Significance
This study discloses specific central and peripheral mechanisms involving cannabinoid type-1 (CB1) receptors in impairing the effect of acute stress on the consolidation of nonemotional memory in rodents. Both hippocampal and peripheral CB1 receptors in dopamine β-hydroxylase–expressing cells (i.e., in adrenergic/noradrenergic cells) are identified as necessary and sufficient for stress-induced memory impairment. Our study provides the foundation for the development of novel potential approaches to tackle cognitive alterations in stress-related disorders.
Keywords: memory consolidation, stress response, cannabinoid receptor, endocannabinoid system, noradrenergic signaling
Abstract
Stressful events can generate emotional memories linked to the traumatic incident, but they also can impair the formation of nonemotional memories. Although the impact of stress on emotional memories is well studied, much less is known about the influence of the emotional state on the formation of nonemotional memories. We used the novel object-recognition task as a model of nonemotional memory in mice to investigate the underlying mechanism of the deleterious effect of stress on memory consolidation. Systemic, hippocampal, and peripheral blockade of cannabinoid type-1 (CB1) receptors abolished the stress-induced memory impairment. Genetic deletion and rescue of CB1 receptors in specific cell types revealed that the CB1 receptor population specifically in dopamine β-hydroxylase (DBH)-expressing cells is both necessary and sufficient for stress-induced impairment of memory consolidation, but CB1 receptors present in other neuronal populations are not involved. Strikingly, pharmacological manipulations in mice expressing CB1 receptors exclusively in DBH+ cells revealed that both hippocampal and peripheral receptors mediate the impact of stress on memory consolidation. Thus, CB1 receptors on adrenergic and noradrenergic cells provide previously unrecognized cross-talk between central and peripheral mechanisms in the stress-dependent regulation of nonemotional memory consolidation, suggesting new potential avenues for the treatment of cognitive aspects on stress-related disorders.
Memory consolidation is sensitive to emotion-related manipulations after acquisition (1). However, the underlying neurobiological mechanisms are only partly understood. Emotions can contribute to memorizing important life events (1, 2); they can also impair memory consolidation (2). Specifically, emotional arousal caused by stress has been studied extensively in animal models and in humans, and it has been reported to produce both facilitation and impairment of memory (3–5). Most studies that investigated the neural mechanisms mediating the effects of stress have focused on emotional memories; the mechanisms underlying the effects of acute stress on nonemotional memories are less understood.
Acute stressful stimuli activate the sympathetic–adrenal system and the hypothalamic–pituitary–adrenal (HPA) axis (6, 7). Increased activity in the sympathetic–adrenal system involves a rapid release of adrenaline and noradrenaline from adrenal chromaffin cells and sympathetic nerve terminals, respectively (7). Moreover, stress-induced activation of the HPA axis involves the synthesis and secretion of glucocorticoids (cortisol in humans and corticosterone in most rodents) from the adrenal cortex (8). Both animal and human studies have shown that these stress hormones have profound effects on cognition by acting on specific brain regions involved in the processing of emotional stimuli (1, 9–12).
The endocannabinoid system is an endogenous neuromodulatory system playing a relevant role in the regulation of the stress response (13–18). Endocannabinoids, such as 2-arachidonoylglycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide; AEA), act mainly at two types of cannabinoid receptors, cannabinoid type-1 (CB1) and type-2 (CB2) receptors. The predominant localization of the CB1 receptor at presynaptic sites has been associated with its role in suppressing neurotransmitter release upon synaptic activity (19). Accordingly, activation of the CB1 receptor in adrenergic and noradrenergic cells is expected to decrease the release of adrenaline and noradrenaline (20–22). Moreover, the endocannabinoid system also participates in the negative feedback regulation of the HPA axis after stress (13, 23). Thus, glucocorticoids enhance the production of endocannabinoids to counteract the activity of the HPA axis in many brain regions, including the hippocampus, the prefrontal cortex, and the hypothalamus (16, 24). Overall, endocannabinoid production is induced by acute stress and acts by buffering stress-induced behavioral and endocrine stress effects (16, 17, 23).
In this study we reveal a mechanism mediating stress-induced impairment of object-recognition memory consolidation. Using a combination of acute systemic and local pharmacological approaches and newly generated mouse lines, we found that peripheral and hippocampal CB1 receptors in dopamine β-hydroxylase (DBH)-expressing cells (i.e., adrenergic/noradrenergic cells) are both necessary and sufficient to impair object-recognition memory consolidation produced by acute stress.
Results
CB1 Receptors Control Stress-Induced Impairment of Memory Consolidation.
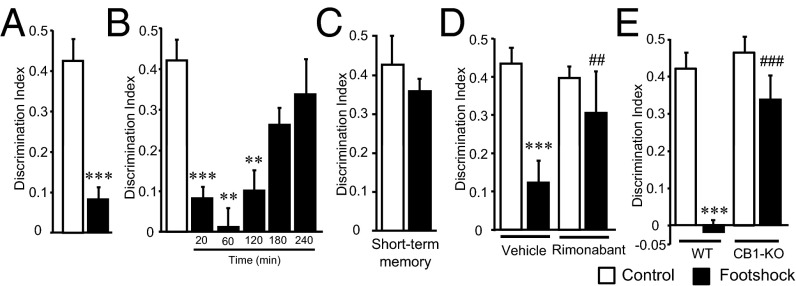
We evaluated the effects of acute stress on the consolidation of long-term (24 h) nonemotional memory by using the novel object-recognition memory paradigm (Fig. S1A). This memory was impaired when mice were exposed to different acutely stressful events [footshock (Fig. 1A) and tail suspension (Fig. S1B)] 20 min after the training session without reducing the overall exploratory behavior during the memory test (Fig. S1 C and D). Under these conditions, c-Fos expression was enhanced in the CA1 region of the hippocampus after mice were exposed to the footshock (Fig. S1E). When the footshock was administered at different time points after memory training, we found that long-term object-recognition memory was progressively less sensitive to footshock exposure (Fig. 1B), indicating that this kind of stress affected the consolidation of object-recognition memory in a specific critical time window after acquisition. Notably, the effects of footshock on object-recognition memory were not observed when short-term memory was tested (60 min after stress exposure) (Fig. 1C), indicating a specific disrupting effect of acute stress on memory consolidation. Consistently, the protein synthesis inhibitor anisomycin (15 mg/kg, i.p.) blocked the long-term memory impairment induced by the footshock (Fig. S2A).
Fig. 1.
Stress-induced memory impairment is mediated by CB1 receptors. (A) Effect of stress in object-recognition memory consolidation [n = 9 or 10; t (17) = 5.492]. (B) Object-recognition memory impairment was reduced when footshock was applied at longer times after training [n = 4–10; F(5, 32) = 15.836]. (C) Object-recognition short-term memory was not affected by the footshock [n = 6; t (10) = 0.86]. (D) DI values obtained on mice treated with the CB1 receptor antagonist rimonabant immediately after the training session (i.e., 20 min before the footshock) [n = 9–15; treatment: F(1, 42) = 3.90, P = 0.054; stress condition: F(1, 42) = 20.61, P = 0.00005; interaction: F(1, 42) = 6.85, P = 0.012]. (E) DI values were obtained from mice lacking the CB1 receptor (CB1-KO) and littermate control (WT) mice. Loss of CB1 receptor obliterated stress-induced memory impairment [n = 6–12; genotype: F(1, 30) = 11.65, P = 0.002; stress condition: F(1, 30) = 24.23, P = 0.00003; interaction: F(1, 30) = 7.94, P = 0.008]. **P < 0.01, ***P < 0.001 compared with nonstress condition; ##P < 0.01, ###P < 0.001 compared with vehicle or WT.
Next, we evaluated whether the endocannabinoid system was involved in the selective modulation of memory consolidation by acute stress in our experimental conditions. The CB1 receptor antagonist rimonabant (1 mg/kg) administered immediately after training prevented the memory impairment produced by both stressors, footshock (Fig. 1D) and tail suspension (Fig. S3), but the CB2 receptor antagonist AM630 (1 mg/kg) had no effect (Fig. S4). Consistently, stress-induced memory impairment was not observed in mutant animals lacking the CB1 receptor (CB1-KO mice) (Fig. 1E).
Hippocampal CB1 Receptors in Stress-Induced Impairment of Memory Consolidation.
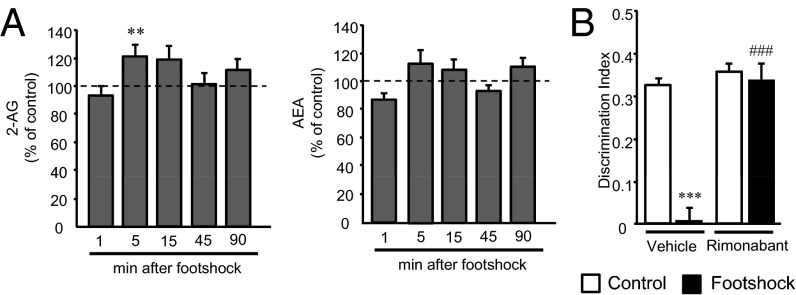
Under our experimental conditions, footshock stress transiently enhanced endocannabinoid levels in the hippocampus (Fig. 2A), in agreement with previous reports (23). For this reason we then focused on the hippocampus to ask whether this brain area is involved in the effects of acute stress on the consolidation of object-recognition memory. Notably, intrahippocampal injections of rimonabant after the acquisition of object-recognition memory prevented the stress-induced memory deficit in WT mice without affecting memory performance in nonstressed mice (Fig. 2B). These data indicated the functional relevance of hippocampal CB1 receptor activation by the elevation of endocannabinoids, specifically 2-AG, in the memory impairment produced by acute stress.
Fig. 2.
Hippocampal CB1 receptors mediate stress-induced memory impairment. (A) Measurements of endocannabinoid levels in the hippocampus at different time points showed a stress-induced rise in 2-AG levels [n = 7–8; 5 min: t (12) = –1.77]. At other time points, footshocked animals did not show significant alterations with respect to controls. (B) Intrahippocampal injection of rimonabant immediately after the training session prevented stress-induced memory impairment in WT mice [n = 7–10; treatment: F(1, 28) = 37.15, P = 0.00001; stress condition: F(1, 28) = 34.22, P = 0.000003; interaction: F(1, 28) = 25.26, P = 0.00003]. Injection sites were confirmed by postmortem histological analysis. **P < 0.01 compared with control; ***P < 0.001 compared with nonstressful condition; ###P < 0.001 compared with vehicle.
Peripheral CB1 Receptors also Contribute to Stress-Induced Memory Impairment.
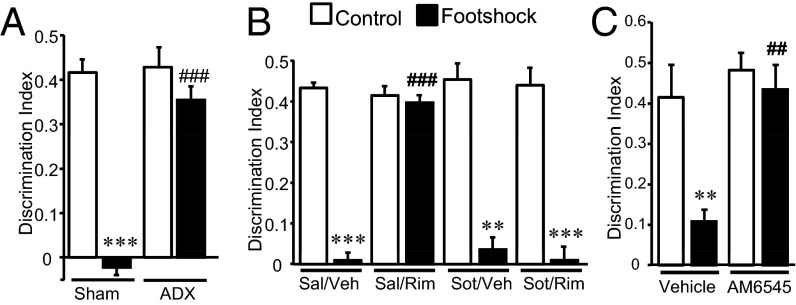
We next investigated the relevance of the peripheral stress response in stress-induced memory impairment using adrenalectomized mice. These animals did not show the memory deficit induced by the footshock compared with sham-operated control mice (Fig. 3A), suggesting that adrenal gland hormones have a crucial role in stress-induced memory impairment. Indeed, under our stress conditions, circulating stress hormones increased transiently above naive-handling conditions (Fig. S5 A–C). Also, in accordance with previous findings (25), the dose of rimonabant that blocked acute stress-induced memory impairment enhanced corticosterone levels in nonstressed mice 90 min after the treatment without affecting adrenaline and noradrenaline levels (Fig. S5 D–F). However, rimonabant administration did not alter memory performance in nonstressed mice (Fig. 2B), suggesting that corticosterone enhancement does not play a major role in the inhibition of stress-induced memory consolidation by CB1 receptor blockade.
Fig. 3.
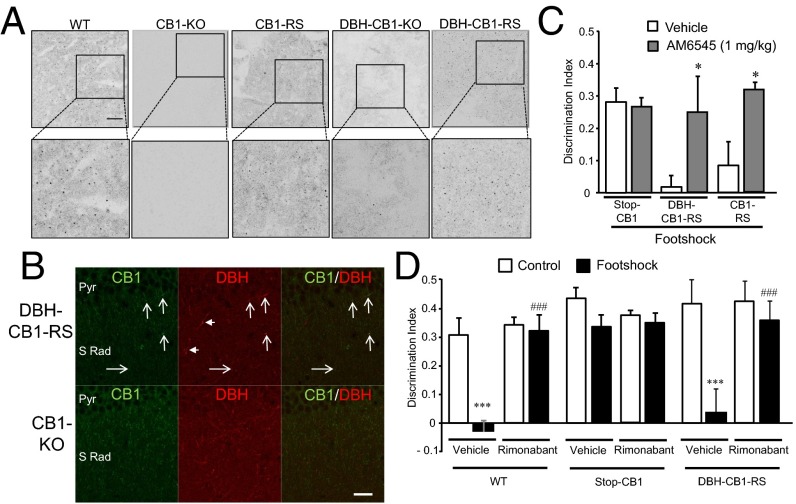
Peripheral CB1 receptors also participate in the stress-induced memory impairment. (A) Stress-induced memory impairment was not observed in adrenalectomized mice [n = 5–10; surgery: F(1, 26) = 23.56, P = 0.00005; stress condition: F(1, 26) = 41.01, P = 0.000001; interaction: F(1, 26) = 20.79, P = 0.0001]. (B) The β-adrenergic receptor antagonist sotalol (sot) alleviated the beneficial effect of rimonabant (Rim) in stress-induced memory impairment [n = 4–6; treatment 1 (sotalol): F(1, 30) = 16.94, P = 0.0003; treatment 2 (rimonabant): F(1, 30) = 19.61, P = 0.0001; stress condition: F(1, 30) = 273.23, P = 0.00001; interaction treatments: F(1, 30) = 27.50, P = 0.00001; interaction treatment 1 × stress condition: F(1, 30) = 25.64, P = 0.000019; interaction treatment 2 × stress condition: F(1, 30) = 25.40, P = 0.00002; three-way interaction: F(1, 30) = 30.97, P = 0.000005]. (C) The peripherally restricted CB1 receptor antagonist AM6545 prevented the stress-induced memory deficit in the object-recognition memory test [n = 4–6; treatment: F(1, 16) = 11.64, P = 0.0035; stress condition: F(1, 16) = 9.47, P = 0.007; interaction: F(1, 16) = 5.05, P = 0.039]. **P < 0.01, ***P < 0.001 compared with nonstress condition; ##P < 0.01, ###P < 0.01 compared with vehicle.
Based on these observations, we consequently focused on the involvement of peripheral adrenergic and noradrenergic transmission in our behavioral paradigm. Mice were trained in the object-recognition test and received the peripherally restricted β-adrenergic receptor antagonist sotalol (10 mg/kg) (26) before rimonabant administration and footshock stress. Under these conditions, sotalol did not affect memory consolidation per se but did prevent the blockade of stress-induced memory impairment by rimonabant (Fig. 3B), suggesting that peripheral β-adrenergic receptor signaling has a role downstream of the systemic blockade of CB1 receptor function.
To assess the involvement of peripheral CB1 receptors in the stress-induced memory impairment, we used AM6545, a CB1 receptor antagonist with limited brain penetrance (27). AM6545 (1 mg/kg) administration before footshock (Fig. 3C) completely prevented the stress-induced memory deficit. AM6545 pretreatment under stress conditions (Fig. S5 G–L) maintained an enhancement of circulating adrenaline and noradrenaline as detected 90 min after footshock (Fig. S5 J–L). These data revealed a crucial role of peripheral CB1 receptors in the stress-induced memory impairment by controlling the adrenergic tone after acute stress.
CB1 Receptors in DBH+ Cells Are Key in the Stress-Induced Memory Deficits.
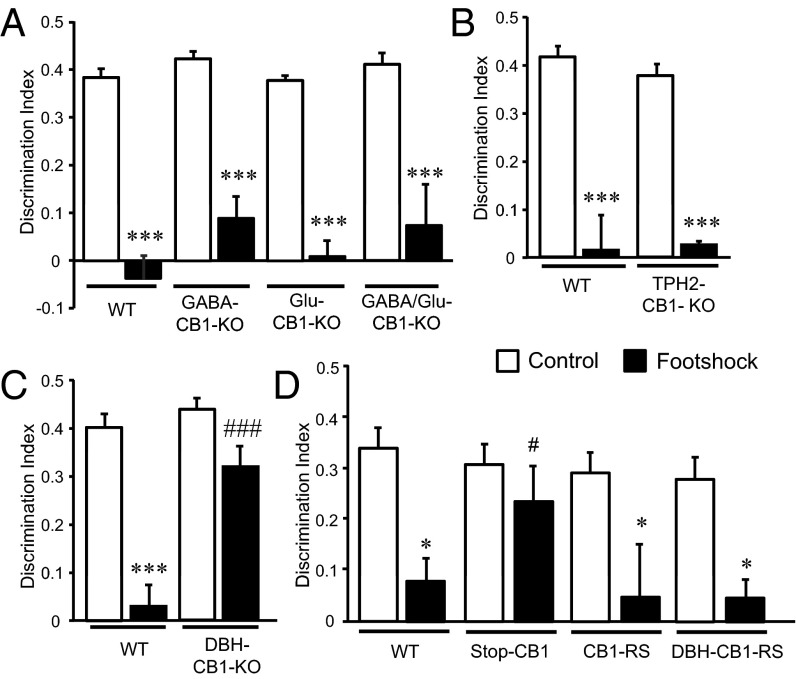
Different CB1 receptor conditional-KO mouse lines were assessed to investigate which specific CB1 receptor populations modulate these processes (Fig. 4). Stress-induced memory impairment was present in mice lacking the CB1 receptor in forebrain GABAergic neurons (GABA-CB1–KO mice) (Fig. 4A) (28), in dorsal telencephalic glutamatergic neurons (Glu-CB1–KO mice) (Fig. 4A) (28), in both GABAergic and glutamatergic neurons (GABA/Glu-CB1–KO mice) (Fig. 4A) (29), and in central serotonergic neurons, i.e., in tryptophan hydroxylase 2+ cells (TPH2-CB1–KO mice) (Fig. 4B) (30, 31). Strikingly, mice lacking the CB1 receptor in DBH cells (DBH-CB1–KO mice) (Fig. S6A) were insensitive to stress-induced nonemotional memory impairment (Fig. 4C), although no genotype differences were observed in contextual and cued fear-conditioning tasks (Fig. S7).
Fig. 4.
CB1 receptors in adrenergic/noradrenergic cells are necessary and sufficient to mediate stress-induced memory impairment. (A) Mice lacking CB1 receptors in forebrain GABAergic neurons (GABA-CB1–KO mice), in dorsal telencephalic/cortical glutamatergic neurons (Glu-CB1–KO mice), or in both GABAergic and glutamatergic neurons (GABA/Glu-CB1–KO mice) [n = 5; genotype: F(3, 32) = 1.84, P = 0.16; stress condition: F(1, 32) = 161.68, P = 0.0000; interaction: F(3, 32) = 0.49, P = 0.69] and (B) in central serotonergic neurons (TPH2-CB1–KO) show the memory impairment caused by stress [n = 5; genotype: F(1, 16) = 0.14, P = 0.712; stress condition: F(1, 16) = 97.12, P = 0.0000; interaction: F(1, 16) = 0.44, P = 0.514]. (C) Object-recognition memory is not affect by stress in mice lacking CB1 receptors in DBH cells (DBH-CB1–KO mice) [n = 9–11; genotype: F(1, 36) = 9.23, P = 0.00009; stress condition: F(1, 36) = 43.23, P = 0.0000; interaction: F(1, 36) = 11.48, P = 0.002]. (D) Effect of footshock stress on novel object-recognition memory in WT CB1 receptor-deficient mice (Stop-CB1 mice), control mice reexpressing CB1 receptors in all cells (CB1-RS mice), and mice reexpressing CB1 receptors exclusively in DBH cells (DBH-CB1–RS mice) [n = 4–6; genotype: F(3, 34) = 2.58, P = 0.26; stress condition: F(1, 34) = 23.25, P = 0.00003; interaction: F(3, 34) = 2.12, P = 0.38]. *P < 0.05, ***P < 0.001 compared with nonstress conditions; #P < 0.05, ###P < 0.001 compared with WT group.
To evaluate whether the expression of CB1 receptor in DBH+ cells is not only necessary but also sufficient (32) to produce stress-induced memory deficits, specific mouse lines were developed to rescue CB1 receptor expression globally (CB1-RS mice) (32) or specifically in DBH+ cells (DBH-CB1–RS mice) (Fig. S6 B and C). Analysis of memory performance after footshock showed that both CB1-RS and DBH-CB1–RS mice exhibited stress-induced memory impairment (Fig. 4D). In contrast, Stop-CB1 mice [mice without CB1 receptor expression, similar to constitutive CB1-KO mice (28)] were not sensitive to the cognitive deficit induced by footshock. Importantly, there were no differences between mouse lines in general activity as measured in the modified Irwin test, in nociceptive sensitivity, or in context conditioning memory (Fig. S8); these findings remove the possibility that confounding factors were induced by developmental alterations resulting from genetic manipulations. Moreover, these data highlight the labile characteristics of a nonemotional memory in comparison with an emotional one, confirm the pivotal and diverse roles of CB1 receptors in memory processing, and demonstrate the crucial role of the CB1 receptors specifically expressed in adrenergic/noradrenergic cells in stress-induced memory impairment.
Peripheral and Hippocampal CB1 Receptors in DBH+ Cells Control Stress-Induced Memory Impairment.
DBH+ cells are localized in the central nervous system (33) and also at peripheral locations, the adrenal gland in particular (33). CB1 receptor mRNA and protein were specifically detected in adrenal medulla (Fig. 5A and Fig. S9B). As expected, CB1 receptor mRNA was reduced in the DBH-CB1–KO mice compared with WT control animals (Fig. S9 A and B). In the hippocampus, CB1 receptor protein was present to different degrees in the CA1 region (Fig. S9C). Notably, the expression of CB1 receptor protein was detected at low levels in the hippocampus of DBH-CB1–RS mice colocalizing to DBH+ fibers (Fig. 5B). This low level of expression contrasted with the strong CB1 receptor expression detected in WT, CB1-RS, and DBH-CB1–KO mice (Fig. S9C). Taken together, these data indicate that DBH+ fibers contain low but functionally important levels of CB1 receptor protein.
Fig. 5.
Peripheral and central CB1 receptors in DBH+ cells are crucial to mediate stress-induced memory impairment. (A) Detection of CB1 receptor protein in the adrenal medulla in the different mouse lines studied. CB1 receptor protein was detected in DBH-CB1–RS mice. (Scale bar, 100 μm.) (B) Detection of CB1 receptors (arrows) in DBH+ fibers in the stratum radiatum (S Rad) and pyramidal layer (Pyr) of the hippocampus in DBH-CB1–RS mice. Arrowheads indicate DBH fibers where CB1 receptors were not detected. CB1-KO mice were used as negative controls. (Scale bar, 25 μm.) (C) The peripherally restricted CB1 receptor antagonist AM6545 prevented the stress-induced memory deficit in DBH-CB1–RS mice [n = 5–8; genotype: F(1, 33) = 2.57, P = 0.09; treatment: F(1, 33) = 11.30, P = 0.002; interaction: F(1, 33) = 3.609, P = 0.038]. (D) Intrahippocampal injection of rimonabant prevented stress-induced memory impairment in DBH-CB1–RS mice, substantiating the specific role of this CB1 receptor population in mediating stress-induced memory impairment [n = 3–6; genotype: F(2, 44) = 9.16, P = 0.0005; treatment: F(1, 44) = 16.08, P = 0.0002; stress condition: F(1, 44) = 30.70, P = 0.000002; interaction genotype × treatment: F(2, 44) = 6.61, P = 0.003; interaction genotype × stress condition: F(2, 44) = 3.09, P = 0.05; interaction treatment × stress condition: F(1, 44) = 17.39, P = 0.0001; three-way interaction: F(2, 44) = 2.29, P = 0.1]. *P < 0.05 compared with vehicle; ***P < 0.001 compared with nonstress condition; ### P < 0.001 compared with vehicle.
Remarkably, the peripheral CB1 receptor antagonist AM6545 prevented the stress-induced memory impairment in DBH-CB1–RS and control CB1-RS mice, strongly suggesting that peripheral CB1 receptors have a crucial role in the effect of stress (Fig. 5C). Similarly, intrahippocampal injections of rimonabant in DBH-CB1–RS mice and in control Stop-CB1 mice fully prevented stress-induced memory impairment (Fig. 5D). Together these data reveal that both peripheral and hippocampal CB1 receptors present in DBH+ cells have a crucial role in stress-induced memory impairment.
Discussion
Our study demonstrates the crucial role of CB1 receptors in the impairment of nonemotional memory consolidation induced by acute stress. We identified the hippocampal and peripheral CB1 receptors present in DBH+ cells as necessary and sufficient determinants for the deficit observed in the object-recognition memory task triggered by stress.
Stress can modulate cognitive performance in opposite ways, depending on its intensity and the type of memory evaluated (1, 34). To understand better the effects of acute stress on a nonemotional memory, we used an object-recognition paradigm, a memory task that allows accurate definition of the memory consolidation period. Interestingly, although different types of acute stress produced a clear long-term memory deficit, short-term object-recognition memory was not affected by our stress paradigm, in agreement with observations in human declarative short-term memory, which is not influenced by emotional arousal (35).
The endocannabinoid system plays a pivotal role both in the modulation of the stress response (13, 36) and in the control of cognitive functions (14, 18). CB1 receptors modulate HPA feedback inhibition secondary to glucocorticoid receptor activation (15, 37), and endocannabinoids can alter both emotional (38) and nonemotional (39) memories. Our data demonstrate that systemic and local pharmacological blockade or complete genetic inactivation of the CB1 receptor prevents the memory deficit triggered by different stressors. According to previous findings (25), pharmacological blockade of CB1 receptors by rimonabant is able to enhance circulating corticosterone levels, although it does not modify circulating amounts of adrenaline or noradrenaline. However, that rimonabant treatment alone did not affect the object-recognition memory and completely blocked the memory-impairing effects of the acute stress suggesting that this corticosterone enhancement does not play a prominent role in the effects of CB1 receptor blockade. Notably, we identified a specific population of CB1 receptors expressed in DBH+ cells as being both necessary and sufficient for stress-induced impairment of object-recognition memory, but other, much more abundant, CB1 receptor populations were not involved in this process. Although developmental changes produced by noninducible genetic manipulations cannot be fully discarded, the observation that mutant mice behaved similarly to controls in other tests, together with our genetic and pharmacological data, indicates that acute activation of CB1 receptors in DBH+ cells is responsible for stress-induced memory impairment.
Importantly, adrenalectomy also blocked stress-induced memory impairment, indicating that the adrenal gland is a key peripheral tissue controlling the memory impairment induced by acute stress. Interestingly, the involvement of the HPA axis in cognitive performance has been described (40), but the mechanisms involving the sympathetic system, in which adrenal glands are also involved, are less well characterized. In this study we propose that CB1 receptors expressed in DBH+ cells of the adrenal medulla, which release adrenaline and noradrenaline upon sympathetic activation (41), are relevant for the object-recognition memory deficit produced by acute footshock. Our proposal is based on the following observations: (i) circulating adrenaline and noradrenaline levels after stress are sustained by AM6545 pretreatment; (ii) stress-induced memory impairment is blocked by AM6545 in the DBH-CB1–RS mice; (iii) CB1 receptor expression in the adrenal medulla was reduced in DBH-CB1–KO animals and was re-expressed in the DBH-CB1–RS mice; (iv) the peripherally restricted β-adrenergic receptor antagonist sotalol prevented the effect of rimonabant in rescuing the consequences of stress on object-recognition memory consolidation, pointing to key peripheral β-adrenergic receptor signaling downstream of the CB1 receptor blockade.
Interestingly, recent data revealed that the anorectic and anxiogenic effects of rimonabant require peripheral activation of sympathetic activity (26). Consistently, the present data indicate that control of adrenergic/noradrenergic transmission by CB1 receptors is involved in the central–peripheral control of behavior. Thus, in agreement with previous findings (23, 36, 42), acute stress enhanced endocannabinoid levels in the hippocampus during the consolidation window of the object-recognition task. Notably, the intrahippocampal administration of rimonabant completely blocked the stress-induced memory impairment in WT mice and, importantly, in DBH-CB1–RS mice also. Although the role of other brain circuits such as the prefrontal cortex (43) or the perirhinal cortex (44) in the object recognition task cannot be discarded, our data strongly suggest that the hippocampus plays a prominent role in mediating the complex impact of endocannabinoid signaling on stress-induced memory impairment.
Our data demonstrate a transient increase in endocannabinoid levels in the hippocampus after acute stress. We hypothesize that endocannabinoids are enhanced at specific synapses, because the 2-AG–synthesizing enzyme diacylglycerol lipase α (DGLα) is heavily expressed in the hippocampus (45). The synthesis of endocannabinoids triggered by stress could be mediated through glucocorticoid receptors in the hippocampus (36) or by the engagement of hippocampal α1-adrenergic receptors and Gq/11-mediated signaling activated by local noradrenaline release (46). Mobilized endocannabinoids, in turn, may act on CB1 receptors expressed at low levels in noradrenergic fibers, as shown by immunohistochemical experiments. At these fibers, which project from the locus coeruleus or the nucleus tractus solitarius (47), CB1 receptors would control noradrenaline transmission, as has been proposed in other brain regions (22, 48). These findings lead us to propose that the endocannabinoid-mediated decrease in noradrenaline release at hippocampal noradrenergic terminals is a key step in modulating nonemotional memory consolidation studied in the object-recognition task. In this regard, it has been shown that noradrenaline is able to modulate synchronized hippocampal activity that can interfere with nonemotional memory consolidation (49, 50).
In summary, our multidisciplinary study revealed the involvement of central and peripheral mechanisms in stress-induced object-recognition memory impairment in which CB1 receptors in adrenergic and noradrenergic cells are key players. The discovery of this mechanism warrants the study of new approaches in the treatment of those cognitive aspects associated to stress-related conditions.
Materials and Methods
Animals.
Male Swiss albino (CD-1) mice (Charles River) and CB1 receptor constitutive-KO mice (8–10 wk of age) and their WT controls in the CD-1 background (51) weighing 29–33 g were used. Conditional-KO mice lacking CB1 receptors in DBH-expressing cells were generated as detailed in SI Materials and Methods. Rescued mice expressing CB1 receptor exclusively in DBH-expressing cells were generated as detailed in SI Materials and Methods. Conditional-KO mice lacking CB1 receptors in forebrain GABAergic neurons (GABA-CB1–KO mice), in dorsal telencephalic glutamatergic neurons (Glu-CB1–KO mice), in both GABAergic and glutamatergic neurons (GABA/Glu-CB1–KO mice), in central serotonergic (tryptophan hydroxylase 2-positive) neurons (TPH2-CB1–KO mice), and their WT (floxed/floxed) littermates (25–30 g) were in a mixed genetic background, with a predominant C57BL/6N contribution (at least seven generations of backcrossing) (28, 29, 31).
Mice were housed in cages holding a maximum of four mice per cage and were maintained at a controlled temperature (21 ± 1 °C) and humidity (55 ± 10%). Food and water were available ad libitum. Lighting was maintained in 12-h light/dark cycles (light on at 8:00 AM and off at 8:00 PM). All experiments were performed during the light phase of the dark/light cycle. Animals were habituated to the experimental room and were handled daily for 1 wk before the experiments began. All animal procedures were conducted in accordance with the standard ethical guidelines (European Communities Directive 2010/63/UE) and were approved by the local ethical committees [Comitè Ètic d'Experimentació Animal, Parc de Recerca Biomedica de Barcelona (PRBB), Spain; Ethical Committee on Animal Care and Use of Rhineland-Palatinate, Germany; and the Committee on Animal Health and Care of INSERM and Ministry of Agriculture and Forestry, France]. The PRBB has an authenticated Public Health Service approved Animal Welfare Assurance (no. A5388-01) granted by the Office of Laboratory Animal Welfare of the National Institutes of Health. All behavioral data were obtained by experimental observers blinded to the experimental conditions.
Drugs and Treatments.
Cremophor-EL, AM6545, and anisomycin were purchased from Sigma. Rimonabant was kindly provided by Sanofi-Aventis Recherche. AM630 was purchased from Tocris Bioscience. Rimonabant and AM630 were dissolved in 5% (vol/vol) ethanol: 5% (vol/vol) cremophor-EL: 90% (vol/vol) saline. Anisomycin was dissolved in saline. AM6545 was dissolved in 0.26% DMSO [4.74% (vol/vol) ethanol: 5% (vol/vol) cremophor-EL: 90% (vol/vol) saline]. All drugs were administered intraperitoneally in a volume of 10 mL/kg. Rimonabant (1 mg/kg), AM6545 (1 mg/kg), and anisomycin (15 mg/kg) were administered 20 min before the exposure to the stressful stimuli. The doses of rimonabant, AM6545, and anisomycin used did not affect nociceptive responses measured in the hot-plate test or anxiety-like behavior measured in the elevated plus-maze test (Fig. S10).
Surgical Procedures.
Surgical procedures for local hippocampal administration and bilateral adrenalectomy are detailed in SI Materials and Methods.
Object-Recognition Task.
Object-recognition memory was assayed in the V-maze (Fig. S1) under dim light conditions as described previously (39). On day 1, mice were habituated to the empty maze for 9 min. On the second day mice were introduced in the maze, in which two identical objects were presented, for 9 min. For the memory test, mice were placed again in the V-maze, in which one of the familiar objects had been replaced by a novel object, at the indicated time points for a period of 9 min, and the total time spent exploring each of the two objects (novel and familiar) was recorded by an experimenter blind to the experimental conditions. Object exploration was defined as orientation of the nose toward the object at a distance of less than 2 cm. A discrimination index (DI) was calculated as the difference between the time spent exploring either the novel (Tn) or familiar (Tf) object divided by the total time spent exploring both objects: (Tn + Tf): DI = (Tn − Tf)/(Tn + Tf). A DI of 0 indicates no preference for either object, and a DI higher than 0.3 was considered to reflect memory retention for the familiar object. Long-term memory was assessed 24 h after the training session or as indicated. Drug administration and stress exposure were always performed after the training session to avoid possible intrinsic effects during the acquisition phase.
Stress Paradigms.
Footshock or tail suspension was applied in different groups of mice 20 min after the training session in the object-recognition memory task. For footshock stress, mice were placed for 150 s in a conditioning chamber (Panlab) with a stainless steel grid floor through which a single electric footshock was delivered (0.5 mA; 2 s). Mice were removed from the chamber 150 s after footshock. Control mice were handled exactly as the footshock-stressed mice but did not receive the footshock in the conditioning chamber. For tail-suspension stress, mice were individually suspended by the tail for 5 min at a height of 35 cm above a cushioned surface. The control/unstressed mice for this stress condition remained undisturbed in their home cages.
Behavioral Characterization of Mouse Lines.
Fear-conditioning paradigms for emotional memory, nociceptive responses to hot and cold stimuli, and details on the modified Irwin test and anxiety-like behavior are given in SI Materials and Methods.
Corticosterone and Adrenaline/Noradrenaline Measurement.
Plasma fractions were obtained from trunk blood samples recovered with EDTA (1 mM) and sodium metabisulphite (4 mM). Plasma corticosterone was measured by ELISA following the manufacturer’s instructions (IBL International). Plasma adrenaline and noradrenaline were measured by ELISA kits following the manufacturer’s instructions (Labor Diagnostika Nord).
Endocannabinoid Extraction and LC/Multiple Reaction Monitoring Quantification.
Hippocampal tissues were rapidly isolated at different time points after footshock stress, weighed, and frozen. Endocannabinoid quantification was then performed as detailed in SI Materials and Methods.
Real-Time Quantitative PCR.
Adrenal glands were removed, cleaned from adhered fat tissues, and rapidly frozen on dry ice for further processing as described in SI Materials and Methods.
Tissue Preparation for Immunofluorescence.
Mice were deeply anesthetized 90 min after stress exposure by intraperitoneal injection (0.2 mL/10 g of body weight) of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) before intracardiac perfusion with 4% (wt/vol) paraformaldehyde in 0.1 M Na2HPO4/NaH2PO4 buffer (PB), pH 7.5, delivered with a peristaltic pump at 19 mL/min for 3 min. Subsequently, brains and adrenal glands were extracted and postfixed in the same fixative solution for 4 h and were cryoprotected overnight at 4 °C in 30% (wt/vol) sucrose in PB. Coronal frozen sections (30 μm) of the dorsal hippocampus (coordinates relative to bregma: −1.22 mm to −1.82 mm) were obtained on a freezing microtome and were stored until used. Adrenal glands were processed in a cryostat (Leica) to obtain 7-μm-thick slices mounted on gelatin-coated glass slides.
Immunofluorescence.
Free-floating brain slices or glass slide-mounted adrenal gland slices were rinsed in PB. Brain slices were washed three times with PB; then coronal brain sections were incubated with 10 mM citrate buffer, pH 6.0, at 95 °C for 30 min. Afterwards, sections were blocked with 3% (vol/vol) normal goat serum and 0.3% Triton X-100 in PB (NGS-T-PB) at room temperature for 2 h and were incubated overnight in the same solution with the primary antibody to CB1 receptor (1:500 rabbit or 1:1,000 guinea pig; both from Frontier Science), DBH (1:500 rabbit; Merck-Millipore), or c-Fos (1:750 rabbit; Calbiochem) at 4 °C. The next day, after three rinses in PB, sections were incubated at room temperature with the secondary antibody to rabbit conjugated to Cy2 or Cy3 (1:500; Jackson ImmunoResearch) in NGS-T-PB for 2 h. After incubation, brain sections were rinsed and mounted immediately onto glass slides coated with gelatin. Mowiol was used as mounting medium.
Image Analysis.
Confocal images were obtained using a Leica TCS SP2 confocal microscope adapted to an inverted Leica DM IRBE microscope. Cy2 and Cy3 were excited with the 488-nm line of an argon laser and the 543-nm line of a green neon laser, respectively. Tissue sections were examined with a 40× or 63× oil-immersion objective. The images (eight-bit color; 1,024 × 1,024 pixels) were analyzed using ImageJ software.
Statistical Analysis.
Comparisons between groups were performed by Student’s t tests only when assessing two-group comparisons and by one-way, two-way, three-way, or repeated-measurement ANOVA, as appropriate, for multiple-group comparisons. Post hoc comparisons were performed by Student–Newman–Keuls test or Bonferroni test only when a significant main effect of one-way ANOVA or a significant interaction between the factors of two- and three-way ANOVA was revealed. All results are expressed as mean ± SEM. Differences were considered significant at P < 0.05. The statistical analysis was performed using the Statistical Package for Social Science program SPSS 19.0 (SPSS Inc.).
SI Materials and Methods
Animals.
Generation of DBH-CB1–KO mice.
Mice lacking the CB1 receptor in DBH-expressing neurons were generated using CB1 floxed mice and mice expressing Cre recombinase in DBH+ cells (Fig. S6). The mouse lines expressing Cre recombinase in DBH-expressing cells (DBH-Cre mice) under the regulatory sequences of the DBH (52) and the mouse line carrying CB1 floxed allele (53) were generated and described earlier. Experimental animals were mice lacking CB1 receptor in DBH-expressing neurons, CB1floxed/floxed;dbh-cre/WT mice (herein, DBH-CB1–KO mice), and the littermate WT controls, CB1floxed/floxed mice. Mice were in a predominant C57BL/6N background (more than seven backcrosses).
Generation of DBH-CB1–RS mice.
To express the CB1 receptor exclusively in DBH+ cells, the recently developed rescue approach was applied (32) whereby CB1 receptor function is suppressed globally in Stop-CB1 mice by a transcriptional Stop cassette flanked by two loxP sites and inserted into the 5′ UTR of the ORF-containing CB1 receptor exon. Crossing Stop-CB1 mice with Cre recombinase-expressing transgenic mice reactivated (i.e., rescued) CB1 receptor function only in Cre-expressing cells. Thus, DBH-CB1–RS mice were generated by mating Stop-CB1 mice with DBH-Cre mice (52), whereas WT equivalent controls (CB1-RS mice) were generated by crossing Stop-CB1 mice with the general Cre-deleter mouse line EIIa-Cre (Fig. S6) (32). The offspring produced were genotyped for the presence of Cre and the Stop cassette. Experimental animals were mice containing the Stop cassette, phenotypically representing a CB1-null allele, CB1stop/stop (Stop-CB1) mice; mice with CB1 receptor rescue in DBH-expressing cells, CB1stop/stop;dbh-cre/WT (herein, DBH-CB1–RS) mice; and mice with CB1 rescue in all cells (CB1-RS mice) (Fig. S6).
Surgical Procedures.
Bilateral adrenalectomy.
Mice were anesthetized by isoflurane inhalation [5% (vol/vol) isoflurane initially and then 3% (vol/vol) with oxygen for maintenance]. A small (1 cm) incision was made in the left and right flanks, and the adrenal glands were identified and removed from the surrounding tissue. Wounds were closed in two layers using 4/0 silk sutures (Alcon). Postoperatively, all animals were given access to 0.9% NaCl to ensure adequate salt balance. The experiments were resumed following a recovery period of 10 d.
Surgery for intrahippocampal microinjection.
Mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg) (Imalgene 500; Merial) and xylazine (10 mg/kg) (Rompun; Bayer) and were placed into a stereotaxic apparatus (David Kopf Instruments) with mouse adaptor and lateral ear bars. Mice were bilaterally implanted with 3-mm stainless steel guide cannulas (Plastics One) targeting the hippocampus with the following coordinates from bregma: anteroposterior, –1.8 mm; mediolateral, ±1.0 mm; dorsoventral, −1.3 mm. Guide cannulas were secured in place with dental cement. Mice were weighed daily and were allowed to recover for 7–10 d in individual cages before the beginning of the experiments.
Surgery and local hippocampal administration.
Mice underwent surgery for the bilateral implantation of guide cannulas. Rimonabant (1 μg/0.5 μL) or its vehicle was injected into the hippocampus using a peristaltic pump (PHD 22/2000 Syringe Pump Infusion; Harvard Apparatus) at a flow rate of 0.3 μL/min. The correct placement of the hippocampal cannulas was verified afterward by histological analysis.
Behavioral Characterization of Mouse Lines.
Fear-conditioning paradigms for emotional memory test.
WT, Stop-CB1, CB1-RS, and DBH-CB1–RS mice were footshocked in the conditioning chamber as described above (39), and context-recognition memory was measured 48 h after conditioning. On the test day, mice were observed for 5 min to measure freezing behavior (lack of movement except for respiration) when exposed to the same conditioning chamber.
DBH-CB1–KO mice and their WT controls were trained in conditioning chambers (Med Associates) with a shock intensity of 1 mA lasting 2 s that coterminated with a cue tone (9 kHz, 80 dB, 20 s). Twenty-four hours after conditioning mice were observed for 5 min to measure freezing behavior when exposed to the same conditioning chamber (context-recognition). Four hours after the context-recognition test, animals were exposed to the cue in a new context, and the initial freezing response to the tone was measured for 20 s.
Plantar test.
Heat hyperalgesia was evaluated by measuring paw-withdrawal latency in response to radiant heat with a plantar test apparatus (Ugo Basile). Mice were placed in Plexiglas boxes (20 cm high, 9 cm in diameter) positioned on a glass surface and were habituated to the environment for 1 h before testing. The mean paw-withdrawal latencies for right and left hind paws were determined from the average of three separate trials separated by intervals of 5–10 min to prevent thermal sensitization. A cut-off time of 20 s was used to prevent tissue damage.
Modified Irwin test.
Experimental mice were individually placed in a Plexiglas cylinder for 3 min to observe their appearance and behavior. Animal behavioral changes and neuromuscular, autonomic, and sensory-motor signs were recorded according to a standardized observation battery (55).
Hot plate test.
The hot-plate test was performed 25 min after drug or vehicle injection in the same animals evaluated in the elevated plus-maze. A glass cylinder was used to keep the animals on the heated surface of the plate, which was kept at a temperature of 52 ± 0.5 °C (Columbus Instruments). The nociceptive threshold was evaluated by measuring the licking and the jumping responses. A cut-off of 240 s was used to prevent tissue damage.
Elevated plus-maze test.
The elevated plus-maze test was performed as previously reported (39). Five-minute test sessions were performed 20 min after drug or vehicle injection.
Endocannabinoid Extraction and LC/Multiple Reaction Monitoring Quantification.
Endocannabinoid extraction.
Frozen brain tissues were transferred to extraction tubes containing cold steel beads. A spiking solution of deuterated endocannabinoids in acetonitrile was mixed with 0.1 M formic acid, which served as homogenization buffer, and was rapidly pipetted to the extraction tubes. Ethylacetate/hexane (9:1) for endocannabinoid extraction then was pipetted manually, and tissues were homogenized for 1 min. Homogenates were centrifuged at 5,000 × g, 4 °C, for 15 min and then were kept at −20 °C for 30 min to freeze the aqueous phase. The upper organic phase was recovered in microtiter plates and was evaporated, and the extracts were reconstituted in 50 µL water:acetonitrile (1:1). The amounts of internal standards and concentration range of calibration curves were tailored to those levels in the hippocampus.
LC/multiple reaction monitoring.
Twenty microliters of the solution of extracted endocannabinoids was injected and separated on a Phenomenex Luna 2.5 µm C18 (2)-HST column, 100 mm × 2 mm, combined with a precolumn (C18, 4 mm × 2 mm) (Phenomenex) by increasing acetonitrile containing 0.1% formic acid over 2 min from 55–90% (vol/vol) and maintaining it at 90% (vol/vol) for 5.5 min. Throughout the analysis samples were maintained at 4 °C in the LC autosampler. The separated endocannabinoids were flow-through analyzed using multiple reaction monitoring on a 5500 QTRAP triple-quadrupole linear ion trap mass spectrometer equipped with a Turbo V Ion Source (AB Sciex). Positive and negative ions were analyzed simultaneously using the positive–negative switching mode. The following multiple reaction monitoring transitions were monitored for quantification of endocannabinoids: AEA, m/z 348.3 to m/z 62.3; AEA-d4, m/z 352.3 to m/z 62.1; 2-AG, m/z 379.1 to m/z 287.2; 2-AG-d5, m/z 384.1 to m/z 287.2; AA, m/z 303.05 to m/z 259.1; and AA-d8, m/z 311.04 to m/z 267.0. Calibration solutions were spiked with a mixture of deuterated endocannabinoids and were run in triplicate. The endocannabinoid concentrations were normalized to the tissue weights.
Real-Time Quantitative PCR.
Adrenal medulla was separated from the cortex under binocular microscope using microscissors, frozen immediately on dry ice, and stored frozen at −80 °C. Total RNA was extracted from tissues using TRIzol (Invitrogen) and cleaned further using the RNeasy kit (Qiagen). cDNA was synthesized from total RNA using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative real-time PCR was run using Taqman probes for CB1 receptor and DBH (Applied Biosystems), and relative mRNA levels were calculated by the comparative cycle threshold (Ct) method normalized to the DBH mRNA level.
Supplementary Material
Acknowledgments
We thank Gal Raz for critical reading of the manuscript; Cristina Fernández-Avilés and Dulce Real for expert technical assistance; and the Laboratori de Neurofarmacologia-NeuroPhar for helpful discussions. We acknowledge a predoctoral fellowship from the Spanish Ministry of Education and a postdoctoral fellowship from the Investments for the Future Programme, Initiative of Excellence Bordeaux, ANR-10-IDEX-03-02 (to A.B.-G.); a postdoctoral fellowship from the Marie Curie action Seventh Framework Programme for Research and Technological Development, FP7-PEOPLE-2013 (to A.B.-G.); the partial support by FRAXA Research Foundation (M.G.-G.); a European Molecular Biology Organization postdoctoral fellowship (to L. Bellocchio); grants from Ministerio de Ciencia e Innovación [BFU2012-33500 and BFU2015-68568P (to A.O.), and SAF2014-59648-P (to R.M.) (MINECO/FEDER, UE)]; Instituto de Salud Carlos III Grant RD06/0001/0001 (to R.M.); Generalitat de Catalunya Grant SGR-2014-1547 (to R.M.); ICREA (Institució Catalana de Recerca i Estudis Avançats) Academia (R.M.); the German Research Council DFG Grants FOR926 (Core Unit Endocannabinoid Measurements) (to B.L.), CRC-TRR58 (to B.L.), and CRC1080 (to B.L.); EU-FP7 Grant REPROBESITY, HEALTH-F2-2008-223713 (to B.L. and G.M.); PainCage Grant HEALTH-2013-INNOVATION-1-603191 (to G.M.); INSERM (G.M.); European Research Council Grant ENDOFOOD, ERC–2010–StG–260515 (to G.M.); Fondation pour la Recherche Medicale (G.M.); Region Aquitaine (G.M.); and LABEX BRAIN Grant ANR-10-LABX-43 (to G.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525066113/-/DCSupplemental.
References
- 1.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125(6):797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neurosci Biobehav Rev. 2012;36(7):1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends Cogn Sci. 2006;10(4):152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 6.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol Rev. 2009;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45(4):292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King SO, 2nd, Williams CL. Novelty-induced arousal enhances memory for cued classical fear conditioning: Interactions between peripheral adrenergic and brainstem glutamatergic systems. Learn Mem. 2009;16(10):625–634. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- 10.Miyashita T, Williams CL. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral beta-adrenergic receptors. Neurobiol Learn Mem. 2006;85(2):116–124. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Gazarini L, Stern CA, Carobrez AP, Bertoglio LJ. Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting α1- and β-adrenergic receptors. Learn Mem. 2013;20(4):210–219. doi: 10.1101/lm.030007.112. [DOI] [PubMed] [Google Scholar]
- 12.Furini CR, et al. Beta-adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory. Hippocampus. 2010;20(5):672–683. doi: 10.1002/hipo.20656. [DOI] [PubMed] [Google Scholar]
- 13.Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14(4):384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- 14.Zanettini C, et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16(12):705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41(1):80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busquets-Garcia A, et al. Dissecting the cannabinergic control of behavior: The where matters. BioEssays. 2015;37(11):1215–1225. doi: 10.1002/bies.201500046. [DOI] [PubMed] [Google Scholar]
- 19.Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Ishac EJ, et al. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederhoffer N, Hansen HH, Fernandez-Ruiz JJ, Szabo B. Effects of cannabinoids on adrenaline release from adrenal medullary cells. Br J Pharmacol. 2001;134(6):1319–1327. doi: 10.1038/sj.bjp.0704359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter H, et al. Presynaptic α₂-adrenoceptors control the inhibitory action of presynaptic CB₁ cannabinoid receptors on prefrontocortical norepinephrine release in the rat. Neuropharmacology. 2012;63(5):784–797. doi: 10.1016/j.neuropharm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Akirav I. Cannabinoids and glucocorticoids modulate emotional memory after stress. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2554–2563. doi: 10.1016/j.neubiorev.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Hill MN, et al. Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. J Neurosci. 2010;30(45):14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner MA, et al. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33(1):54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellocchio L, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB₁ receptor blockade. Proc Natl Acad Sci USA. 2013;110(12):4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120(8):2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monory K, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51(4):455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellocchio L, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13(3):281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- 30.Dubreucq S, et al. Cannabinoid type 1 receptors located on single-minded 1-expressing neurons control emotional behaviors. Neuroscience. 2012;204:230–244. doi: 10.1016/j.neuroscience.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 31.Häring M, et al. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front Behav Neurosci. 2015;9:235. doi: 10.3389/fnbeh.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruehle S, et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: Distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci. 2013;33(25):10264–10277. doi: 10.1523/JNEUROSCI.4171-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7(5):703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- 34.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quevedo J, et al. Differential effects of emotional arousal in short- and long-term memory in healthy adults. Neurobiol Learn Mem. 2003;79(2):132–135. doi: 10.1016/s1074-7427(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 36.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchoa ET, et al. Novel aspects of glucocorticoid actions. J Neuroendocrinol. 2014;26(9):557–572. doi: 10.1111/jne.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morena M, et al. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc Natl Acad Sci USA. 2014;111(51):18333–18338. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busquets-Garcia A, et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70(5):479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handbook Exp Pharmacol. 2005;1(168):327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, et al. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26(1):56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16(12):1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- 44.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Uchigashima M, et al. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31(21):7700–7714. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirilly E, Hunyady L, Bagdy G. Opposing local effects of endocannabinoids on the activity of noradrenergic neurons and release of noradrenaline: Relevance for their role in depression and in the actions of CB(1) receptor antagonists. J Neural Transm (Vienna) 2013;120(1):177–186. doi: 10.1007/s00702-012-0900-1. [DOI] [PubMed] [Google Scholar]
- 47.Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: Relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cathel AM, et al. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci. 2014;40(8):3202–3214. doi: 10.1111/ejn.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;21(3):452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Ul Haq R, et al. Adrenergic modulation of sharp wave-ripple activity in rat hippocampal slices. Hippocampus. 2012;22(3):516–533. doi: 10.1002/hipo.20918. [DOI] [PubMed] [Google Scholar]
- 51.Ledent C, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283(5400):401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 52.Parlato R, Otto C, Begus Y, Stotz S, Schütz G. Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development. 2007;134(9):1663–1670. doi: 10.1242/dev.02838. [DOI] [PubMed] [Google Scholar]
- 53.Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 54.Soria-Gómez E, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17(3):407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 55.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacology (Berl) 1968;13(3):222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.