliW inhibits CsrA by a mechanism that does not involve competition for the residues required for RNA binding.
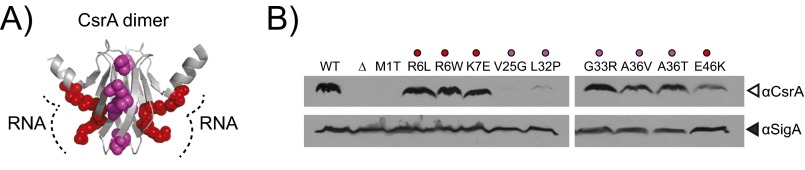
Fig. S1.
CsrAlof alleles in the RNA binding pocket are stable. (A) Each residue mutated in a CsrA lof allele, originally isolated as suppressors of fliW (sow), is highlighted on the structure of the homologous protein RsmA from Pseudomonas aeruginosa (15, 38). The RNA binding surface is indicated by a dotted line. (B) Western blot analysis of B. subtilis cell lysates of WT (3610), and 10 unique missense CsrAlof mutants (DS6529, DS6604, DS6608, DS6632, DS6633, DS6634, DS8579, DS8580, DS8584, DS8592) separately probed with anti-CsrA, and anti-SigA primary antibodies.
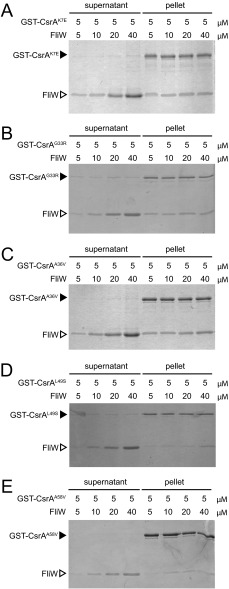
Fig. S2.
Protein–protein interaction between FliW and the CsrAlof or CsrAcrw proteins. (A–E) Protein pull-down assay using the indicated amounts of GST–CsrAlof (K7E, G33R, A36V) or GST–CsrAcrw fusion proteins (L49S, A58V) (black caret), loaded onto a glutathione-Sepharose column with the indicated amounts of FliW protein (open caret) added. Gels were stained with Coomassie Brilliant Blue. “Supernatant” indicates the proteins that failed to bind to the beads, and “pellet” indicates the proteins that remained bound to the beads after a series of washes.
FliW Binds to a Surface of CsrA Distinct from Its RNA Binding Pocket.
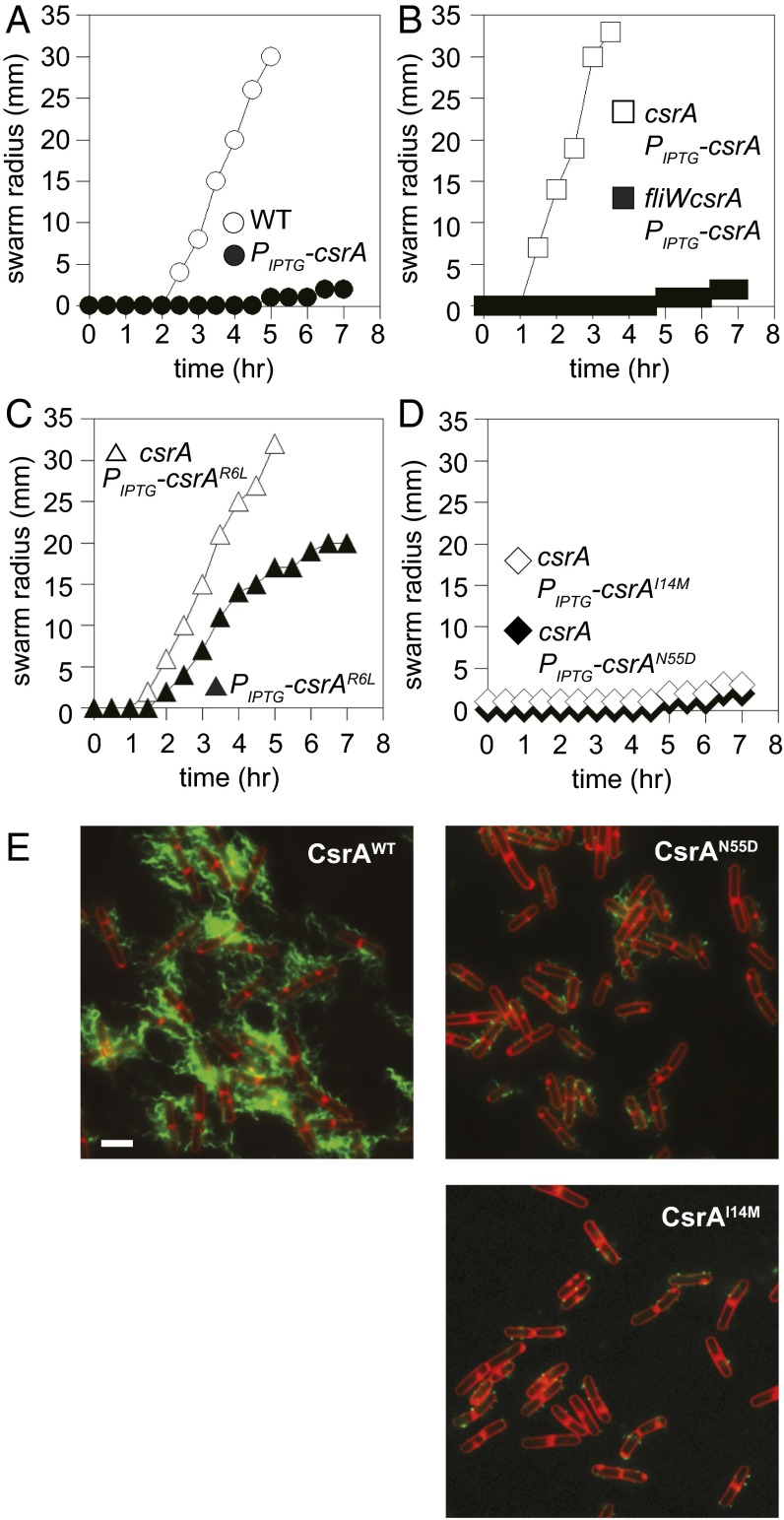
To identify CsrA residues required for inhibition by FliW, a forward genetic screen was devised on the basis of the following biological observations: when CsrA was artificially expressed from the isopropyl β-d-thiogalactopyranoside (IPTG)-inducible promoter Physpank and integrated at an ectopic locus as a merodiploid (amyE::Physpank-csrA), motility was inhibited in an IPTG-dependent manner (Fig. 3A). We hypothesized that in the merodiploid, the combined pool of IPTG-expressed CsrA and natively expressed CsrA was sufficient to titrate the pool of FliW, bind hag mRNA, and inhibit its translation. Consistent with this interpretation, when the IPTG-inducible CsrA construct was expressed in a strain in which the native copy of the csrA gene had been deleted, motility was not inhibited in the presence of IPTG, which is likely the result of a failure to achieve stoichiometric excess of CsrA to allow both FliW antagonism and repression of hag translation (Fig. 3B). Consistent with FliW antagonism, motility inhibition was restored to the IPTG-inducible CsrA construct in the presence of IPTG when both the csrA and fliW genes were deleted simultaneously (Fig. 3B). As final evidence for titration of FliW, ectopic expression of a CsrAR6L lof RNA binding mutant allele modestly inhibited motility in the merodiploid, but not when the native copy of csrA was deleted (Fig. 3C). We infer that the CsrAR6L lof protein, despite being unable to bind hag mRNA and inhibit its translation, competed with the WT protein for FliW binding and liberated a subpopulation of WT CsrA that mediated motility inhibition. We conclude that ectopically expressed CsrA was antagonized by FliW when the native copy of csrA was deleted.
Fig. 3.
CsrAcrw alleles inhibit motility and filament assembly. (A) Quantitative swarm expansion assays for strains: WT (○, 3610), and Physpank-csrA in the presence of 1 mM IPTG (●, DS4940). (B) Quantitative swarm expansion assays for strains: ΔcsrA Physpank-csrA in the presence of 1 mM IPTG (□, DK1522) and ΔfliWcsrA Physpank-csrA in the presence of 1 mM IPTG (■, DK1469). (C) Quantitative swarm expansion assays for strains: Physpank-csrAR6L in the presence of 1 mM IPTG (▲, DK1616) and ΔcsrA Physpank-csrAR6L in the presence of 1 mM IPTG (△, DK3713). Note that the allele presented in C is a lof for RNA binding, but not FliW binding, and thus ectopic expression of the allele partially inhibits motility by titrating FliW from WT protein expressed from the native locus. (D) Quantitative swarm expansion assays for strains: ΔcsrA Physpank-csrAI14M in the presence of 1 mM IPTG (♢, DK3004) and ΔcsrA Physpank-csrAN55D in the presence of 1 mM IPTG (♦, DK3005). Each point is the average of three replicas. (E) Fluorescence micrograph overlays of WT (DS9502), ΔcsrA Physpank-csrAN55D (DK2442), and ΔcsrA Physpank-csrAI14M (DK2441) in the presence of 1 mM IPTG, membrane stained with FM4-64 (false colored red) and flagella stained (false colored green). (Scale bar, 2 µm.)
To identify alleles of CsrA resistant to FliW antagonism (crw alleles), the ectopic Physpank-csrA construct was mutated by error-prone PCR and introduced into a strain in which the native copy of the csrA gene was deleted. Next, 1,600 transformants were obtained and patched onto swarm agar in the presence of IPTG. Most of the transformants were motile, similar to the WT control, and were discarded. Twenty-nine transformants representing five unique crw mutant alleles were isolated (CsrAI14M, CsrAG15S, CsrAL49S, CsrAN55D, and CsrAA58V), which, unlike the WT or lof csrA alleles, inhibited swarming motility in an IPTG-dependent manner (Fig. 3D and Fig. S3A). When plotted on a 3D structure of CsrA from Pseudomonas aeruginosa (15), the analogous residues mutated in the crw alleles were all found on a surface of the CsrA protein that has heretofore not been ascribed a function in Gram-negative bacteria (Fig. 1B). Furthermore, each of the CsrA crw mutant alleles, but not the lof alleles, inhibited assembly of the flagellar filament in the presence of IPTG, resembling filament assembly inhibition observed in the absence of FliW (Fig. 3E and Fig. S3 B–E). We infer that the crw alleles rendered CsrA resistant to FliW antagonism but maintained their ability to bind to and inhibit translation of hag mRNA.
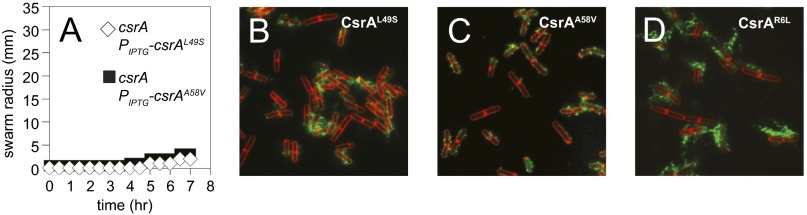
Fig. S3.
Motility and flagellar filament assembly phenotypes of CsrAcrw alleles. (A) Quantitative swarm expansion assays for strains: csrA Physpank-csrAL49S in the presence of 1 mM IPTG (♢, DK3006) and csrA Physpank-csrAA58V in the presence of 1 mM IPTG (■, DK3007). (B–D) Fluorescence micrograph overlays of ΔcsrA Physpank-csrAL49S (DK2443; B), ΔcsrA Physpank-csrAA58V (DK2444; C), and Physpank-csrAR6L (DK1616; D), in the presence of 1 mM IPTG, membrane stained with FM4-64 (false colored red) and flagella stained (false colored green). (Magnification: 1,000×.)
One way in which the CsrA crw alleles might be resistant to FliW antagonism is if they were defective in the ability to bind to FliW protein. To test interaction with FliW, each of the CsrA crw alleles was cloned and expressed as a GST-fusion protein in E. coli (except for CsrAG15S, which failed to yield stable protein in vitro) and loaded on GSH beads in the FliW pulldown assay. When GST–CsrAL49S, GST–CsrAN55D, and GST–CsrAA58V were loaded on the beads, FliW was poorly retained in the pellet fraction (Fig. 2D and Fig. S2). We conclude that CsrA residues L49, N55, and A58 are important for interaction with FliW and, when mutated, reduce FliW binding. In contrast, when GST–CsrAI14M was loaded on the beads, FliW was retained in the pellet fraction at equimolar concentrations and only appeared in the supernatant when in molar excess (Fig. 2E). Thus, it appeared that CsrAI14M was functionally different from the other crw alleles, as it could still bind FliW in a manner comparable to the WT protein in vitro.
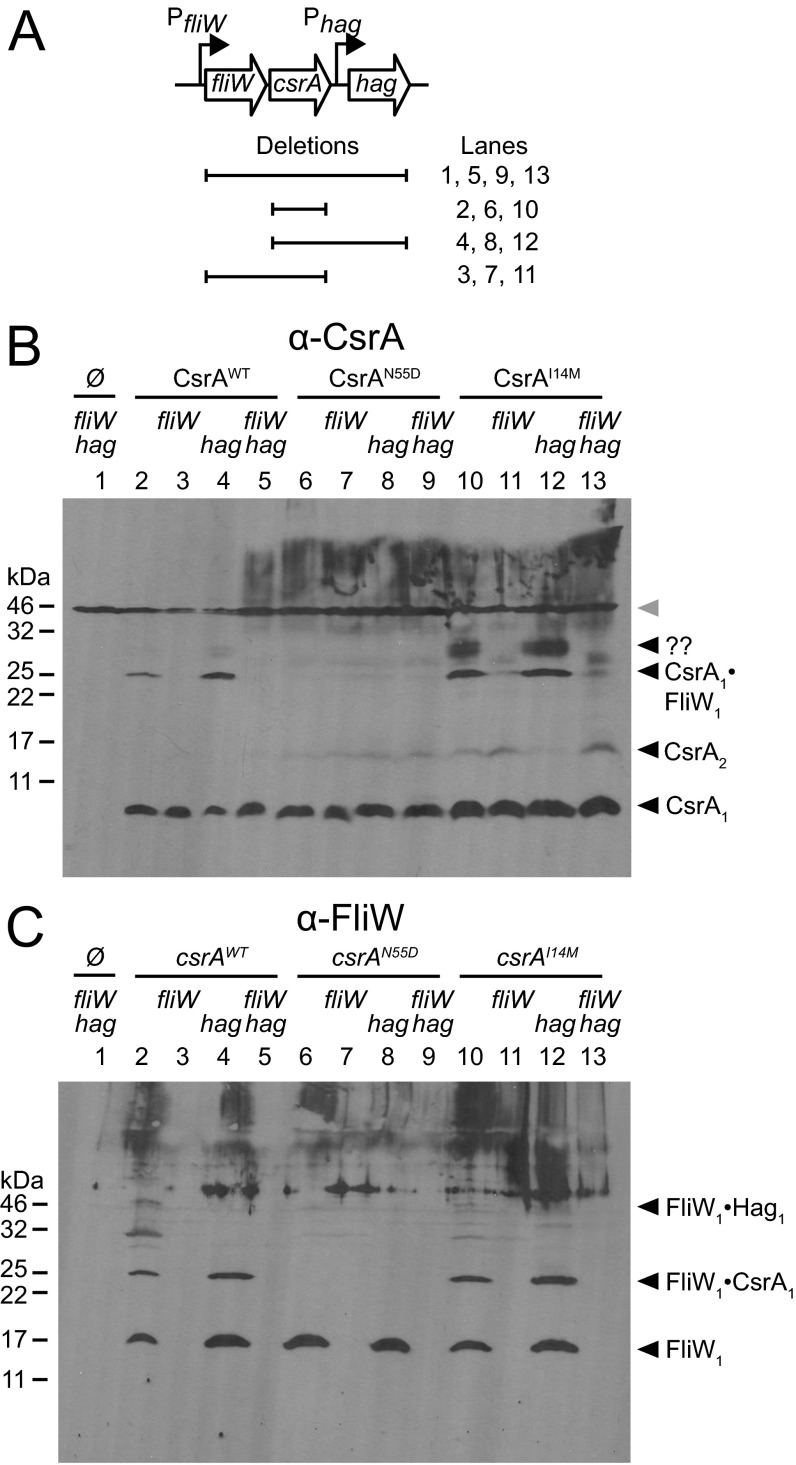
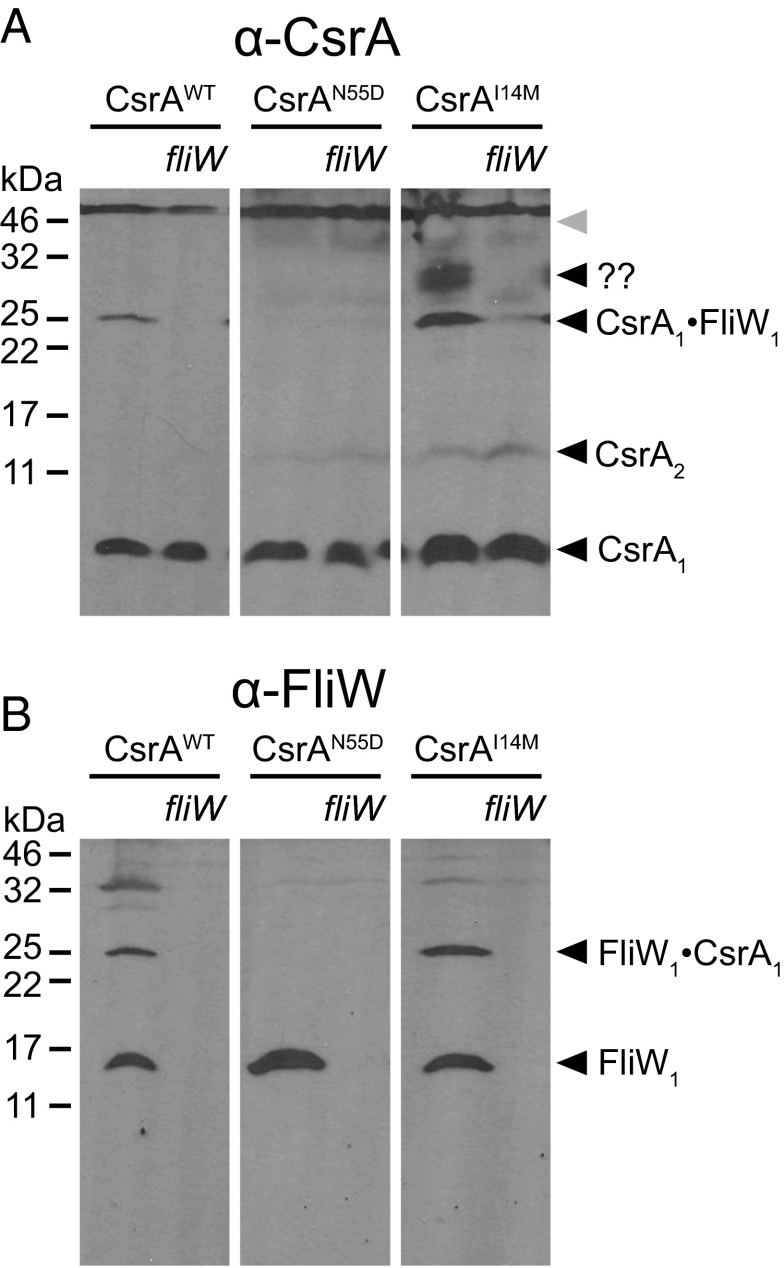
To determine whether CsrAI14M could bind FliW in vivo, Western blots were conducted on lysates of cells treated with the chemical cross-linker formaldehyde and probed with antibodies raised against either CsrA or FliW (Fig. S4). Despite CsrA being a dimer in solution, WT CsrA resolved primarily as a monomer (8 kDa), likely because of the minimal tethering distance of formaldehyde as a cross-linker (Fig. 4A, CsrA1). FliW similarly resolved as a monomer (16 kDa), but whether FliW exists primarily as a monomer or dimer in solution is unknown (Fig. 4B, FliW1). A band that reacted with both anti-CsrA and anti-FliW antibodies resolved at ∼24 kDa, and we interpret this band to be a 1:1 complex of CsrA and FliW (Fig. 4 A and B, CsrA1·FliW1). The 24-kDa band was dependent on FliW (Fig. 4B) and was abolished in the presence of CsrAN55D, consistent with the inability of CsrAN55D to bind FliW in vitro (Fig. 2D). Finally, the 24-kDa band was preserved in the presence of CsrAI14M (Fig. 4A), consistent with the ability of CsrAI14M to bind FliW in vitro (Fig. 2E). We conclude that residue I14 is required for antagonism by, but not interaction with, FliW and we infer that the CsrAI14M mutant allele may permit simultaneous binding of FliW and the hag mRNA 5′UTR.
Fig. S4.
CsrAI14M mutant is resistant to inhibition by FliW but still binds FliW in vivo. (A) Genetic neighborhood containing the fliW, csrA, and hag genes. Large open arrows represent ORFs (lengths not to scale). Bent arrows indicate promoters. Horizontal lines indicate the boundaries of the in-frame deletion mutations corresponding to the lane numbers of Western blots shown in B and C. (B and C) Western blots of whole-cell lysates grown in the presence of 1 mM IPTG and crosslinked with 0.3% formaldehyde were developed using primary antibodies against CsrA (B) and FliW (C), respectively. All lanes contain a deletion of the native csrA gene. Some lanes are also deleted for either fliW and/or hag, as indicated. CsrA proteins indicated above the line were expressed in trans or no CsrA protein was provided as indicated by the null symbol (Ø). The following strains were used to generate lysates for both panels: lane 1, ΔfliWcsrAhag (DK1483); lane 2, ΔcsrA Physpank-csrA (DK1522); lane 3, ΔcsrAfliW Physpank-csrA (DK1469); lane 4, ΔcsrAhag Physpank-csrA (DK1503); lane 5, ΔfliWcsrAhag Physpank-csrA (DK3608); lane 6, ΔcsrA Physpank-csrAN55D (DK3005); lane 7, ΔfliWcsrA Physpank-csrAN55D (DK3011); lane 8, ΔcsrAhag Physpank-csrAN55D (DK3628); lane 9, ΔfliWcsrAhag Physpank-csrAN55D (DK3614); lane 10, ΔcsrA Physpank-csrAI14M (DK3004); lane 11, ΔfliWcsrA Physpank-csrAI14M (DK3010); lane 12, ΔcsrAhag Physpank-csrAI14M (DK3627); and lane 13, ΔfliWcsrAhag Physpank-csrAI14M (DK3613).
Fig. 4.
CsrAI14M mutant is resistant to inhibition by FliW but still binds FliW in vivo. Western blots of whole-cell lysates grown in the presence of 1 mM IPTG and crosslinked with 0.3% formaldehyde were developed using primary antibodies against CsrA (A) and FliW (B), respectively. Samples in all lanes were prepared from strains containing a deletion of the native csrA gene plus deletion of fliW, as indicated. CsrA proteins of the allele indicated above the line were expressed in trans. The following strains were used to generate lysates for both panels: ΔcsrA Physpank-csrA (DK1522), ΔcsrAfliW Physpank-csrA (DK1469), ΔcsrA Physpank-csrAN55D (DK3005), ΔfliWcsrA Physpank-csrAN55D (DK3011), ΔcsrA Physpank-csrAI14M (DK3004), and ΔfliWcsrA Physpank-csrAI14M (DK3010). All panels cropped from two larger gels shown in Fig. S4.
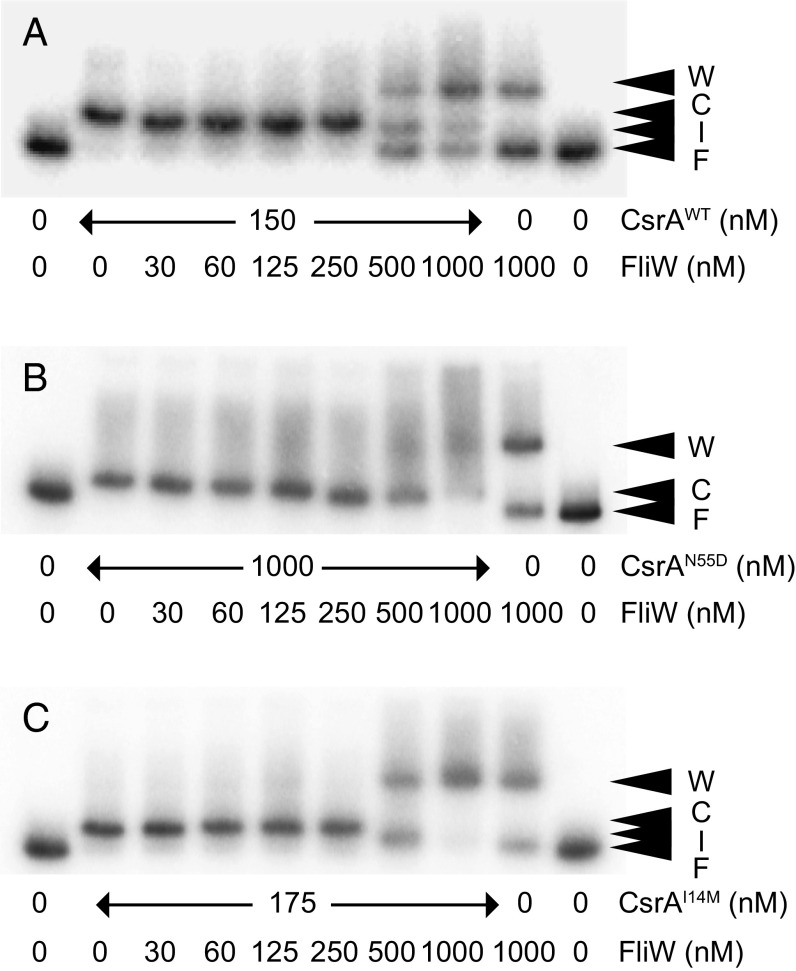
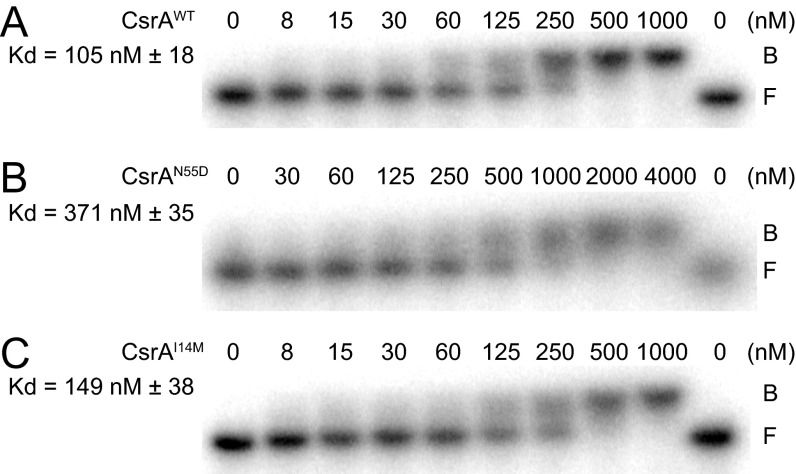
To determine the consequence of the CsrAcrw proteins on hag mRNA 5′UTR binding, an RNA electrophoretic mobility shift assay (EMSA) was conducted. The addition of 150 nM WT CsrA was sufficient to retard migration of radiolabeled hag 5′UTR RNA (Fig. 5A, caret C, and Fig. S4). Addition of FliW antagonized CsrA, resulting in a band corresponding to unbound free RNA (Fig. 5A, caret F) and a band that might be a binding intermediate located between free and bound RNA (Fig. 5A, caret I). At high concentrations, FliW caused an RNA shift even in the absence of CsrA (Fig. 5A, caret W), as previously reported (38). Although a fourfold greater concentration of CsrAN55D was required to retard RNA mobility (Fig. S5), it was fully resistant to FliW, as no amount of FliW tested was sufficient to antagonize CsrA and restore unbound RNA (Fig. 5B). The CsrAI14M protein had a similar affinity for hag mRNA as WT CsrA (Fig. S5), and similar to CsrAN55D, no amount of FliW tested was sufficient to restore unbound RNA (Fig. 5C). At the highest concentration of FliW added to CsrAI14M, both the high-mobility signals representing the CsrA–RNA complex and free RNA were dramatically reduced. We note that at the highest FliW concentration, a broader, more intense signal at a size similar to the band shift caused by FliW alone was observed, perhaps indicative of an RNA–CsrAI14M–FliW supercomplex (Fig. 5C). We conclude that these two crw alleles of CsrA were resistant to FliW inhibition by different mechanisms: CsrAN55D does not bind FliW, and we speculate that CsrAI14M may be able to bind to FliW and RNA simultaneously. We further conclude that FliW antagonizes CsrA by noncompetitive binding to an allosteric site defined by L49, N55, and A58 and requires residue I14 for inhibition.
Fig. 5.
CsrAcrw proteins bind to RNA in vitro. (A–C) RNA EMSA, using WT hag 5′UTR RNA and the indicated amounts of CsrA–His6 (A), CsrAN55D–His6 (B), or CsrAI14M–His6 (C) and FliW protein. “F” indicates unbound RNA, “C” indicates CsrA–RNA complex, and “W” indicates FliW–RNA complex. ”I” indicates a band with slightly increased mobility that might suggest a release intermediate.
Fig. S5.
RNA binding affinity of CsrAcrw proteins. RNA EMSA using WT hag 5′UTR RNA (A–C) and the indicated amounts of CsrA–His6 (A), CsrAN55D–His6 (B), and CsrAI14M–His6 (C). F indicates free probe, and B indicates probe bound by CsrA. The apparent Kd values of CsrA–hag RNA interaction are indicated.
Discussion
CsrA (also known as RsmA) is a conserved RNA binding protein that regulates translation and/or mRNA stability by binding to the 5′UTR of target transcripts. Studied almost exclusively in the context of the Gram-negative γ-proteobacteria, CsrA activity is inhibited by sRNAs encoding iterations of the CsrA binding site (CsrB/CsrC, RsmY/RsmZ), which competitively sequester CsrA from its native mRNA targets (1). Recently, however, CsrA-hag mRNA interaction was found to be antagonized by the protein FliW in the firmicute B. subtilis, but the mechanism of FliW-mediated antagonism was unknown (38). Here we randomly mutagenized CsrA to obtain alleles resistant to FliW antagonism and found residues that occupy an allosteric surface of CsrA separate from its mRNA binding pocket. In addition, CsrA lof mutants defective for RNA binding retained the ability to bind FliW. Thus, unlike the sRNAs of γ-proteobacteria, FliW does not compete with RNA for CsrA binding. We conclude that when FliW binds to the CsrA allosteric site, it either causes a conformational change or otherwise remotely impairs CsrA’s ability to bind RNA.
Some of the alleles that rendered CsrA resistant to FliW antagonism mutated residues near the C terminus of CsrA and resulted in a failure to bind FliW protein. The failure to bind FliW not only accounts for the in vivo phenotypes of swarming motility inhibition and flagellar assembly defects but also genetically mapped the FliW binding surface of CsrA. Organisms that encode FliW appear to have CsrA proteins with C-terminal extensions of the primary sequence (Fig. 1A). Although most of the extension is poorly conserved, one highly conserved residue (N55) is essential for binding FliW and may be a general determinant of the CsrA–FliW interaction. At least two other poorly conserved residues (L49 and A58) were also required for FliW binding, perhaps conferring species-specific contacts or cognate specificity if an organism encodes multiple FliW and CsrA paralogs. Thus, at least one function of the extended C terminus of CsrA found in bacteria outside the γ-proteobacteria is to serve as a binding site for FliW.
One allele of CsrA, CsrAI14M, exhibited the same lack of FliW antagonism in vivo as CsrAN55D, but retained the ability to bind FliW, as determined by in vitro pulldown assays with purified proteins and by in vivo cross-linking assays in the context of the cytoplasm. Thus, we infer that I14M contorts the CsrA conformation such that simultaneous binding of RNA and FliW is possible, perhaps generating a hag RNA–CsrA–FliW supercomplex that may have been observed in RNA EMSA experiments (Fig. 5C). As further evidence of altered CsrA conformation in the cross-linked Western blot assays, CsrAI14M resolved as a doublet of intense bands at 28–30 kDa that were FliW-dependent but did not react with anti-FliW antibodies (Fig. 4). Thus, FliW is required for the formation of a special CsrA complex that was also weakly detected in the WT CsrA strain when the hag 5′UTR and coding region were deleted (Fig. S4). Perhaps the I14M mutation stabilizes an otherwise transient conformation of CsrA that serves as a precursor to FliW binding. Finally, we note that the I14 residue occupies a surface proximal to the FliW binding site but is highly conserved in CsrA proteins, regardless of whether FliW is also encoded in the genome, and thus may illuminate fundamental aspects of CsrA activity (Fig. 1A).
CsrA is highly conserved, but the targets it controls and the mechanism by which it is regulated are diverse. In γ-proteobacteria, CsrA governs highly pleiotropic phenotypes by targeting a wide array of transcripts and is regulated by sRNAs that function as competitive inhibitors. The sRNAs themselves are regulated by a variety of inputs including, but not limited to, two-component systems, integration host factor, DeaD box helicases, the stringent response [(p)ppGpp and DksA], and RNA turnover, but the environmental inputs that control CsrA activity are poorly understood (20, 30, 33, 34, 40, 41). In contrast, B. subtilis CsrA is a specific inhibitor of hag translation. Furthermore, CsrA activity is indirectly inhibited by Hag protein via the CsrA–FliW–Hag partner switching mechanism to create a homeostatic autoinhibitory loop governing flagellar filament assembly (16, 37, 38). FliW is the first known protein antagonist of CsrA, which we here show binds to CsrA at an allosteric site and noncompetitively inhibits RNA binding. We speculate that the noncompetitive mechanism of FliW antagonism may be necessary because it would be difficult for sRNA, or perhaps other mRNA targets should they exist, to regulate CsrA by competing with the highly abundant hag transcript (39, 42–44).
In sum, FliW inhibits CsrA by a noncompetitive mechanism and we infer that FliW regulation may be ancestral to, and more common than, the sRNA-mediated competitive inhibition of CsrA in bacteria. Phylogenetic analysis indicates that FliW is coinherited with, and often encoded adjacent to, CsrA in widely diverse bacteria (34, 38, 39). Recent work in the distantly related C. jejuni is consistent with the B. subtilis model, in which FliW antagonizes CsrA and CsrA inhibits flagellin translation (39). Thus, the noncompetitive mechanism of inhibition that governs the CsrA–FliW–flagellin module is likely to be directly applicable to all FliW-encoding organisms, including members of the firmicutes, the spirochetes, and the deep branches of the proteobacteria (34, 39, 45–48). In contrast, competitive inhibition of CsrA by sRNA has been extensively studied, but only in bacteria belonging to the family of γ-proteobacteria (29). The phylogenetic distribution of sRNA competitors is poorly understood because of difficulties in predicting sRNA presence, expression, and function, but the conservation of the BarA–UvrY two component system that regulates csrB and csrC expression is largely restricted to the γ-proteobacteria, suggesting the sRNA mechanism of CsrA antagonism is narrowly distributed (34, 38, 49). Finally we note that, whether an organism uses sRNAs or FliW for regulation, CsrA regulates virulence factors in each pathogen in which it has been studied (1, 45–48). We suggest that CsrA is a candidate therapeutic target and that the precedent for noncompetitive inhibition can be exploited to isolate noncompetitive inhibitors, which are typically effective at lower doses than competitive counterparts.
Experimental Procedures
All strains used in this study are listed in Table S1. All plasmids used in this study are listed in Table S2. All primers used in this study are listed in Table S3.
Table S1.
Strains
| Strain | Genotype |
| 3610 | WT |
| DS4940 | amyE::Physpank-csrA spec (7) |
| DS6188 | ΔcsrA (7) |
| DS6529 | ΔfliW sow2 (CsrAR6L) (7) |
| DS6604 | ΔfliW sow4 (CsrAA36V) (7) |
| DS6608 | ΔfliW sow8 (CsrAA36T) (7) |
| DS6632 | ΔfliW sow10 (CsrAL32P) (7) |
| DS6633 | ΔfliW sow11 (CsrAG33R) (7) |
| DS6634 | ΔfliW sow12 (CsrAK7E) (7) |
| DS8579 | ΔfliW sow15 (CsrAE46K) (7) |
| DS8580 | ΔfliW sow16 (CsrAM1T) (7) |
| DS8584 | ΔfliW sow20 (CsrAV25G) (7) |
| DS8592 | ΔfliW sow28 (CsrAR6W) (7) |
| DS9540 | hagT209C |
| DK1469 | ΔfliWcsrA amyE::Physpank-csrA spec |
| DK1483 | ΔfliWcsrAhag |
| DK1503 | ΔcsrAhag amyE::Physpank-csrA spec |
| DK1522 | ΔcsrA amyE::Physpank-csrA spec |
| DK1616 | hagT209C amyE::Physpank-csrAR6L spec |
| DK1650 | comIQ12L amyE::Physpank-csrAE46K spec |
| DK2441 | ΔcsrA amyE::Physpank-csrAI14M spec lacA::Phag-hagT209C mls |
| DK2442 | ΔcsrA amyE::Physpank-csrAN55D spec lacA::Phag-hagT209C mls |
| DK2443 | ΔcsrA amyE::Physpank-csrAL49S spec lacA::Phag-hagT209C mls |
| DK2444 | ΔcsrA amyE::Physpank-csrAA58V spec lacA::Phag-hagT209C mls |
| DK3004 | ΔcsrA amyE::Physpank-csrAI14M spec |
| DK3005 | ΔcsrA amyE::Physpank-csrAN55D spec |
| DK3006 | ΔcsrA amyE::Physpank-csrAL49S spec |
| DK3007 | ΔcsrA amyE::Physpank-csrAA58V spec |
| DK3008 | ΔcsrA amyE::Physpank-csrAG15S spec |
| DK3010 | ΔfliWcsrA amyE::Physpank-csrAI14M spec |
| DK3011 | ΔfliWcsrA amyE::Physpank-csrAN55D spec |
| DK3608 | ΔfliWcsrAhag amyE::Physpank-csrA spec |
| DK3613 | ΔfliWcsrAhag amyE::Physpank-csrAI14M spec |
| DK3614 | ΔfliWcsrAhag amyE::Physpank-csrAN55D spec |
| DK3627 | ΔcsrAhag amyE::Physpank-csrAI14M spec |
| DK3628 | ΔcsrAhag amyE::Physpank-csrAN55D spec |
Table S2.
Plasmids
| Plasmid | Genotype |
| pCSB9 | PT7-CsrA-His6 amp (8) |
| pET21a | PT7-His6 amp (Novagen) |
| pGEX-2TK | PT7-GST amp (GE Healthcare) |
| pRO11 | PT7-CsrAI14M-His6 amp |
| pRO12 | PT7-CsrAN55D-His6 amp |
| pSM6 | PT7-GST-CsrA amp |
| pSM12 | PT7-His6-SUMO-FliW amp |
| pSM96 | PT7-GST-CsrAR6L amp |
| pSM97 | PT7-GST-CsrAK7E amp |
| pSM98 | PT7-GST-CsrAG33R amp |
| pSM99 | PT7-GST-CsrAA36V amp |
| pSM100 | PT7-GST-CsrAE46K amp |
| pSM104 | PT7-GST-CsrAI14M amp |
| pSM105 | PT7-GST-CsrAN55D amp |
| pSM106 | PT7-GST-CsrAL49S amp |
| pSM107 | PT7-GST-CsrAA58V amp |
| pSM108 | PT7-GST-CsrAG15S amp |
Table S3.
Primers
| Primer | Sequence |
| 688 | TATGCAGCAATGGCAAGAACGTTG |
| 848 | CTCCTGGATCCTGAGGAATGATTAGGAGATAGAAATTT |
| 1565 | CACGTTGAAGGATCGCATGAG |
| 2093 | AGGAGGAATTCGATTATGCATAGTGTTCAAGGAAAAAT |
| 2140 | AGGAGGGATCCATGCTAGTTTTATCGCGGAAAATAAAC |
| 2141 | CTCCTGAATTCTCACTTTTTTTGTGAGGATAATGCGG |
| 3177 | CTAATTCAAGGCGTGTCTCAC |
| 3180 | GCGGTATTCCGTATGTCAAG |
| 4554 | GGAGGAGAATCATATGCTAGTTTTATCG |
| 4555 | AAAAAAATCCTCGAGCTTTTTTTGTGAGGA |
Generation of csrA Mutant Pool.
To generate a pool of csrA mutants, primer pair 3177/3180 was used to amplify the csrA reading frame, using DS4940 chromosomal DNA as a template and Expand polymerase with Expand Buffer 1 (Roche). The mutant PCR library was directly transformed into the cured ancestral strain DS2569 by natural competence, and phage lysates were generated from the resulting transformants. The phage lysates were used to transduce strains of B. subtilis for screening of crw alleles of csrA.
In Vivo Cross-Linking.
B. subtilis was grown to midexponential phase at 37 °C in LB broth. Ten milliliters of culture was harvested by centrifugation and resuspended in 10 mL of pH 7.4 PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4). Formaldehyde (0.3% final concentration) was added to samples and rocked at room temperature for 1 h. Next, 0.75 mL 2 M glycine was added to samples and rocked at room temperature for 10 min to quench the cross-linker. Samples were centrifuged and resuspended to 50 OD600 in lysis buffer (20 mM Tris⋅HCl at pH 7.0, 10 mM EDTA, 1 mg⋅mL−1 lysozyme, 10 μg⋅mL−1 DNase I, and 1 mM PMSF] and incubated at 37 °C for 1 h. 6× SDS loading dye was added and incubated for 15 min at room temperature. Samples were separated by 15% (vol/vol) acrylamide SDS/PAGE. Blots were probed with either α-CsrA (1:2,500) or α-FliW (1:5,000) antibodies.
SI Experimental Procedures
Strains and Growth Conditions.
Bacillus subtilis strains were grown in lysogeny broth (LB) (10 g tryptone, 5 g yeast extract, 5 g NaCl/L) broth or on LB plates fortified with 1.5% (wt/vol) Bacto agar at 37 °C. When appropriate, antibiotics were included at the following concentrations: 10 µg/mL tetracycline, 100 µg/mL spectinomycin, 5 µg/mL chloramphenicol, 5 µg/mL kanamycin, and 1 µg/mL erythromycin plus 25 µg/mL lincomycin (mls). IPTG (Sigma) was added to the medium at the indicated concentration when appropriate.
Strain Construction.
All constructs were first introduced into the cured ancestral strain DS2569 by natural competence and then transferred to the 3610 background, using SPP1-mediated generalized phage transduction (50). All strains used in this study are listed in Table S1. All plasmids used in this study are listed in Table S2. All primers used in this study are listed in Table S3.
GST–CsrAcrw fusion protein expression vector.
To generate the translational fusion of CsrAcrw to the GST tag, a fragment containing csrAcrw was amplified using chromosomal DNA from the corresponding B. subtilis strain isolated from CsrA resistant to FliW (crw) screen as a template and the primer pair 2140/2141 and was digested with EcoRI and BamHI. The fragment was ligated into the EcoRI and BamHI sites of pGEX-2TK containing an ampicillin resistance cassette.
CsrAcrw–6His fusion protein expression vector.
To express and purify the CsrAcrw-6His construct, a fragment containing csrAcrw was amplified using the primer pair 4554/4555 and the same chromosomal DNA used to generate the GST tag translational fusions. The fragment was digested with XhoI and NdeI and ligated into the XhoI and NdeI sites of pET21a containing an ampicillin resistance cassette.
Swarm Expansion Assay.
Cells were grown to midexponential phase at 37 °C in LB broth and resuspended to 10 OD600 in pH 8.0 PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4) containing 0.5% India ink (Higgins). Freshly prepared LB containing 0.7% Bacto agar (25 mL/plate) was dried for 10 min in a laminar flow hood, centrally inoculated with 10 µL of the cell suspension, dried for another 10 min, and incubated at 37 °C. The India ink demarks the origin of the colony, and the swarm radius was measured relative to the origin. For consistency, an axis was drawn on the back of the plate, and swarm radii measurements were taken along this transect. For experiments including IPTG, cells were propagated in broth in the presence of IPTG, and IPTG was included in the swarm agar plates.
Microscopy.
Fluorescence microscopy was performed with a Nikon 80i microscope with a phase contrast objective Nikon Plan Apo 100× and an Excite 120 metal halide lamp. FM4-64 was visualized with a C-FL HYQ Texas Red Filter Cube (excitation filter, 532–587 nm; barrier filter, >590 nm).
For fluorescent microscopy of flagella, 1 mL of broth culture was harvested at 0.5–1.0 OD600, resuspended in 50 µL PBS buffer containing 5 µg/mL Alexa Fluor 488 C5 maleimide (Molecular Probes), and incubated for 3 min at room temperature (51). Cells were then washed twice with 500 µL PBS buffer. When appropriate, membranes were stained by resuspension in 50 µL PBS buffer containing 5 µg/mL FM4-64 (Molecular Probes) and incubated for 5 min at room temperature. Four microliters of suspension were placed on a microscope slide and immobilized with a poly-l-lysine–treated coverslip.
Sequencing csrAlof Alleles.
The region around fliW was amplified using the primer pair 2093/848 and sequenced with primer 1565.
Sequencing csrAcrw Alleles.
The csrA open-reading frame was amplified using the amyE construct specific primer pair 3177/3180 and sequenced with primers 688 and 2141.
FliW Protein Purification.
The FliW protein expression vector pSM12 was transformed into Rosetta gami Escherichia coli, grown to ∼0.7 OD600 in 500 mL of terrific broth, induced with 1 mM IPTG and grown overnight at 16 °C. Cells were pelleted and resuspended in lysis buffer (50 mM Na2HPO4, 300 mM NaCl, 10 mM imidazole), treated with lysozyme and lysed by sonication. Lysed cells were centrifuged at 14,000 × g for 30 min. Cleared supernatant was combined with Ni-NTA resin (Novagen) and incubated for 1 h at 4 °C. The bead/lysate mixture was poured onto a 1-cm separation column (Bio-Rad), the resin was allowed to pack, and was washed with Wash Buffer (50 mM Na2HPO4, 300 mM NaCl, 30 mM imidazole). His-SUMO-FliW bound to the resin was then eluted using a stepwise elution of Wash Buffer with 50–500 mM imidazole. Eluted proteins were separated by SDS/PAGE and Coomassie stained to verify purification of the His-SUMO-FliW fusion. Purified His-SUMO-FliW was combined with ubiquitin ligase (protease) and cleavage buffer and incubated overnight at 4 °C to cleave the SUMO tag from the FliW protein. The cleavage reaction was combined with Ni-NTA beads, incubated for 2 h at 4 °C and centrifuged to pellet the resin. Supernatant was removed and dialized in 50 mM Tris⋅HCl at pH 8.0, 150 mM NaCl, 10% (vol/vol) glycerol, 1 mM DTT and stored at −20 °C. Removal of the SUMO tag was verified by SDS/PAGE and Coomassie staining.
GST–CsrAWT/crw Protein Purification.
The GST–CsrA protein expression vector pSM6 was transformed into Rosetta gami E. coli, grown to ∼0.7 OD600 in 500 mL of LB broth, induced with 1 mM IPTG and grown for 3 h at 37 °C. Cells were pelleted and resuspended in lysis buffer (25 mM Tris⋅HCl at pH 8.0, 1 mM DTT, 1 mM EDTA, 0.1% Triton X-100, 150 mM NaCl, 250 µg/mL lysozyme, 0.5 mM PMSF, 5 µg/mL leupeptin), and frozen overnight at −80 °C. The frozen cell pellet was thawed and lysed by sonication. Lysed cells were centrifuged at 18,000 × g for 30 min at 4 °C. Cleared supernatant was combined with glutathione-Sepharose (GE Healthcare) and incubated for 3 h at 4 °C. The bead/lysate mixture was poured onto a 1-cm separation column (Bio-Rad), the resin was allowed to pack and was washed with wash buffer (25 mM Tris⋅HCl at pH 8.0, 1 mM DTT, 1 mM EDTA, 0.1% Nonidet P-40, 250 mM NaCl, 10% glycerol, 0.5 mM PMSF, 5 µg/mL leupeptin). GST–CsrA bound to the resin was then eluted using GST-elution buffer [25 mM Tris⋅HCl at pH 8.5, 20 mM Glutathione, 1 mM DTT, 1 mM EDTA, 250 mM NaCl, 10% (vol/vol) glycerol, 0.5 mM PMSF, 5 µg/mL leupeptin]. Eluted proteins were separated by SDS/PAGE and Coomassie stained to verify purification of the GST–CsrA fusion. GST–CsrA was dialyzed into 25 mM Tris⋅HCl at pH 8.0, 1 mM DTT, 1 mM EDTA, 250 mM NaCl, 10% (vol/vol) glycerol, 20% (wt/vol) sucrose, 0.5 mM PMSF, 5 µg/mL leupeptin.
CsrAWT/crw–6His Protein Purification.
The CsrAwt–6His expression vector pCSB9 (16) was transformed into BL21 E. coli, and CsrAI14M/N55D-6His expression vectors pRO11/pRO12 were transformed into Rosetta gami E. coli, grown to ∼0.7 OD600 in 1 L terrific broth at 37 °C, induced with 1 mM IPTG and incubated at 16 °C overnight. Cells were pelleted and resuspended in lysis buffer (50 mM Na2HPO4, 300 mM NaCl, 10 mM imidazole), treated with lysozyme, and lysed by sonication. Lysed cells were centrifuged at 14,000 × g for 30 min at 4C. Cleared supernatant was combined with Ni-NTA resin (Novagen) and incubated for 1 h at 4 °C. The bead/lysate mixture was poured onto a 1-cm separation column (Bio-Rad), the resin was allowed to pack and was washed with wash buffer (50 mM Na2HPO4, 300 mM NaCl, 30 mM Imidazole). CsrAWT/Crw bound to the resin was then eluted using a stepwise elution of wash buffer with 50–500 mM imidazole. Elutions were separated by SDS/PAGE and Coomassie stained to verify purification of the CsrAWT/Crw–6His. Purified CsrAWT/Crw-6His was dialized into 50 mM Tris⋅HCl at pH 8.0, 150 mM NaCl, 10% (vol/vol) glycerol, 1 mM DTT and stored at −20 °C.
GST–CsrAWT/crw and FliW Interaction Pull-Down Assay.
Glutathione-Sepharose beads were washed with 0.1× T buffer; 1× T buffer is 25 mM Tris⋅HCl at pH 8.0, 20% (vol/vol) glycerol, 100 mM NaCl, 1 mM DTT, and 1× protease inhibitor mixture (Roche). Thirty microliters washed beads were mixed with 30 µL 5 µM GST–CsrA protein and rotated on labquake at 4 °C for 2 h. Next, the beads bound to GST–CsrA protein were centrifuged at 125 × g for 2 min, and the pellet was washed twice with 1× T buffer and again twice with 0.1× T buffer. Then 30 µL of increasing concentrations of FliW (5, 10, 20, and 40 µM) were added to the washed beads bound to GST–CsrA and rotated on labquake at 4 °C for 2 h. The samples were then centrifuged at 125 × g for 2 min, and the supernatant was saved. The pellet was washed four times with 0.1× T buffer. The supernatant and pellet fractions were subjected to SDS/PAGE analysis and stained with Coomassie Brilliant Blue.
Anti-CsrA Antibody Preparation.
One milligram of purified GST–CsrA protein was sent to Cocalico Biologicals Inc. for serial injection into a rabbit host for antibody generation. Anti-CsrA serum was mixed with CsrA-conjugated Affigel-10 beads and incubated overnight at 4 °C. Beads were packed onto a 1-cm column (Bio-Rad) and then washed with 100 mM glycine at pH 2.5 to release the antibody, and immediately neutralized with 2 M Tris base. Purification of the antibody was verified by SDS/PAGE. Purified anti-CsrA antibody was dialized into 1× PBS, 50% (vol/vol) glycerol, and stored at −80 °C.
Anti-FliW Antibody Preparation.
One milligram of purified FliW protein was sent to Cocalico Biologicals Inc. for serial injection into a rabbit host for antibody generation. The third bleed has been used for Western analyses.
RNA Electrophoretic Mobility Shift Assay.
Quantitative electrophoretic mobility shift assays used to examine CsrA-hag RNA followed a published procedure (14). DNA templates for in vitro transcription were synthesized by PCR using chromosomal DNA from B. subtilis strains 3610. Each template contained a T7 RNA polymerase promoter and hag-specific sequences (+1 to +103, relative to the start of hag transcription). RNA was synthesized in vitro with the Stratagene RNA-Maxx transcription kit. Gel-purified RNA was dephosphorylated with calf intestinal alkaline phosphatase and subsequently 5′ end-labeled using [γ-32P]ATP and polynucleotide kinase. Labeled RNA was gel-purified, suspended in TE buffer (10 mM Tris⋅HCl at pH 8.0, 1 mM EDTA), and renatured by heating to 85 °C and slowly cooling to room temperature. Binding reaction mixtures (10 µL) contained 10 mM Tris⋅HCl at pH 7.5, 100 mM KCl, 10 mM MgCl2, 2 µg yeast RNA, 10% (vol/vol) glycerol, 20 mM DTT, 4 U RNase inhibitor (Promega), 0.2 nM 5′ end-labeled hag RNA, various concentrations of WT or mutant CsrA, and 0.1 mg xylene cyanol per milliliter. After incubation for 30 min at 37 °C, samples were fractionated on native 15% (vol/vol) polyacrylamide gels. Radioactive bands were visualized with a Phosphorimager (Molecular Dynamics). Apparent equilibrium binding constants (Kd) of CsrA-hag RNA interaction were calculated as described previously (52).
Acknowledgments
We thank Amilcar Perez for technical assistance. The work was funded by NIH Grants R01 GM093030 (to D.B.K.), and R01 GM059969 and R01 GM098399 (to P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602455113/-/DCSupplemental.
References
- 1.Vakulskas CA, Potts AH, Babitzke P, Ahmer BMM, Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79(2):193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimmann C, Valverde C, Kay E, Haas D. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J Bacteriol. 2005;187(1):276–285. doi: 10.1128/JB.187.1.276-285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris ER, et al. Structural rearrangement in an RsmA/CsrA ortholog of Pseudomonas aeruginosa creates a dimeric RNA-binding protein, RsmN. Structure. 2013;21(9):1659–1671. doi: 10.1016/j.str.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marden JN, et al. An unusual CsrA family member operates in series with RsmA to amplify posttranscriptional responses in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2013;110(37):15055–15060. doi: 10.1073/pnas.1307217110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RD, et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42(Database issue):D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott ZD, et al. csrT represents a new class of csrA-like regulatory genes associated with integrative conjugative elements of Legionella pneumophila. J Bacteriol. 2015;198(3):553–564. doi: 10.1128/JB.00732-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez P, et al. Solution structure of the carbon storage regulator protein CsrA from Escherichia coli. J Bacteriol. 2005;187(10):3496–3501. doi: 10.1128/JB.187.10.3496-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rife C, et al. Crystal structure of the global regulatory protein CsrA from Pseudomonas putida at 2.05 A resolution reveals a new fold. Proteins. 2005;61(2):449–453. doi: 10.1002/prot.20502. [DOI] [PubMed] [Google Scholar]
- 9.Heeb S, et al. Functional analysis of the post-transcriptional regulator RsmA reveals a novel RNA-binding site. J Mol Biol. 2006;355(5):1026–1036. doi: 10.1016/j.jmb.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Mercante J, Suzuki K, Cheng X, Babitzke P, Romeo T. Comprehensive alanine-scanning mutagenesis of Escherichia coli CsrA defines two subdomains of critical functional importance. J Biol Chem. 2006;281(42):31832–31842. doi: 10.1074/jbc.M606057200. [DOI] [PubMed] [Google Scholar]
- 11.Mercante J, Edwards AN, Dubey AK, Babitzke P, Romeo T. Molecular geometry of CsrA (RsmA) binding to RNA and its implications for regulated expression. J Mol Biol. 2009;392(2):511–528. doi: 10.1016/j.jmb.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MY, Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J Bacteriol. 1997;179(14):4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker CS, Morozov I, Suzuki K, Romeo T, Babitzke P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol Microbiol. 2002;44(6):1599–1610. doi: 10.1046/j.1365-2958.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 14.Dubey AK, Baker CS, Romeo T, Babitzke P. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA. 2005;11(10):1579–1587. doi: 10.1261/rna.2990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert M, et al. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nat Struct Mol Biol. 2007;14(9):807–813. doi: 10.1038/nsmb1285. [DOI] [PubMed] [Google Scholar]
- 16.Yakhnin H, et al. CsrA of Bacillus subtilis regulates translation initiation of the gene encoding the flagellin protein (hag) by blocking ribosome binding. Mol Microbiol. 2007;64(6):1605–1620. doi: 10.1111/j.1365-2958.2007.05765.x. [DOI] [PubMed] [Google Scholar]
- 17.Romeo T, Gong M, Liu MY, Brun-Zinkernagel A-M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175(15):4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawhon SD, et al. Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol. 2003;48(6):1633–1645. doi: 10.1046/j.1365-2958.2003.03535.x. [DOI] [PubMed] [Google Scholar]
- 19.Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol. 2009;72(3):612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards AN, et al. Circuitry linking the Csr and stringent response global regulatory systems. Mol Microbiol. 2011;80(6):1561–1580. doi: 10.1111/j.1365-2958.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molofsky AB, Swanson MS. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol Microbiol. 2003;50(2):445–461. doi: 10.1046/j.1365-2958.2003.03706.x. [DOI] [PubMed] [Google Scholar]
- 22.Lucchetti-Miganeh C, Burrowes E, Baysse C, Ermel G. The post-transcriptional regulator CsrA plays a central role in the adaptation of bacterial pathogens to different stages of infection in animal hosts. Microbiology. 2008;154(Pt 1):16–29. doi: 10.1099/mic.0.2007/012286-0. [DOI] [PubMed] [Google Scholar]
- 23.Heroven AK, Böhme K, Dersch P. The Csr/Rsm system of Yersinia and related pathogens: A post-transcriptional strategy for managing virulence. RNA Biol. 2012;9(4):379–391. doi: 10.4161/rna.19333. [DOI] [PubMed] [Google Scholar]
- 24.Liu MY, et al. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem. 1997;272(28):17502–17510. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 25.Weilbacher T, et al. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol Microbiol. 2003;48(3):657–670. doi: 10.1046/j.1365-2958.2003.03459.x. [DOI] [PubMed] [Google Scholar]
- 26.Lenz DH, Miller MB, Zhu J, Kulkarni RV, Bassler BL. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol Microbiol. 2005;58(4):1186–1202. doi: 10.1111/j.1365-2958.2005.04902.x. [DOI] [PubMed] [Google Scholar]
- 27.Kay E, Dubuis C, Haas D. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci USA. 2005;102(47):17136–17141. doi: 10.1073/pnas.0505673102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duss O, et al. Structural basis of the non-coding RNA RsmZ acting as a protein sponge. Nature. 2014;509(7502):588–592. doi: 10.1038/nature13271. [DOI] [PubMed] [Google Scholar]
- 29.Babitzke P, Romeo T. CsrB sRNA family: Sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol. 2007;10(2):156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, et al. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J Bacteriol. 2002;184(18):5130–5140. doi: 10.1128/JB.184.18.5130-5140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ Microbiol. 2011;13(12):3128–3138. doi: 10.1111/j.1462-2920.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 32.Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol. 2014;196(2):357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K, Babitzke P, Kushner SR, Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20(18):2605–2617. doi: 10.1101/gad.1461606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zere TR, et al. Genomic targets and features of BarA-UvrY (-SirA) signal transduction systems. PLoS One. 2015;10(12):e0145035. doi: 10.1371/journal.pone.0145035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavez RG, Alvarez AF, Romeo T, Georgellis D. The physiological stimulus for the BarA sensor kinase. J Bacteriol. 2010;192(7):2009–2012. doi: 10.1128/JB.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titz B, Rajagopala SV, Ester C, Häuser R, Uetz P. Novel conserved assembly factor of the bacterial flagellum. J Bacteriol. 2006;188(21):7700–7706. doi: 10.1128/JB.00820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Babitzke P, Kearns DB. FliW and FliS function independently to control cytoplasmic flagellin levels in Bacillus subtilis. J Bacteriol. 2013;195(2):297–306. doi: 10.1128/JB.01654-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, et al. CsrA-FliW interaction governs flagellin homeostasis and a checkpoint on flagellar morphogenesis in Bacillus subtilis. Mol Microbiol. 2011;82(2):447–461. doi: 10.1111/j.1365-2958.2011.07822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dugar G, et al. The CsrA-FliW network controls polar localization of the dual-function flagellin mRNA in Campylobacter jejuni. Nat Commun. 2016;7:11667. doi: 10.1038/ncomms11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonas K, et al. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ Microbiol. 2010;12(2):524–540. doi: 10.1111/j.1462-2920.2009.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vakulskas CA, et al. Global effects of the DEAD-box RNA helicase DeaD (CsdA) on gene expression over a broad range of temperatures. Mol Microbiol. 2014;92(5):945–958. doi: 10.1111/mmi.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterzenbach T, et al. A novel CsrA titration mechanism regulates fimbrial gene expression in Salmonella typhimurium. EMBO J. 2013;32(21):2872–2883. doi: 10.1038/emboj.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolas P, et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012;335(6072):1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 44.Mondal S, Yakhnin AV, Sebastien A, Albert I, Babitzke P. NusA-dependent transcription termination prevents misregulation of global gene expression. Nature Microbiol. 2016;1:15007. doi: 10.1038/nmicrobiol.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnard FM, et al. Global regulation of virulence and the stress response by CsrA in the highly adapted human gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;51(1):15–32. doi: 10.1046/j.1365-2958.2003.03788.x. [DOI] [PubMed] [Google Scholar]
- 46.Fields JA, Thompson SA. Campylobacter jejuni CsrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J Bacteriol. 2008;190(9):3411–3416. doi: 10.1128/JB.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sze CW, et al. Carbon storage regulator A (CsrA(Bb)) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol Microbiol. 2011;82(4):851–864. doi: 10.1111/j.1365-2958.2011.07853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karna SLR, Prabhu RG, Lin Y-H, Miller CL, Seshu J. Contributions of environmental signals and conserved residues to the functions of carbon storage regulator A of Borrelia burgdorferi. Infect Immun. 2013;81(8):2972–2985. doi: 10.1128/IAI.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni PR, Cui X, Williams JW, Stevens AM, Kulkarni RV. Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri. Nucleic Acids Res. 2006;34(11):3361–3369. doi: 10.1093/nar/gkl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasbin RE, Young FE. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol. 1974;14(6):1343–1348. doi: 10.1128/jvi.14.6.1343-1348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science. 2008;320(5883):1636–1638. doi: 10.1126/science.1157877. [DOI] [PubMed] [Google Scholar]
- 52.Yakhnin AV, Trimble JJ, Chiaro CR, Babitzke P. Effects of mutations in the L-tryptophan binding pocket of the Trp RNA-binding attenuation protein of Bacillus subtilis. J Biol Chem. 2000;275(6):4519–4524. doi: 10.1074/jbc.275.6.4519. [DOI] [PubMed] [Google Scholar]