Significance
The intracellular and extracellular trafficking of heme, a hydrophobic and potentially cytotoxic cofactor in proteins such as hemoglobin, remains an underexplored area. While cellular heme can be derived exogenously or from de novo synthesis, it is unclear if there is differential trafficking of heme from these two sources. To critically examine this possibility, we developed peroxidase-based enzymatic reporters for heme and deployed them in subcellular compartments in mammalian cells and in several tissues in the Caenorhabditis elegans animal model. Our results show that extracellular and endogenous heme is trafficked to virtually all intracellular compartments via distinct cellular routes and that interorgan heme transport is essential for systemic regulation of heme homeostasis in C. elegans.
Keywords: heme, tetrapyrroles, iron, trafficking, subcellular
Abstract
Heme is an essential prosthetic group in proteins that reside in virtually every subcellular compartment performing diverse biological functions. Irrespective of whether heme is synthesized in the mitochondria or imported from the environment, this hydrophobic and potentially toxic metalloporphyrin has to be trafficked across membrane barriers, a concept heretofore poorly understood. Here we show, using subcellular-targeted, genetically encoded hemoprotein peroxidase reporters, that both extracellular and endogenous heme contribute to cellular labile heme and that extracellular heme can be transported and used in toto by hemoproteins in all six subcellular compartments examined. The reporters are robust, show large signal-to-background ratio, and provide sufficient range to detect changes in intracellular labile heme. Restoration of reporter activity by heme is organelle-specific, with the Golgi and endoplasmic reticulum being important sites for both exogenous and endogenous heme trafficking. Expression of peroxidase reporters in Caenorhabditis elegans shows that environmental heme influences labile heme in a tissue-dependent manner; reporter activity in the intestine shows a linear increase compared with muscle or hypodermis, with the lowest heme threshold in neurons. Our results demonstrate that the trafficking pathways for exogenous and endogenous heme are distinct, with intrinsic preference for specific subcellular compartments. We anticipate our results will serve as a heuristic paradigm for more sophisticated studies on heme trafficking in cellular and whole-animal models.
Heme is an essential but toxic macrocycle (1, 2) that serves as a protein prosthetic group and signaling molecule. As a cofactor, it enables diverse functions that include electron transfer, chemical catalysis, and gas binding/transport. As a signaling molecule, heme regulates the antioxidant response, circadian rhythms, microRNA processing, and cell differentiation and proliferation (3–9). All heme-dependent processes require that heme is trafficked from its site of synthesis in the mitochondria or its point of entry into the cell, to distinct hemoproteins located in numerous subcellular compartments. Further, heme transfer between cells and tissues is required for systemic heme homeostasis and organismal development (10–15). Given that free heme is cytotoxic and hydrophobic (16, 17), the trafficking of heme for insertion and/or signaling is likely coordinated between heme transporters, chaperones, and carrier proteins (1).
Genetically engineered heme proteins have been developed in bacteria to study heme transport and coordination between heme proteins, i.e., cytochrome biosynthesis (18, 19). By using natural or engineered heme synthesis-deficient bacterial strains, researchers previously showed exogenous heme is directly incorporated into heme proteins (20, 21). However, such tools are not available to probe how mammalian cells handle heme, especially because hemoproteins reside in several subcellular membrane-enclosed compartments (1). Regardless of whether heme is synthesized in the mitochondria or imported from the environment, heme has to be translocated across membrane barriers (22, 23). It has been suggested that the majority of extracellular heme is degraded through the heme oxygenase pathway to extract iron from the porphyrin in mammalian cells (22, 24–28). Therefore, it is of significant interest to investigate whether exogenous heme is ever used in toto for cellular hemoproteins in animals and if there is a hierarchical preference by subcellular compartments to use one form of heme over another, i.e., from de novo synthesis or from exogenous sources.
Although the identity of heme transporters and trafficking factors has seen some headway due to genetic studies in model systems within the past decade (10, 13, 14, 29, 30), the lack of proper physical tools to probe heme availability and trafficking at the cell biological level has greatly hindered our progress in understanding the intricacies of cellular and organismal heme homeostasis. To overcome this obstacle, we developed peroxidase-based enzymatic reporters for heme and deployed them in subcellular compartments in mammalian cell lines and in several tissues in the Caenorhabditis elegans animal model. Our results show that extracellular heme is used intact for incorporation into hemoproteins in virtually all intracellular compartments and that endogenous and exogenous heme trafficking is mediated by distinct cellular pathways. Furthermore, genetic studies in C. elegans validate the utility of these hemoprotein reporters and demonstrate that interorgan heme transport is essential for organismal heme homeostasis.
Results
Subcellular Targeting of Peroxidase Reporters.
To monitor labile heme that is trafficked to various subcellular organelles, we needed to first develop appropriate reporters that would measure heme levels uniformly, irrespective of the intracellular membrane compartment. More importantly, these reporters should have suitable affinity for heme. We used genetically encoded peroxidase-based enzymatic reporters because horseradish peroxidase (HRP) activity is dependent on a heme prosthetic group, and it can also be reconstituted into an active form in vitro using the apoprotein and heme (31) (Fig. 1A). The HRP–heme dissociation constant (Kd) was determined by difference absorption spectroscopy to be 270 ± 40 nM, similar to previously reported 155 ± 27 nM using surface plasmon resonance technique (32) (SI Appendix, Fig. S1A). This value is weaker than constituent heme proteins and enzymes (e.g., globins, cytochromes, and catalase) that have heme affinity ranges between 10−12 and 10−15 M (33), making HRP an ideal reporter for labile heme. Studies have used purified apoHRP to bind bioavailable heme in cell-free extracts as a way to measure regulatory heme (34–38). This in vitro HRP reconstitution method was found to be more sensitive than the traditional pyridine hemochromogen method (39), with minimal interference from fluorescent porphyrins (40). However, a major drawback for this method was that the biological material must first be disrupted and then mixed with apoHRP to measure the conversion of apo to holo, thereby preventing analysis of subcellular heme distribution (34, 35).
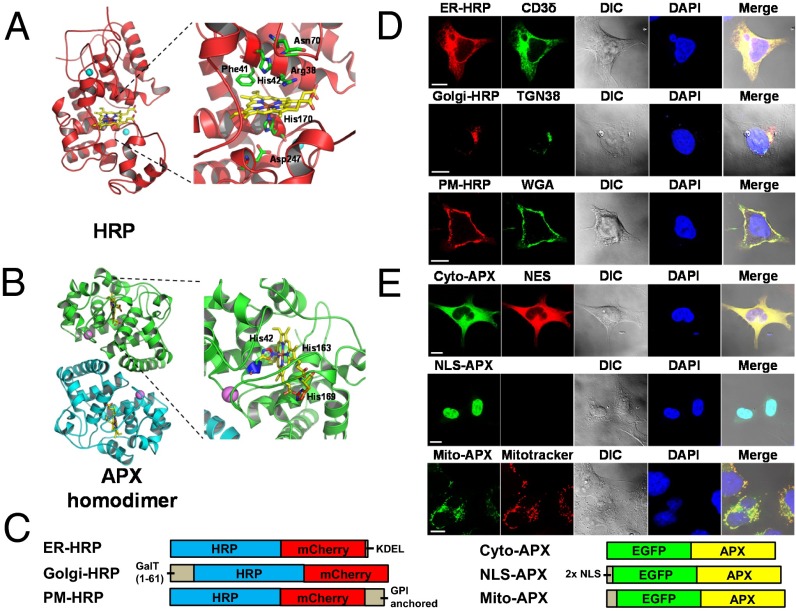
Fig. 1.
Synthesis of peroxidase-based reporters for interrogating intracellular labile heme. (A) Structure and heme binding sites of HRP [from Protein Data Bank (PDB) ID code 1H5A (91)] and (B) APX [from PDB ID code 1APX (42)]. The heme cofactor is shown in yellow. Residues involved in heme binding are indicated and displayed with side chains. APX forms a noncovalent homodimer with each monomer binding one heme. (C) HRP and APX reporters were fused to mCherry and EGFP, respectively. The sorting signals used for intracellular targeting are described in Materials and Methods. (D) Localization of targeted HRP and (E) APX reporters in HEK293 cells. CD3δ-EGFP (CD3δ) (92, 93), EGFP-TGN38 (TGN38) (94), Alexa-Fluor-488–conjugated wheat germ agglutinin (WGA), nuclear export sequence fused mCherry (NES), DAPI, and MitoTracker Red were used as colocalization markers for ER, Golgi, plasma membrane (PM), cytosol (Cyto), nucleus (NLS), and mitochondrial matrix (Mito), respectively. HEK293 cells were fixed 42 h posttransfection, counterstained with DAPI, and imaged using a Zeiss LSM710 confocal microscope under a 63× oil immersion objective. (Scale bar, 10 μm.)
HRP variants were created by using specific subcellular targeting sequences to direct the expressed protein to the cytosol, mitochondrial matrix, peroxisome, endoplasmic reticulum (ER), Golgi complex, and plasma membrane (PM; GPI anchored) (SI Appendix, Table S1). To ensure the correct targeting of these genetically engineered probes, HRP variants were tagged with mCherry at the carboxyl-terminus (Fig. 1 C and D and SI Appendix, Fig. S2A). However, only the ER, Golgi, and PM-targeted HRP was found to be active (SI Appendix, Fig. S2B). The nonactive HRP fusions migrated faster than the active forms and were detectable by anti-mCherry antibodies but not with antibodies generated against holo-HRP (SI Appendix, Fig. S2 C and D), suggesting that this class III peroxidase, which is glycosylated, requires the secretory pathway for proper folding (41). To generate reporters for the remaining compartments, we used the cytosolic ascorbate peroxidase (APX), a class I heme-containing homodimer (42, 43) (Fig. 1 B and C). The APX–heme Kd was determined to be 360 ± 40 nM, a value comparable to HRP (SI Appendix, Fig. S1B).
Expression of both proteins in mammalian HEK293 cells found them to colocalize with the expected subcellular markers using confocal microscopy (Fig. 1 D and E and SI Appendix, Fig. S3). In addition, targeting APX to the ER showed comparable expression levels to other APX probes, demonstrating that ER-HRP could be substituted by ER-APX in the secretory pathway (SI Appendix, Fig. S4). Thus, HRP and APX are both suitable as subcellular reporters for measuring labile heme.
Subcellular Labile Heme Dictates Reporter Activity.
We tested the activity of our genetically targeted probes as a function of heme concentrations in HEK293 cells. Cells expressing engineered HRP exhibited 2,000- to 11,000-fold greater peroxidase activity (Fig. 2A). By contrast, APX reporters showed 50- to 60-fold greater activity. Activity was not detected in a monomeric version of APX (APEX) or a mutant in which the active site was mutated to mimic HRP (APXH; Fig. 2A) (44). In situ histochemical staining detected functional HRP and APX activities in all six subcellular compartments (Fig. 2B).
Fig. 2.
Subcellular labile heme dictates reporter activity. (A) HEK293 cells grown at basal condition were harvested 42 h after transfection with engineered HRP/APX constructs and assayed for peroxidase activity by adding o-dianisidine and H2O2 as the substrates. Peroxidase activity was normalized for total cellular protein content. APEX, humanized monomeric version of APX; APXH, humanized mutant in which the active site has been mutated to mimic HRP. Error bars represent the SEM from three biological independent experiments. ns, nonsignificant; *P < 0.05, **P < 0.01, ***P < 0.001. (B) Reporter transfected HEK293 cells grown at basal condition were fixed 42 h posttransfection with 4% PFA and stained for in situ peroxidase activity. Brightfield images were taken on a Leica DMIRE2 microscope using 63× oil immersion lens. (Scale bar, 10 μm.) (C) Transfected HEK293 cell lysates were analyzed by in-gel peroxidase activity staining of native PAGE gel (Upper Left), immunoblotting of native PAGE gel with anti-EGFP antibody (Upper Right), in-gel activity staining of SDS/PAGE gel (Lower Left), or immunoblotting of SDS/PAGE gel with anti-EGFP antibody (Lower Right). (D) Transfected HEK293 cells were deprived of heme for 24 h followed by heme supplementation for the indicated heme concentrations for 18 h. Cell lysates were analyzed by in-gel DAB staining and immunoblotting with anti-EGFP antibody on native PAGE gels (circles indicate inactive APX monomers; triangles indicate active APX dimers).
The large signal-to-background ratio observed for our peroxidase reporters could provide sufficient range to detect changes in intracellular labile heme. Because labile heme has been estimated by mixing purified apoHRP with cell lysate in vitro (38), it is possible that our genetically targeted peroxidase reporters may inadvertently bind heme released during cell lysis and not authentically reflect subcellular labile heme. To evaluate this possibility, we depleted intracellular heme levels by removing exogenous sources of heme from the media by using heme-depleted serum (HD) and treating the cells with succinylacetone (SA), an inhibitor of heme synthesis that blocks the formation of porphobilinogen (PBG). Consequently, apo-reporters formed by heme depletion in cellula (ER-HRP and cyto-APX were chosen as representatives) were able to be reconstituted into holoenzymes by supplementation of the cell lysate with hemin (SI Appendix, Fig. S5 A and B) but not with lysates from HEK293 cells preloaded with heme (SI Appendix, Fig. S5 C–F). These results show that the genetically encoded reporters do not steal heme bound by other hemoproteins after cell lysis.
Native HRP is a monomeric glycoprotein that only dimerizes when expressed as a nonglycosylated catalytically active protein in Escherichia coli. The equilibrium between monomeric and dimeric recombinant HRP is affected by the peroxidase substrates (45, 46). By contrast, APX is not glycosylated and forms a noncovalent homodimer in a concentration-dependent manner (47, 48). Because the monomer–dimer equilibria of APX could interfere with our interpretation of changes in labile heme, we sought to characterize the active APX species in our cell-based assay conditions (48). We analyzed targeted APX reporters expressed in mammalian cells using an in-gel peroxidase activity assay and immunoblotting. APX reporters were active on native PAGE but not on SDS/PAGE (Fig. 2C, Left). APX reporters migrated as monomers and dimers on native PAGE gels and disassociated into monomers on SDS/PAGE (Fig. 2C, Right). Monomeric mutant APEX migrated exclusively as a monomer on both native and SDS/PAGE (Fig. 2C, lanes 4 and 9); by contrast, APXH migrated exclusively as a dimer on native PAGE and disassociated into monomers on SDS/PAGE (Fig. 2C, lanes 5 and 10). Furthermore, immunoblotting revealed that the active APX on native PAGE corresponded to dimers, whereas the monomers had no detectable activity (Fig. 2C).
To further investigate the effect of heme on the stability of APX dimers, we examined APX reporters expressed in HEK293 cells grown in different heme concentrations. In HD + SA conditions, APX migrated almost exclusively in its monomeric form (Fig. 2D, lane 4). Heme supplementation resulted in gradual conversion of APX from a monomer to a dimer on native PAGE. At 16 μM heme, the majority of APX in the cytoplasm, nucleus, and mitochondria was active and migrated as a dimer (Fig. 2D, lane 8). These results clearly demonstrate that APX can be converted from apo to holo by heme incorporation from extracellular sources.
De Novo Synthesis and Extracellular Heme Influences Labile Heme.
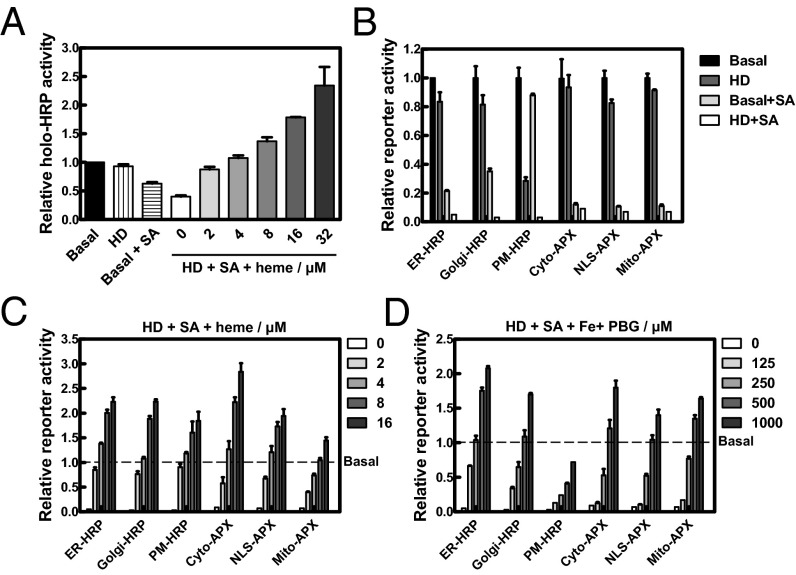
We sought to determine the source of labile heme by first creating a cellular heme deficiency condition using HD + SA. Total labile heme in HEK293 cells was assessed by mixing the cell lysates with commercially available apoHRP. The HD condition by itself only had a modest effect on intracellular labile heme, whereas cells treated with succinylacetone reduced labile heme by one-third (Fig. 3A). Furthermore, the combination of HD + SA decreased labile heme to 40% of basal values and could be fully restored by supplementation with 2 µM exogenous heme (Fig. 3A). Indeed, HPLC measurements of total cellular heme confirmed that HD + SA decreased total heme by 60–70% of basal levels (SI Appendix, Fig. S6A and Table S2). Moreover, heme supplementation resulted in a greater net change in total cellular heme in heme-depleted cells compared with cells grown in basal medium (SI Appendix, Fig. S6 B and C). The labile heme concentration in HEK293 cells as measured by apoHRP in basal condition was found to be 433 ± 125 nM (SI Appendix, Fig. S6D).
Fig. 3.
Intracellular labile heme is influenced by both endogenous and exogenous heme. (A) HEK293 cells were deprived of heme for 24 h followed by repletion with indicated concentrations of heme for 18 h. Cells were harvested, and total labile heme in the lysates was analyzed by reconstituting purified apoHRP activity using o-dianisidine and H2O2 as the substrates. (B) Transfected HEK293 cells were heme deprived for 24 h, treated with 0.1 mg/mL cycloheximide (CHX) for 30 min, and then grown at the conditions indicated in B or exposed to various concentrations of (C) heme or (D) PBG plus 0.1 mM Fe:NTA for additional 18 h in the presence of 0.1 mg/mL CHX. Cells were lysed and assayed for peroxidase activity using o-dianisidine. In cellula peroxidase activity was calculated by subtracting blank readings at baseline (A600) and activity (A440) and then normalized to assay time, followed by a second normalization for reporter expression levels using fluorescence measurements (RFU) from the GFP/mCherry tags or total protein (TP) . Reporter activity of different growth conditions was baseline corrected for activity observed in basal medium (dashed line). Error bars represent SEM from three biological independent experiments.
The incomplete depletion of intracellular heme by the HD + SA combination treatment could be because either cellular labile heme can be reduced only to a certain threshold or cells may require longer depletion periods (>40 h). Moreover, there is the possibility that an existing pool of endogenous labile heme persists even after HD + SA treatment, and imported exogenous heme triggers the release of an existing intracellular heme pool that gets incorporated into the reporter proteins. To address this question, we grew HEK293 cells in the presence of HD + SA and supplemented with metalloporphyrins as a surrogate for exogenous heme. Compared with other metalloporphyrins, swapping manganese protoporphyrin IX (MnPPIX) for heme caused APX to migrate as dimers, but these MnPPIX–APX dimers lacked any detectable enzymatic activity (SI Appendix, Fig. S7 A–C). Even though MnPPIX–APX dimers form stable complexes as heme–APX dimers (SI Appendix, Fig. S7D), increasing the concentrations of exogenous MnPPIX (2–16 µM) did not trigger heme release from a preexisting heme pool, because APX showed no activity at any of the MnPPIX concentrations (SI Appendix, Fig. S7 E and F). These results confirm that exogenous heme does not mobilize residual cellular heme stores for incorporation into APX.
We next determined the effect of intracellular heme perturbation on subcellular heme trafficking using our reporters. Unlike the in vitro reconstitution of apoHRP, genetically encoded HRP and APX activities were undetectable in HD + SA (Fig. 3B). The heme depletion was highly effective across all six intracellular compartments and persisted even in the presence of iron supplementation (SI Appendix, Fig. S8). PM-HRP activity was the only reporter that was significantly reduced by HD alone, whereas the remaining reporters showed greatly reduced activity only when heme synthesis was inhibited by succinylacetone (Fig. 3B). To determine whether the reporters interrogate subcellular labile heme, cells were first grown in the presence of HD + SA so they accumulated sufficient apo-peroxidase, followed by cycloheximide (CHX) treatment to inhibit new reporter synthesis. Supplementation with 4 µM heme to these cells fully restored peroxidase activity in all six compartments as assessed by in situ DAB staining (SI Appendix, Fig. S9). Moreover, restoration of reporter activity by exogenous heme appeared to be compartment-specific with the mitochondrial matrix requiring twice as much heme to fully restore reporter activity to basal conditions (Fig. 3C). A different preference was observed when PBG was supplemented to the grown medium to bypass the succinylacetone block. Under these conditions, 500 µM PBG was sufficient to restore the reporter activity to basal conditions in all compartments except the PM-HRP, which was engineered with a GPI anchor, and the active site faces the extracellular milieu (Fig. 3D). PBG supplementation restored reporter activity in the following order of ER > Golgi ≈ mitochondria ≥ cytoplasm ≈ nucleus > PM, suggesting that the secretory pathway may play a significant role in the trafficking of mitochondria-derived heme. Together, these results indicate that both exogenous and endogenous heme contribute to cellular labile heme and that exogenous heme can be used intact by hemoproteins.
Exogenous and Endogenous Heme Are Trafficked by Distinct Pathways.
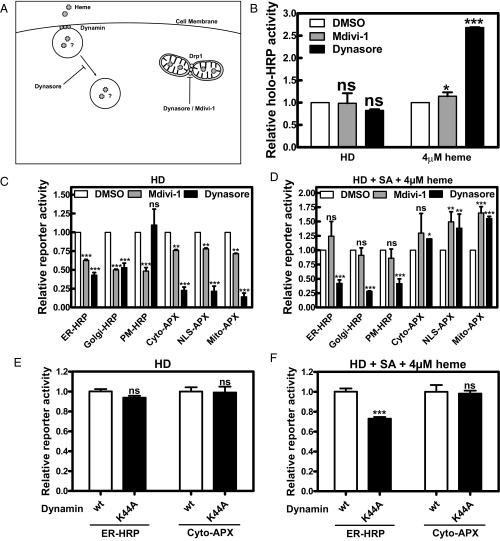
To differentiate how exogenous versus endogenous heme is transported and trafficked, we used Dynasore, a small molecule GTPase inhibitor that targets dynamin (49) (Fig. 4A). Treatment of Dynasore efficiently diminished uptake of fluorescent transferrin, a standard probe of clathrin-mediated endocytosis (50) (SI Appendix, Fig. S10A). Little or no effect was observed on HRP or APX activities when Dynasore was mixed with cells either after lysis or when cells were lysed immediately after Dynasore treatment (SI Appendix, Fig. S11 A and B). To restrict the source of intracellular heme, we grew the cells in either HD or HD + SA + 4 µM heme because both conditions have similar levels of intracellular heme (SI Appendix, Fig. S6). Total labile heme measured by in vitro reconstitution of apoHRP activity showed no difference between control and inhibitor treated cells under HD condition (Fig. 4B) but showed a significantly greater HRP activity in the presence of Dynasore and 4 µM heme (Fig. 4B).
Fig. 4.
Endogenous and exogenous heme trafficking are differentially allocated at the subcellular level. (A) Illustration of pathways affected by Dynasore and Mdivi-1. (B) Untransfected HEK293 cells were deprived of heme for 24 h, repleted with either endogenous heme (HD medium) or exogenous heme (HD + SA + 4 µM heme) in the presence of 80 µM dynasore or 50 µM Mdivi-1 for 18 h, and harvested, and total labile heme in the lysates was analyzed by apoHRP reconstitution assay. For inhibitor assays, transfected HEK293 cells were heme deprived for 24 h, treated with 0.1 mg/mL CHX for 30 min, and repleted with either endogenous heme (HD medium; C) or exogenous heme (HD + SA + 4 µM heme; D) in the presence of 0.1 mg/mL CHX and 80 µM Dynasore or 50 µM Mdivi-1 for 18 h before measuring peroxidase activity. For dynamin coexpression assays, cotransfected HEK293 cells were heme deprived for 24 h, treated with 0.1 mg/mL CHX for 30 min, and repleted with either endogenous heme (HD medium; E) or exogenous heme (HD + SA + 4 µM heme; F) in the presence of 0.1 mg/mL CHX for 18 h before measuring peroxidase activity. In cellula peroxidase activity was normalized as in Fig. 3. Reporter activity of different treatments was baseline corrected for activity observed with DMSO condition. Error bars represent SEM from three biological independent experiments. ns, nonsignificant; *P < 0.05, **P < 0.01, ***P < 0.001.
We next examined the effects of these inhibitors on heme trafficking and compartmentalization using HRP/APX reporters. Even though the overall labile heme had not changed in the HD conditions, with the exception of PM-HRP, all other reporters showed a significant reduction in activity with Dynasore (Fig. 4C and SI Appendix, Fig. S11C). This reduction in endogenous heme compartmentalization could be because Dynasore also disrupts the mitochondrial dynamin Drp1 and consequently interferes with mitochondrial fission and formation of the ER–mitochondria encounter structure (ERMES) (49, 51). Surprisingly, cells supplemented with exogenous heme in the presence of Dynasore showed full restoration of cytoplasmic, nuclear, and mitochondrial reporter activities but not for the ER, Golgi, and PM reporters (Fig. 4D and SI Appendix, Fig. S11D). To differentiate the effect of Dynasore on endosomal dynamin from mitochondrial dynamin, we used Mdivi-1, a small molecule inhibitor that specifically blocks Drp1 function (52). Reporter activities in all compartments were significantly decreased in Mdivi-1 treated cells only in HD conditions (Fig. 4C), and exogenous heme supplementation reversed this effect (Fig. 4D). This result is consistent with the finding that Mdivi-1 does not impair clathrin-mediated endocytosis (SI Appendix, Fig. S10A). Because Dynasore has been reported to impair not only clathrin-mediated endocytosis, but also fluid-phase endocytosis (53), we used a dominant-negative dynamin mutant (K44A) that blocks clathrin-mediated endocytosis (54–56). Expression of dynamin K44A in HEK293 cells inhibited uptake of fluorescent transferrin (SI Appendix, Fig. S10B) and specifically reduced ER-HRP but not cyto-APX activity in the presence of exogenous heme (Fig. 4 E and F). These results further support our proposition that subcellular trafficking of endogenous and exogenous heme occurs via distinct pathways.
Measuring Heme Homeostasis in Vivo.
To measure subcellular heme in various organs within an intact animal, we exploited C. elegans because it is a heme auxotroph. Heme levels can be experimentally manipulated by either nutritional supplementation or genetically regulating the heme trafficking pathways mediated by HRGs (13, 14, 29, 30, 57). We generated transgenic worms expressing HRP and APX reporters under the control of tissue-specific promoters. Transgenic worms expressing the HRP reporters showed greater activity, so we characterized worms expressing ER-HRP driven from the intestinal (Pvha-6), hypodermal (Pdpy-7), muscle (Pmyo-3), and pan-neuronal (Punc-119) promoters (Fig. 5A and SI Appendix, Fig. S12).
Fig. 5.
Tissue-specific peroxidase reporters in C. elegans show regulation of heme trafficking. (A) Tissue-specific expression of reporters in transgenic worms. Live young adult worms on OP50 seeded NGM plates were imaged using a Leica MZ16FA fluorescent stereomicroscope. (Scale bar, 50 μm.) (B) Synchronized L1 larvae were grown in mCeHR-2 axenic liquid medium supplemented with various concentrations of heme (x axis), harvested at young adult stage, lysed, and subjected to labile heme and reporter activity analysis using o-dianisidine and H2O2 as the substrates. Total labile heme (black circles, right y axis) was determined by reconstituting purified apoHRP activity. Reporter activity in different tissues (left y axis) was baseline corrected for activity observed at 1.5 μM heme. (C) Synchronized L1 larvae carrying the transgene for the heat shock inducible reporter (Phsp-16.2::ER-HRP-mCherry) were grown in mCeHR-2 axenic medium supplemented with different concentrations of heme at 20 °C. Worms were heat-shocked for 30 min at 37 °C, allowed to recover at 20 °C for various lengths of time, and harvested, and the lysates were analyzed for peroxidase activity. (D) Synchronized transgenic L1 larvae were exposed to RNAi by feeding HT115 bacteria expressing dsRNA against control vector, hrp, and mrp-5; harvested at young adult stage; lysed; and subjected to peroxidase activity assay. Peroxidase activity was normalized by RFU. Error bars represent SEM from three biological independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Wild-type (WT) worms were grown in mCeHR2 liquid axenic culture supplemented with various concentrations of heme, and total worm homogenates were incubated with apoHRP to measure labile heme (57). Although there was a direct correlation between apoHRP reconstitution and increasing heme (R2 = 0.996), at concentrations ≥100 µM the ratio of HRP activity to heme was almost 5:1 (slope = 4.9), indicating that a significant portion of heme was present as labile heme under these conditions (SI Appendix, Fig. S13A).
Transgenic worms expressing ER-HRP showed a heme-dependent increase in activity that was tissue dependent (intestine > hypodermis > neuron > muscle; SI Appendix, Fig. S13B). This variation in activity could be due to intrinsic differences in either tissue heme levels or transgene expression. We assessed the influence of variations in transgene expression by comparing two transgenic strains that express ER-HRP at different levels in the muscle (SI Appendix, Fig. S14 A and B). The high-expression strain showed greater than fivefold greater activity at 20 μM heme compared with the low-expression strain (SI Appendix, Fig. S14C). However, when HRP activity at various heme concentrations was normalized to activity at 1.5 μM, the lowest concentration of heme that permits worm growth, no discernable differences were observed in both transgenic strains in their response to environmental heme (SI Appendix, Fig. S14D). This result suggests that the effect of transgene expression levels can be minimized by appropriate baseline correction.
Worms expressing intestinal HRP showed a linear increase in activity at concentrations ≥50 µM heme, although the magnitude was not as great as apoHRP reconstitution by labile heme (Fig. 5B). Activity of HRP reporters expressed from extraintestinal tissues plateaued at much lower heme concentrations, typically around 20 μM. Neuronal HRP exhibited only modest changes in activity over a broad range of heme concentrations. Worms grown at 500 μM heme showed a 10-fold increase in neuronal HRP activity compared with worms grown at 1.5 μM (Fig. 5B), indicating that the neuronal threshold for heme is lower than nonneuronal tissues.
We next assessed whether heme incorporation into HRP could be regulated in a controlled manner by expressing ER-HRP-mCherry from the heat shock inducible promoter (Phsp-16.2). HRP was transiently expressed by exposing the worms to 37 °C for 30 min. Expression of HRP-mCherry reporter activity could be easily quantified within 1 h postinduction, and maximal activity was observed at 4 h in worms grown at the optimal heme concentration of 20 µM (Fig. 5C). These studies show that temporally controlled reporters could be ideal to evaluate the effects of environmental–gene interactions on organismal heme homeostasis.
Regulation of Interorgan Heme Homeostasis by Intestinal Heme Export.
To directly measure tissue heme perturbations in genetically altered worms, we used the transgenic worm reporter strains. In C. elegans, knockdown of mrp-5 causes embryonic lethality that is suppressed by heme supplementation supporting a role for MRP-5 in heme export from the intestine to extraintestinal tissues (13). Depletion of the hrp transgene suppresses HRP activity in the intestine, hypodermis, and muscle but has less of an effect on the neurons because most neurons in C. elegans are resistant to RNAi (58). However, mrp-5 depletion resulted in a striking increase in intestinal HRP activity concomitant with a significant decrease in HRP activity in the hypodermis, muscle, and neurons (Fig. 5D). Together, these results conclusively show that loss of MRP-5, the major intestinal heme exporter, causes heme accumulation in the intestine concomitant with heme deficiency in the extraintestinal tissues.
Discussion
Free heme is not readily soluble at neutral pH and is cytotoxic, causing tissue damage (16, 17, 59, 60). These chemical properties of heme necessitate that cellular heme levels are tightly regulated while retaining transitory pools of regulatory heme. Most of the evidence to date indicates that total heme pool within the cell comprises at least two components (15, 60). A portion of the total heme pool is committed to various housekeeping heme proteins and enzymes that are essential for the survival of the cell. For instance, c-type cytochromes bind heme irreversibly via covalent linkages to thiol groups on the heme vinyl substituents. Other heme proteins, such as b-type cytochromes and globins, bind heme noncovalently, but this interaction is still essentially irreversible under physiological conditions by virtue of strong coordinate bond(s) to axial heme ligands (usually His, Met, or Cys). These proteins have high affinity for heme, usually less than pM for the globins (61) and in the nM range for heme oxygenase (62, 63). The remaining portion is in the form of a regulatory heme pool, which is available for trafficking around the cell by as yet unspecified mechanisms. It is clear that this pool of exchangeable heme can be mobilized to regulate more complex biological events such as ion channel regulation, transcriptional events, and circadian rhythm (64–66). Although it is unknown as to what form the heme exists in this pool, evidence to date indicates that it is bound noncovalently and reversibly (with Kd values in the μM range) to heme-binding chaperone proteins (i.e., heme-regulated inhibitor) (67). The modes of interaction of heme with these regulatory/trafficking proteins are different from those of the housekeeping heme proteins, consistent with their weaker affinity for heme. A number of heme-binding interactions have been suggested, including Cys/Pro (CP) motifs or Cys/His motifs (66, 68–70).
In accordance with the attributes of the well-documented labile iron pool (71), we termed this regulatory form of heme as labile heme—a metabolically active form of heme associated with macromolecules or low-molecular weight ligands and exchangeable with acceptor proteins. Intracellular labile heme has not been precisely estimated due to its relatively low abundance and lack of proper analytical tools applied at the subcellular level. Using anion-exchange chromatography, free heme was estimated to be 0.1–0.15 µM in normal human erythrocytes (72). Given that the affinity of heme to intracellular and extracellular heme-binding proteins varies from 1 µM to <1 pM (3, 73), and the Km of heme oxygenase-1 is ∼1 µM, cytosolic labile heme concentrations are likely to be less than 1 µM (33). Because heme can be transferred from one protein to another with a higher heme affinity (74, 75), in vitro reconstitution of purified apoHRP, which has a heme affinity lower than housekeeping proteins that bind protoheme or heme b, has been previously used to determine labile heme in IMR90 lung fibroblast cell extracts (34, 35). This method estimated labile heme to be 614 ± 214 nM, in close approximation to our estimate of 433 ± 125 nM in HEK293 cells. It is noteworthy, however, that severe heme restriction imposed by HD + SA treatment for 40 h shows a 60% reduction in labile heme by the in vitro reconstitution method, whereas in cellula activity for subcellular-targeted HRP and APX reporters is virtually undetectable (Fig. 3 A and B). One simple explanation for this discrepancy could be that there are intrinsic differences in the process for in vitro versus subcellular hemylation, i.e., conversion of apoHRP to holoHRP by heme. Another plausible explanation could be that labile heme is stored in an intracellular compartment and inaccessible to the subcellular HRP/APX reporters during heme deprivation.
Early heme absorption studies using dog intestines showed that heme is translocated intact across the membrane and collected in secondary endolysosomes in a subapical region colocalizing extensively with heme oxygenase-2 (HO-2) (76–80). Heme absorption in live digest cells of the cattle tick Boophilus microplus showed that ingested hemoglobin entered cells through endocytosis after being degraded in primary lysosomes; the released heme was transported into the cytosol while still bound to protein(s), and unbound heme finally accumulated in specialized membrane-bound organelles called hemosomes to prevent heme toxicity (81). We found that HEK293 cells supplied exclusively with exogenous heme accumulated 2.5-fold more labile heme when treated with Dynasore, resulting in attenuation of secretory pathway reporters without effect on nonsecretory pathway reporters (Fig. 4 B and D). These results suggest that a dynamin-dependent endocytic pathway may exist for heme compartmentalization in mammalian cells. Although we do not know the precise nature or composition of this compartment, developing additional heme sensors that can report labile heme changes in these heme storage compartments would be of great importance.
With the exception of heme-salvaging tissues such as the liver, extracellular heme is typically believed to be entirely degraded to liberate iron for endogenous heme synthesis in mammalian cells (15, 22, 24–28). Our results counter this generalized model and show that cells have the ability to transport and incorporate exogenous heme as an intact macrocycle; extracellular heme in the growth media restored reporter activity in all of the six subcellular compartments in the absence of endogenous heme synthesis (Fig. 3C). These findings may at least partially explain the phenomenon for why i.v. heme, administered as a therapeutic treatment to acute porphyria patients, increases heme-dependent enzyme activity in the liver because hepatocytes may import and use the injected heme in toto (82) in addition to its role in regulating transcription, translation, and import of ALAS into the mitochondria. Even yeast may have the capability to distinguish endogenously produced from exogenously acquired heme because overexpression of Pug1p, a heme transporter in Saccharomyces cerevisiae, selectively suppresses cell growth when exogenous heme is the sole heme source (83).
We found that ER appear to be an important locale for trafficking of both extracellular and de novo synthesized heme (Fig. 3 C and D), implying that heme allocation to various subcellular compartments may be hierarchical, a concept recently reported for copper allocation in Chlamydomonas (84). Using a dynamin K44A mutant to inhibit clathrin-mediated endocytosis, we found that labile heme decreases in the secretory pathway without affecting the cytosolic or nuclear compartments when cells rely solely on extracellular heme (Fig. 4D). Given the contiguous relationship between the endocytic and secretory pathways, it is conceivable that exogenous heme is imported via endocytosis and enters the secretory pathway through dynamin-dependent vesicular trafficking, whereas cytosolic hemoproteins acquire heme through a dynamin-independent process. Although a role for the secretory pathway would be consistent for import of extracellular heme, why is it important for heme synthesized in the mitochondria? A potential explanation could be that heme transport requires specialized structures such as the mitochondrial-associated membranes or the ERMES (1, 85, 86). These structures facilitate calcium transport and lipid trafficking between the ER and mitochondria. Ferrochelatase, heme-binding protein-1 (HBP1), and HO-2 were all detected at these interorganelle contact points (87, 88). Thus, a presumptive heme trafficking route via the ER and bypassing the cytosol can partially explain our observations that Dynasore-treated cells under HD conditions showed less perturbation of secretory pathway targeted reporters than their counterparts in other compartments. Likewise, Mdivi-1–treated cells showed a greater heme reduction in the secretory pathway, presumably because inhibition of mitochondrial fission also impaired mitochondria heme export (Fig. 4C). Although a truncated isoform of the plasma membrane FLVCR1a heme exporter termed FLVCR1b was shown to perform this function (89, 90), organisms such as yeast lack FLVCR homologs, raising the possibility that interorganelle transport may serve as alternate modes of mitochondrial heme transport.
Materials and Methods
All cell lines, culture conditions, reagents, and methods are described in SI Appendix, SI Materials and Methods. Heme-depleted FBS was prepared by treating FBS with ascorbic acid for ∼7–8 h, followed by dialysis against PBS and filter sterilization. HEK293 cells were maintained in Dulbecco’s modified medium (DMEM) with 10% (vol/vol) FBS, 1% penicillin-streptomycin, and glutamine (PSG). HD medium was prepared as DMEM with heme-depleted 10% (vol/vol) FBS and 1% PSG. SA and HD + SA medium was prepared by adding 0.5 mM SA in basal or HD medium, respectively. C. elegans strains were maintained on nematode growth medium (NGM) agar plates seeded with OP50 bacteria or in axenic liquid mCeHR-2 medium supplemented with 20 μM hemin at 20 °C.
Supplementary Material
Acknowledgments
We thank Hector Bergonia for assistance with the cellular heme content experiments; Jaswir Basran for assistance with the heme binding experiments; Harry Dailey for critical discussions and reading the manuscript; and Jennifer Lippincott-Schwartz for providing pCD3δ-EGFP, pEGFP-TGN38, and pEGFP-Dynamin (WT and K44A) plasmids. This work was supported by National Institutes of Health Grant DK074797, supplement to Grants DK074797 (to I.H.), DK090257 (to J.D.P.), and ES025661 (to A.R.R. and I.H.); and National Science Foundation Grant MCB1552791 (to A.R.R.).
Footnotes
Conflict of interest statement: I.H. is the president and founder of Rakta Therapeutics Inc. (College Park, MD), a company involved in the development of heme transporter-related diagnostics. I.H. declares no other competing financial interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609865113/-/DCSupplemental.
References
- 1.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109(10):4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamza I. Intracellular trafficking of porphyrins. ACS Chem Biol. 2006;1(10):627–629. doi: 10.1021/cb600442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995;14(2):313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8(1-2):107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20(11):2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14(12):1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen J, et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Reports. 2014;7(1):180–193. doi: 10.1016/j.celrep.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang SY, Kim MK, Kim JC. Cloning of hHRI, human heme-regulated eukaryotic initiation factor 2alpha kinase: Down-regulated in epithelial ovarian cancers. Mol Cells. 2000;10(5):584–591. doi: 10.1007/s10059-000-0584-5. [DOI] [PubMed] [Google Scholar]
- 9.Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14(1):23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- 10.Keel SB, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319(5864):825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 11.Haldar M, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156(6):1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh-Nakadai A, et al. The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program. Nat Immunol. 2014;15(12):1171–1180. doi: 10.1038/ni.3024. [DOI] [PubMed] [Google Scholar]
- 13.Korolnek T, Zhang J, Beardsley S, Scheffer GL, Hamza I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014;19(6):1008–1019. doi: 10.1016/j.cmet.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal A, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453(7198):1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller-Eberhard U, Vincent SH. Concepts of heme distribution within hepatocytes. Biochem Pharmacol. 1985;34(6):719–725. doi: 10.1016/0006-2952(85)90749-x. [DOI] [PubMed] [Google Scholar]
- 16.Wagener FA, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 17.Jeney V, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 18.Goldman BS, Gabbert KK, Kranz RG. Use of heme reporters for studies of cytochrome biosynthesis and heme transport. J Bacteriol. 1996;178(21):6338–6347. doi: 10.1128/jb.178.21.6338-6347.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman BS, Beck DL, Monika EM, Kranz RG. Transmembrane heme delivery systems. Proc Natl Acad Sci USA. 1998;95(9):5003–5008. doi: 10.1073/pnas.95.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard-Fogal CL, Frawley ER, Feissner RE, Kranz RG. Heme concentration dependence and metalloporphyrin inhibition of the system I and II cytochrome c assembly pathways. J Bacteriol. 2007;189(2):455–463. doi: 10.1128/JB.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson MS, Pelzel SE, Latimer J, Muller-Eberhard U, Hansen EJ. Identification of a genetic locus of Haemophilus influenzae type b necessary for the binding and utilization of heme bound to human hemopexin. Proc Natl Acad Sci USA. 1992;89(5):1973–1977. doi: 10.1073/pnas.89.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korolnek T, Hamza I. Like iron in the blood of the people: The requirement for heme trafficking in iron metabolism. Front Pharmacol. 2014;5:126. doi: 10.3389/fphar.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamza I, Dailey HA. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823(9):1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheftel AD, Mason AB, Ponka P. The long history of iron in the universe and in health and disease. Biochim Biophys Acta. 2012;1820(3):161–187. doi: 10.1016/j.bbagen.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yachie A, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103(1):129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshinaga T, Sassa S, Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982;257(13):7778–7785. [PubMed] [Google Scholar]
- 27.Ferris CD, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1(3):152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 28.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18(2):414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Samuel TK, Krause M, Dailey HA, Hamza I. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2. J Biol Chem. 2012;287(12):9601–9612. doi: 10.1074/jbc.M111.307694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145(5):720–731. doi: 10.1016/j.cell.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNello RK, Dolphin DH. Substituted hemins as probes for structure-function relationships in horseradish peroxidase. J Biol Chem. 1981;256(13):6903–6912. [PubMed] [Google Scholar]
- 32.Fruk L, Kuhlmann J, Niemeyer CM. Analysis of heme-reconstitution of apoenzymes by means of surface plasmon resonance. Chem Commun (Camb) 2009;2009(2):230–232. doi: 10.1039/b817206d. [DOI] [PubMed] [Google Scholar]
- 33.Sassa S. Why heme needs to be degraded to iron, biliverdin IXalpha, and carbon monoxide? Antioxid Redox Signal. 2004;6(5):819–824. doi: 10.1089/ars.2004.6.819. [DOI] [PubMed] [Google Scholar]
- 34.Masuda T, Takahashi S. Chemiluminescent-based method for heme determination by reconstitution with horseradish peroxidase apo-enzyme. Anal Biochem. 2006;355(2):307–309. doi: 10.1016/j.ab.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Atamna H, Brahmbhatt M, Atamna W, Shanower GA, Dhahbi JM. ApoHRP-based assay to measure intracellular regulatory heme. Metallomics. 2015;7(2):309–321. doi: 10.1039/c4mt00246f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinas NA, Kobayashi K, Takahashi S, Mochizuki N, Masuda T. Evaluation of unbound free heme in plant cells by differential acetone extraction. Plant Cell Physiol. 2012;53(7):1344–1354. doi: 10.1093/pcp/pcs067. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Masuda T. High throughput heme assay by detection of chemiluminescence of reconstituted horseradish peroxidase. Comb Chem High Throughput Screen. 2009;12(5):532–535. doi: 10.2174/138620709788489028. [DOI] [PubMed] [Google Scholar]
- 38.Thomas J, Weinstein JD. Measurement of heme efflux and heme content in isolated developing chloroplasts. Plant Physiol. 1990;94(3):1414–1423. doi: 10.1104/pp.94.3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porra RJ, Jones OT. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veitch NC. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry. 2004;65(3):249–259. doi: 10.1016/j.phytochem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Patterson WR, Poulos TL. Crystal structure of recombinant pea cytosolic ascorbate peroxidase. Biochemistry. 1995;34(13):4331–4341. doi: 10.1021/bi00013a023. [DOI] [PubMed] [Google Scholar]
- 43.Sharp KH, Mewies M, Moody PC, Raven EL. Crystal structure of the ascorbate peroxidase-ascorbate complex. Nat Struct Biol. 2003;10(4):303–307. doi: 10.1038/nsb913. [DOI] [PubMed] [Google Scholar]
- 44.Martell JD, et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol. 2012;30(11):1143–1148. doi: 10.1038/nbt.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klyachko NL, Dulkis YuK, Gazaryan IG, Ouporov IV, Levashov AV (1997) Dimerization of recombinant horseradish peroxidase in a reversed micellar system. Biochemistry (Mosc) 62(10):1128–1134. [PubMed]
- 46.Gazaryan IG, Klyachko NL, Dulkis YK, Ouporov IV, Levashov AV. Formation and properties of dimeric recombinant horseradish peroxidase in a system of reversed micelles. Biochem J. 1997;328(Pt 2):643–647. doi: 10.1042/bj3280643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson WR, Poulos TL. Characterization and crystallization of recombinant pea cytosolic ascorbate peroxidase. J Biol Chem. 1994;269(25):17020–17024. [PubMed] [Google Scholar]
- 48.Mandelman D, Schwarz FP, Li H, Poulos TL. The role of quaternary interactions on the stability and activity of ascorbate peroxidase. Protein Sci. 1998;7(10):2089–2098. doi: 10.1002/pro.5560071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 50.van Renswoude J, Bridges KR, Harford JB, Klausner RD. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: Identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci USA. 1982;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang A, John Peter AT, Kornmann B. ER-mitochondria contact sites in yeast: Beyond the myths of ERMES. Curr Opin Cell Biol. 2015;35:7–12. doi: 10.1016/j.ceb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Cassidy-Stone A, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park RJ, et al. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126(Pt 22):5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122(3):565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127(4):915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wigge P, Vallis Y, McMahon HT. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr Biol. 1997;7(8):554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]
- 57.Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci USA. 2005;102(12):4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asikainen S, Vartiainen S, Lakso M, Nass R, Wong G. Selective sensitivity of Caenorhabditis elegans neurons to RNA interference. Neuroreport. 2005;16(18):1995–1999. doi: 10.1097/00001756-200512190-00005. [DOI] [PubMed] [Google Scholar]
- 59.Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20(11):1754–1769. doi: 10.1089/ars.2013.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller-Eberhard U, Fraig M. Bioactivity of heme and its containment. Am J Hematol. 1993;42(1):59–62. doi: 10.1002/ajh.2830420112. [DOI] [PubMed] [Google Scholar]
- 61.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Reactions with Ligands. North Holland; Amsterdam: 1971. pp. 10–12. [Google Scholar]
- 62.Varfaj F, Lampe JN, Ortiz de Montellano PR. Role of cysteine residues in heme binding to human heme oxygenase-2 elucidated by two-dimensional NMR spectroscopy. J Biol Chem. 2012;287(42):35181–35191. doi: 10.1074/jbc.M112.378042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yi L, Ragsdale SW. Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/disulfide redox switch regulating heme binding. J Biol Chem. 2007;282(29):21056–21067. doi: 10.1074/jbc.M700664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou S, Reynolds MF, Horrigan FT, Heinemann SH, Hoshi T. Reversible binding of heme to proteins in cellular signal transduction. Acc Chem Res. 2006;39(12):918–924. doi: 10.1021/ar040020w. [DOI] [PubMed] [Google Scholar]
- 65.Gilles-Gonzalez MA, Gonzalez G. Heme-based sensors: Defining characteristics, recent developments, and regulatory hypotheses. J Inorg Biochem. 2005;99(1):1–22. doi: 10.1016/j.jinorgbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Shimizu T, et al. Gaseous O2, NO, and CO in signal transduction: Structure and function relationships of heme-based gas sensors and heme-redox sensors. Chem Rev. 2015;115(13):6491–6533. doi: 10.1021/acs.chemrev.5b00018. [DOI] [PubMed] [Google Scholar]
- 67.Miksanova M, et al. Characterization of heme-regulated eIF2alpha kinase: Roles of the N-terminal domain in the oligomeric state, heme binding, catalysis, and inhibition. Biochemistry. 2006;45(32):9894–9905. doi: 10.1021/bi060556k. [DOI] [PubMed] [Google Scholar]
- 68.Kühl T, et al. Analysis of Fe(III) heme binding to cysteine-containing heme-regulatory motifs in proteins. ACS Chem Biol. 2013;8(8):1785–1793. doi: 10.1021/cb400317x. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu T. Binding of cysteine thiolate to the Fe(III) heme complex is critical for the function of heme sensor proteins. J Inorg Biochem. 2012;108:171–177. doi: 10.1016/j.jinorgbio.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Smith AT, et al. Functional divergence of heme-thiolate proteins: A classification based on spectroscopic attributes. Chem Rev. 2015;115(7):2532–2558. doi: 10.1021/cr500056m. [DOI] [PubMed] [Google Scholar]
- 71.Kakhlon O, Cabantchik ZI. The labile iron pool: Characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33(8):1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 72.Liu SC, Zhai S, Palek J. Detection of hemin release during hemoglobin S denaturation. Blood. 1988;71(6):1755–1758. [PubMed] [Google Scholar]
- 73.Yuan X, Fleming MD, Hamza I. Heme transport and erythropoiesis. Curr Opin Chem Biol. 2013;17(2):204–211. doi: 10.1016/j.cbpa.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan WT, Liem HH, Sutor RP, Muller-Ebergard U. Transfer of heme from heme-albumin to hemopexin. Biochim Biophys Acta. 1976;444(2):435–445. doi: 10.1016/0304-4165(76)90387-1. [DOI] [PubMed] [Google Scholar]
- 75.Liu M, Lei B. Heme transfer from streptococcal cell surface protein Shp to HtsA of transporter HtsABC. Infect Immun. 2005;73(8):5086–5092. doi: 10.1128/IAI.73.8.5086-5092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West AR, Oates PS. Subcellular location of heme oxygenase 1 and 2 and divalent metal transporter 1 in relation to endocytotic markers during heme iron absorption. J Gastroenterol Hepatol. 2008;23(1):150–158. doi: 10.1111/j.1440-1746.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- 77.Gottlieb Y, Truman M, Cohen LA, Leichtmann-Bardoogo Y, Meyron-Holtz EG. Endoplasmic reticulum anchored heme-oxygenase 1 faces the cytosol. Haematologica. 2012;97(10):1489–1493. doi: 10.3324/haematol.2012.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown EB, Hwang YF, Nicol S, Ternberg J. Absorption of radiation-labeled hemoglobin by dogs. J Lab Clin Med. 1968;72(1):58–64. [PubMed] [Google Scholar]
- 79.Parmley RT, Barton JC, Conrad ME, Austin RL, Holland RM. Ultrastructural cytochemistry and radioautography of hemoglobin--iron absorption. Exp Mol Pathol. 1981;34(2):131–144. doi: 10.1016/0014-4800(81)90070-8. [DOI] [PubMed] [Google Scholar]
- 80.Wyllie JC, Kaufman N. An electron microscopic study of heme uptake by rat duodenum. Lab Invest. 1982;47(5):471–476. [PubMed] [Google Scholar]
- 81.Lara FA, Lins U, Bechara GH, Oliveira PL. Tracing heme in a living cell: Hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J Exp Biol. 2005;208(Pt 16):3093–3101. doi: 10.1242/jeb.01749. [DOI] [PubMed] [Google Scholar]
- 82.Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375(9718):924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 83.Protchenko O, et al. Role of PUG1 in inducible porphyrin and heme transport in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7(5):859–871. doi: 10.1128/EC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kropat J, et al. Copper economy in Chlamydomonas: Prioritized allocation and reallocation of copper to respiration vs. photosynthesis. Proc Natl Acad Sci USA. 2015;112(9):2644–2651. doi: 10.1073/pnas.1422492112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci USA. 2011;108(34):14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kornmann B, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325(5939):477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poston CN, Duong E, Cao Y, Bazemore-Walker CR. Proteomic analysis of lipid raft-enriched membranes isolated from internal organelles. Biochem Biophys Res Commun. 2011;415(2):355–360. doi: 10.1016/j.bbrc.2011.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang A, et al. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics. 2011;10(10):M111 009936. doi: 10.1074/mcp.M111.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quigley JG, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118(6):757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Chiabrando D, et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J Clin Invest. 2012;122(12):4569–4579. doi: 10.1172/JCI62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berglund GI, et al. The catalytic pathway of horseradish peroxidase at high resolution. Nature. 2002;417(6887):463–468. doi: 10.1038/417463a. [DOI] [PubMed] [Google Scholar]
- 92.Lorenz H, Hailey DW, Lippincott-Schwartz J. Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat Methods. 2006;3(3):205–210. doi: 10.1038/nmeth857. [DOI] [PubMed] [Google Scholar]
- 93.Yang M, Omura S, Bonifacino JS, Weissman AM. Novel aspects of degradation of T cell receptor subunits from the endoplasmic reticulum (ER) in T cells: importance of oligosaccharide processing, ubiquitination, and proteasome-dependent removal from ER membranes. J Exp Med. 1998;187(6):835–846. doi: 10.1084/jem.187.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keller P, Toomre D, Díaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3(2):140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.