Abstract
Background
Epilepsy is a neurological disorder characterized by epileptic seizures as a result of excessive neuronal activity in the brain. Approximately 65 million people worldwide suffer from epilepsy; 20–40% of them are refractory to medication therapy. Early detection of disease is crucial in the management of patients with epilepsy. Correct localization of the ictal onset zone is associated with a better surgical outcome. The modern non-invasive techniques used for structural-functional localization of the seizure focus includes electroencephalography (EEG) monitoring, magnetic resonance imaging (MRI), single photon emission tomography/computed tomography (SPECT/CT) and positron emission tomography/computed tomography (PET/CT). PET/CT can predict surgical outcome in patients with refractory epilepsy. The aim of the article is to review the current role of routinely used tracer 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) as well as non routinely used 18F-Flumazenil (18F-FMZ) tracers PET/CT in patients with refractory epilepsy.
Conclusions
Functional information delivered by PET and the morphologic information delivered by CT or MRI are essential in presurgical evaluation of epilepsy. Nowadays 18F-FDG PET/CT is a routinely performed imaging modality in localization of the ictal onset zone in patients with refractory epilepsy who are unresponsive to medication therapy. Unfortunately, 18F-FDG is not an ideal PET tracer regarding the management of patients with epilepsy: areas of glucose hypometabolism do not correlate precisely with the proven degree of change within hippocampal sclerosis, as observed by histopathology or MRI. Benzodiazepine-receptor imaging is a promising alternative in nuclear medicine imaging of epileptogenic focus. The use of 11C-FMZ in clinical practice has been limited by its short half-life and necessitating an on-site cyclotron for production. Therefore, 18F-FMZ might be established as one of the tracers of choice for patients with refractory epilepsy because of better sensitivity and anatomical resolution.
Keywords: epilepsy, nuclear medicine, PET/CT, 18F-FDG, 18F-Flumazenil
Introduction
Epilepsy is a neurological disorder characterized by epileptic seizures as a result of excessive neuronal activity in the brain (the word “epilepsy” is derived from the Greek word meaning to be attacked or seized). For diagnosis of epilepsy at least two unprovoked seizures are required. Metabolic, genetic or structural conditions can be recognized as causes of epilepsy, but in 60% of patients, the cause remains unknown.1 Approximately 65 million people worldwide suffer from epilepsy2, 20–40% of them are refractory to medication therapy.3 Depending on the epilepsy syndrome, dietary changes, neurostimulation or surgery may be considered as treatment options in patients whose seizures do not respond to medication therapy. With the advancement of surgical techniques and devices, surgical treatment has become the treatment of choice in patients who are unresponsive to medication therapy. In selected patients with focal structural lesions such as cortical dysplasia, mesial temporal sclerosis, vascular malformations and in some paediatric epilepsy syndromes, surgery may substantially reduce the frequency of epileptic seizures and improve the patient´s quality of life. Several studies over the last decades support the statement that surgical treatment significantly improved long-term outcomes of seizure control.4-8
Early detection of disease is crucial in the management of patients with epilepsy. Correct localization of the ictal onset zone is associated with a better surgical outcome. Epilepsy surgery may lead to seizure freedom. When “surgical cure” is impossible, epilepsy surgery may help achieve palliative goals such as minimizing the frequency and severity of seizures. Following successful epilepsy surgery, quality of life, cognition and behaviour may improve substantially.9 However, careful patient selection and weighing of risks and benefits are of paramount importance, as surgery may not only fail in terms of improving seizure control, but come with serious adverse events such as intracranial bleeding. Despite international guidelines recommending early and systematic assessment of patient´s eligibility, epilepsy surgery is still being underused and referral of patients with drug refractory epilepsy is often delayed with deleterious consequences on outcome and quality of life.10
The scope of this paper on PET imaging in refractory epilepsy patients cannot cover all aspects of patient management including the range of indications, specific issues in children and adults, surgical techniques or predictors of outcome. For this, the interested reader is referred to a recent review of Ryvlin et al.11
Different modalities in diagnosisof epilepsy
Non-invasive focus localisation of seizures precedes invasive intracranial electrodes procedures. The modern non-invasive techniques used for structural-functional localization of the seizure focus includes electroencephalography (EEG) monitoring, magnetic resonance imaging (MRI), single photon emission tomography/computed tomography (SPECT/CT) and positron emission tomography/computed tomography (PET/CT).
EEG has low sensitivity in the diagnosis of seizure disorders (25–56%).
MRI is a sensitive and specific imaging modality for identifying hippocampal sclerosis as well as other lesions responsible for epilepsy. However 1–1.5 T MRI still fails to reveal approximately 20% of abnormalities in patients with medically refractory epilepsy12; MRI may miss mild changes and subtle lesions. In patients with intractable extratemporal epilepsy, the most common underlying pathology is microscopic cortical dysplasia which sometimes cannot be detected by MRI and hence may pass unnoticed by 18F-FDG PET/CT as well. A recent study of 194 adult patients with medically refractory focal epilepsy showed that 18F-FDG PET/CT is helpful for decision making in 53% of presurgical patients with normal or discordant MRI.13 Advanced MRI technologies (MR spectroscopy, MR volumetry, MR perfusion) may provide additional and more precise information.
In recent years brain perfusion SPECT imaging has been widely used for detection of epileptic focus. In their meta-analysis, Devous et al. reported a 44% (interictal), 75% (postictal) and 97% (ictal) sensitivity of SPECT in patients with temporal lobe epilepsy.14 Extratemporal lobe epilepsy showed SPECT sensitivity in 66% (ictal) and in 40% (interictal) of patients.15,16
PET procedures in patients with epilepsy
In recent years, multiple studies17-22 have demonstrated that 18F-FDG PET/CT can predict surgical outcome in patients with refractory epilepsy. Actually 18F-FDG PET/CT has proven to be the most sensitive imaging technique for presurgical localization of epileptogenic foci in patients with medically refractory partial epilepsy who have non-contributory MRI and EEG. In comparison to SPECT, PET technology provides much better resolution and allows quantitative measurement.23-26
There is a variety of PET tracers used for imaging of epileptic focus: tracers that measure glucose metabolism, serotonine receptors and transport, oxygen metabolism, cerebral blood flow and other receptor binding.27 PET scan findings at the area of a seizure focus are different according to the PET tracer used (Table 1). Multiple PET radiotracers for neurotransmitter and neuromodulator systems still form part of preclinical trials.
Table 1.
Some PET tracers used for imaging of epileptic focus and PET scan findings
| PET scan | Findings on PET scan |
|---|---|
| 18F-FDG interictal | Decreased metabolism |
| 18F-FDG ictal | Increased and decreased metabolism (complex pattern) |
| 18F-FDG postictal | Increased and decreased metabolism (complex pattern) |
| Serotonin receptor (e.g. 18F-Mefway) | Reduced binding |
| Dopamine receptor (e.g. 18F-Fallypride) | Reduced binding |
| 18F-Flumazenil (GABA receptor) | Decreased binding |
| 15O-H2O interictal | Reduced perfusion |
| 15O-H2O ictal | Increased perfusion |
In this review article we focus on the routinely used tracer 18F-FDG as well as on 18F-labelled flumazenil tracers which are less available but becoming popular.
18F-FDG PET/CT in patients with epilepsy
The first application of 18F-FDG PET/CT in patients with epilepsy dates back to the early 1980s.28,29 As glucose is the main energy source for the brain, a radioactive glucose analogue has been the most widely used tracer for PET imaging in patients with refractory epilepsy. There is a good match of glucose metabolism and neuronal activity. Glucose transporters (predominantly GLUT1) transfer 18F-FDG from the blood into cells. Once in the cell, FDG is phosphorylated by hexokinase and forms FDG-6-phosphate. Further metabolism of FDG-6-phosphate is stopped and FDG-6-phosphate is essentially trapped in the cell.
In 2009 the European Association of Nuclear Medicine established an imaging protocol for 18F-FDG PET/CT imaging of the brain.30 According to this protocol patients should fast at least 4 hours before scanning. Psychotropic pharmaceuticals may influence reagional metabolism of the glucose. Serum glucose levels must be checked before 18F-FDG PET/CT examination. If the value is greater than 160 mg/dl, the patient must be rescheduled. Diabetic patients should undergo scanning in euglycemic state. At least 30 minutes before examination as well as 30 minutes after tracer injection patients must rest in a quiet dimly-lighted room. They should be instructed not to talk, read or to be otherwise active. 18F-FDG dose for adults patients is 300–600 MBq in 2-D mode and 125–250 MBq in 3-D mode. For children, dose is calculated by body wight (EANM dosage card). Continuous EEG recording is required 2 hours before tracer injection (to exclude that tracer is not injected postictal state) and at least until 20 minutes after tracer injection. Static PET scan acquisition starts 30 up to 60 minutes after tracer injection and lasts for 15–30 minutes. There are small variations in the protocols depending on quality of imaging technology. In PET scans interpretation, combination of visual inspection and semiquantitive analysis is crucial. Semiquantitative analysis helps to detect abnormalities which are not present on visual inspection.
Physiological18F-FDG distribution is as follows: high in cerebral and cerebellar cortices and subcortical grey matter and mild in the white matter. In children and with normal aging there is decrease in cerebral metabolic rates (particularly in lateral, medial frontal cortex and anterior cingulate cortex).31,32 Regions involving epileptic foci may present increased, reduced, or, absent metabolic activity.
18F-FDG PET/CT scans in patients with epilepsy are usually obtained in the interictal phase. Ictal 18F-FDG PET/CT appears to be highly sensitive (Figure 1), but is difficult to obtain because of unpredictability and very rapid onset of major seizures. In addition, medical personnel and the radiotracer must be available at the patient´s bedside at the time of seizure onset. Postictal 18F-FDG PET/ CT scans can be very complex, and it represents a mishmash of increased and/or decreased metabolism, depending on the time of 18F-FDG injection after the onset of seizure.
Figure 1.
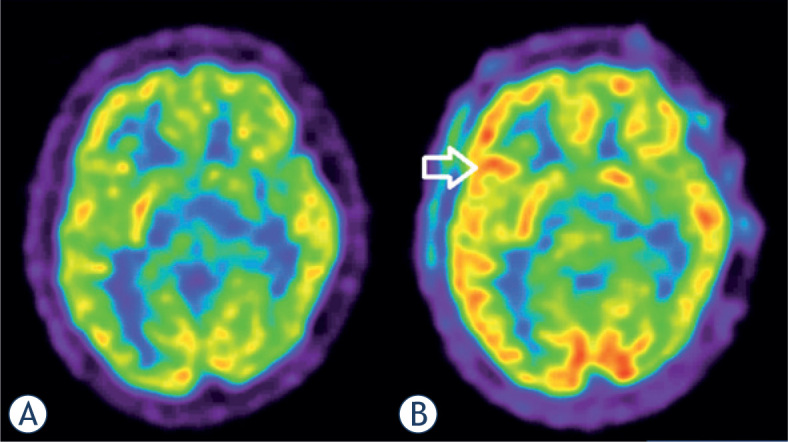
Focal epilepsy in 17 year old male patient. Interictal 18F-FDG PET (A): physiological distribution of 18F-FDG in the brain. Ictal 18F-FDG PET (B): hypermetabolism frontolateral in the right hemisphere (arrow).
Interictal 18F-FDG PET/CT in patients with epilepsy
The goal of implementing 18F-FDG PET/CT in the management of patients with drug refractory epilepsy was to obtain functional imaging of interictal brain glucose metabolism. Multiple studies have shown that interictal 18F-FDG PET/CT is more sensitive than interictal perfusion SPECT33-34,15, because in the interictal period, reduction of regional glucose cerebral metabolism is more pronounced than reduction of cerebral perfusion.35-37
Neuronal loss due to chronic seizure activity, reduction in density of synapses, inhibitory processes in the interictal period and diaschisis also influence glucose hypometabolism, the characteristic sign of epilepsy on interictal 18F-FDG PET/ CT. Interictal 18F-FDG PET/CT cannot precisely define the surgical margins, because some areas of hypometabolism extend beyond epileptic zones. Drzezga et al. showed that automated analysis of 18F-FDG PET/CT scans in patients with epilepsy is more sensitive than visual analysis in patients with temporal lobe epilepsy and extra temporal lobe epilepsy.36 The combination of MRI and interictal 18F-FDG PET/CT will probably evolve as the future modality of choice to get the best results for presurgical evaluation.
Ictal 18F-FDG PET/CT in patients with epilepsy
Ictal 18F-FDG PET in the presurgical workup of refractory epilepsy is rarely performed; it is performed either in status epilepticus or in a status of induced epileptic seizures. As status epilepticus is defined as seizure longer than 30 minutes or more than one seizure within thirty minutes without the person returning to normal, 18F-FDG PET is difficult to be obtained in those moments. Despite technical difficulties ictal 18F-FDG PET has the advantage of a high spatial resolution. Ictal 18F-FDG PET/CT shows hypermetabolism, although an ictal scan reveals a complex combination of hyper and hypometabolism and for that reason it is important to continuously monitor the patient with scalp EEG. A study from 2013 showed that in patients with incompatible EEG, MRI and clinical features, ictal 18F-FDG PET/CT helped to localize the origin of status epilepticus.38 Definition of an ictal onset zone is usually made visually with the support of semiquantitative analysis. A difference above 15% between the affected and the contralateral side suggests significant physiological asymmetry.
Unfortunately, 18F-FDG is not an ideal PET tracer regarding the management of patients with epilepsy. Areas of glucose hypometabolism do not correlate with the proven degree of change within hippocampal sclerosis, as observed by histopathology or MRI; the area of hypometabolism may be larger than the pathological seizure focus.39 So, the presurgical definition of epileptogenic foci cannot be based on 18F-FDG PET/CT imaging alone, because 18F-FDG changes may appear larger than the real ictal onset zone. The reason for this anatomical-functional discrepancy has not been explained yet. However, 18F-FDG PET/CT can reveal and confirm surgical targets and therefore represents a valuable tool in the management of patients with intractable epilepsy.
18F- Flumazenil tracers for PET/CT of patients with epilepsy
Gamma-aminobutyric acid (GABA) is the most important inhibitory neurotransmitter in the central nervous system.40-43 Its main role consists of reducing neuronal excitability and regulating muscle tone. There are two types of GABA receptors: GABAA and GABAB; benzodiazepine receptors are modulatory sites on GABAA receptors. Benzodiazepine distribution in the human brain includes the occipital cortex, temporal cortex, cerebellum, thalamus, and the pons. The majority of GABAA receptors are benzodiazepine-sensitive, but there are also GABAA receptors which are insensitive to classical benzodiazepines. Benzodiazepines enhance the action of GABA on its receptors, thus having anticonvulsant, sedative, hypnotic, anxiolytic and muscle-relaxant effects.
Flumazenil blocks the benzodiazepine sites on GABAA receptors and thus antagonizes the action benzodiazepines have on the central nervous system. Flumazenil was introduced in 1987 and over time it was used as an antidote for the treatment of benzodiazepine overdoses. In epileptogenic regions a reduced level of benzodiazepine receptors is found44; also, irreversible ischemic cortical damage after stroke45, Alzheimer disease46,47 chronic alcoholism48 and schizophrenia49 may influence benzodiazepine complex density.
In the 1980s, Flumazenil was proposed as a promising new marker for imaging of benzodiazepine receptors by PET. The substance was initially labelled with Carbon-11 (11C-FMZ)50 and used in some studies: the majority of patients with seizures refractory to medical treatment had hippocampal sclerosis characterized by neuronal loss and gliosis, which was detected by this positron-labelled GABAA receptor antagonist.51 Six years later a SPECT agent, 123I-iomazenil, was synthetized.52,53 Both compounds showed high benzodiazepine binding potential in the brain. However, use of 11C-FMZ in clinical practice has been limited by its short half-life, necessitating an on-site cyclotron for production, and multiple syntheses if several subjects have to be examined. The first paper on a fluorinated analogue of flumazenil was published in 1992.54 18F-labelled PET radiotracers are more suited for clinical use. Also, the shorter positron range of 18F provides better image resolution, which may enhance the detection of small pathological complexes such as epileptic foci. Following this, several 18F-labelled FMZ tracers, including 5-(2′-[18F]fluoroethyl) flumazenil (18F-FEFMZ), 3-(2′-[18F]fluoroflumazenil (18F-FFMZ), 5-(2’-[18F]fluoroethyl)flumazenil (18F-FEF) and [18F]flumazenil (18F–FMZ) have been developed.55-60 Compared to 11C-FMZ, 18F-FEFMZ has lower receptor affinity, and higher nonspecific binding due to faster metabolism and faster kinetics. 18F-FFMZ also has faster kinetics than 11C-FMZ. Compared to 11C-FMZ, 18F-FEF has lower affinity to benzodiazepine receptors, rapid kinetics in the brain and faster metabolism.55-60 18F-FMZ, with an identical structure to 11C-FMZ, has been shown to have similar pharmacokinetics and peripheral metabolism and PET imaging characteristics as 11C-FMZ.
18F-FMZ has been established as the tracer of choice for patients with refractory epilepsy in some neurological centres in Europe. PET/CT imaging using flumazenil tracers identify a more restricted region of abnormality in the epileptogenic zone than 18F-FDG PET/CT.61 In comparison to 18F-FDG PET/CT scans benzodiazepine-receptor scans appears “sharper” (Figure 2, 3).
Figure 2.
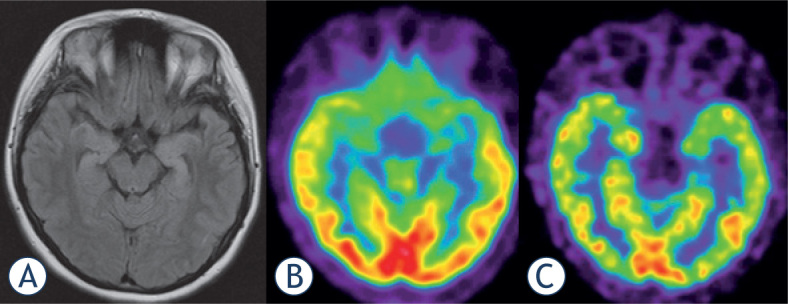
18F-FDG PET (B)scan vs. 18F-Flumazenil PET (C)in a 19 year old female patient with bilateral hippocampal sclerosis as shown by MRI (A). Both PET modalities present low temporomesial uptake being larger on the left side, but benzodiazepinereceptor imaging appears sharper and presents a focal defect.
Figure 3.
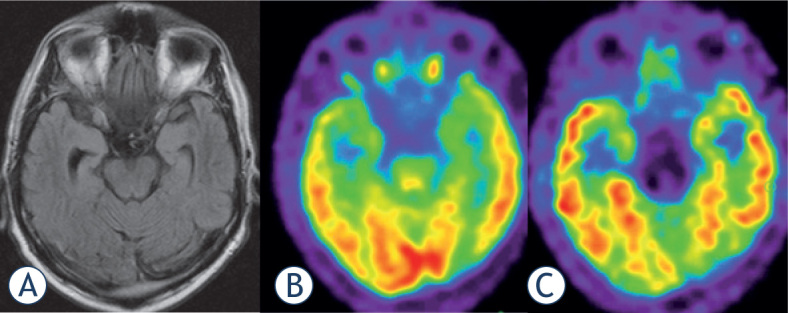
Focal epilepsy in 56 year old male patient. (A)MRI: astrogliosis of the right hippocampus (later proven by histology); (B)18F-FDG PET: minal temporomesial hypometabolism of both sides; (C)18F-Flumazenil PET: focal defect of tracer uptake at the right hippocampus.
Imaging protocol for PET/CT with 18F-labelledflumazenil radiotracers is almost similar to protocol for 18F-FDG PET/CT, except no serum glucose level measurement is required. All flumazenil tracers bind non-selectively to all benzodiazepine receptor subtypes, so pre-treatment with unlabelled flumazenil can result in reduced tracer uptake on benzodiazepine receptors. As a young modality, benzodiazepine-receptor PET imaging is still strengthening its place in neurology.
Conclusions
Functional information delivered by PET and the morphologic information delivered by CT or MR are essential in presurgical evaluation of epilepsy. Nowadays 18F-FDG PET/CT is a routinely performed imaging modality in localization of the ictal onset zone in patients with refractory epilepsy who are unresponsive to medication therapy. Unfortunately, 18F-FDG is not an ideal PET tracer regarding the management of patients with epilepsy: areas of glucose hypometabolism do not correlate precisely with the proven degree of change within hippocampal sclerosis, as observed by histopathology or MRI. Benzodiazepine-receptor imaging is a promising alternative in nuclear medicine imaging of epileptogenic focus. The use of 11C-FMZ in clinical practice has been limited by its short half-life and necessitating an on-site cyclotron for production. Therefore, 18F-FMZ might be established as one of the tracers of choice for patients with refractory epilepsy because of better sensitivity and anatomical resolution.
Multiple new PET tracers for presurgical evaluation of patients with epilepsy are still under preclinical investigations; tracers for neurotransmitter and neuromodulator systems, including the GABA, serotonin, dopamine, glutamate, acetylcholine, adenosine and opioid systems.
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.National Institute for Health and Clinical Excellence. Chapter 1. Introduction. In: National Clinical Guideline Centre, editors. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. London: Royal College of Physicians (UK); 2012. pp. 21–8. [Google Scholar]
- 2.Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D. et al. ILAE commission on epidemiology. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52 (Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 4.Kelly KM, Chung SS. Surgical treatment for refractory epilepsy: review of patient evaluation and surgical options. Epilepsy Res Treat. 2011;2011:303–624. doi: 10.1155/2011/303624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou CC, Shih YH, Yen DJ, Kwan SY, Yu HY. Long-term health-related quality of life in drug-resistant temporal lobe epilepsy after anterior temporal lobectomy. Epileptic Disord. 2015;17:177–83. doi: 10.1684/epd.2015.0744. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Alonso J, Bellas-Lamas P. Surgical treatment for drug-resistant epilepsy. JAMA. 2015;313:1572. doi: 10.1001/jama.2015.2883. [DOI] [PubMed] [Google Scholar]
- 7.Liu SY, Yang XL, Chen B, Hou Z, An N, Yang MH. et al. Clinical outcomes and quality of life following surgical treatment for refractory epilepsy: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e500. doi: 10.1097/MD.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomised, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:365–7. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 9.Taft C, Sager Magnusson E, Ekstedt G, Malmgren K. Health-related quality of life, mood, and patient satisfaction after epilepsy surgery in Sweden-a prospective controlled observational study. Epilepsia. 2014;55:878–85. doi: 10.1111/epi.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D. et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 11.Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13:1114–26. doi: 10.1016/S1474-4422(14)70156-5. [DOI] [PubMed] [Google Scholar]
- 12.Lehericy S, Semah F, Hasboun D, Dormont D, Clemenceau S, Granat O. et al. Temporal lobe epilepsy with varying severity: MRI study of 222 patients. Neuroradiology. 1997;39:788–96. doi: 10.1007/s002340050507. [DOI] [PubMed] [Google Scholar]
- 13.Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108:1306–14. doi: 10.1016/j.eplepsyres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Devous M, Thisted R, Morgan G, Leroy R, Rowe C. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285–93. [PubMed] [Google Scholar]
- 15.Spencer S. The relative contributions of MRI, SPECT and PET imaging in epilepsy. Epilepsia. 1994;35:S72–89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 16.Weil S, Noachtar S, Arnold S, Yousry TA, Winkler PA, Tatsch K. Ictal ECDSPECT differentiate between temporal and extratemporal epilepsy: confirmation by excellent postoperative seizure control. Nucl Med Commun. 2001;22:233–7. doi: 10.1097/00006231-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Blum DE, Ehsan T, Dungan D, Karis JP, Fisher RS. Bilateral temporal hypometabolism in epilepsy. Epilepsia. 1998;39:651–9. doi: 10.1111/j.1528-1157.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 18.Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy. A meta-analysis. Seizure. 2007;16:509–20. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Taft C, Sager Magnusson E, Ekstedt G, Malmgren K. Health-related quality of life, mood, and patient satisfaction after epilepsy surgery in Sweden-a prospective controlled observational study. Epilepsia. 2014;55:878–85. doi: 10.1111/epi.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swartz BE, Tomiyasu U, Delgado-Escueta AV, Mandelkern M, Khonsari A. Neuroimaging in temporal lobe epilepsy: test sensitivity and relationships to pathology and post-surgical outcome. Epilepsia. 1992;33:624–34. doi: 10.1111/j.1528-1157.1992.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 21.Manno EM, Sperling MR, Ding X, Jaggi J, Alavi A, O’Connor MJ. et al. Predictors of outcome after anterior temporal lobectomy: Positron emission tomography. Neurology. 1994;44:2331–6. doi: 10.1212/wnl.44.12.2321. [DOI] [PubMed] [Google Scholar]
- 22.Theodore WH, Sato S, Kufta C, Balish MB, Bromfield EB, Leiderman DB. Temporal lobectomy for uncontrolled seizures: The role of positron emission tomography. Ann Neurol. 1992;32:789–94. doi: 10.1002/ana.410320613. [DOI] [PubMed] [Google Scholar]
- 23.Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108:1306–14. doi: 10.1016/j.eplepsyres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Singhal T, Alavi A, Kim CK. Brain: positron emission tomography tracers beyond [¹⁸F]fluorodeoxyglucose. PET Clin. 2014;9:267–76. doi: 10.1016/j.cpet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Inaji M, Maehara T. PET, SPECT, and MEG in the diagnosis of epilepsy. Nihon Rinsho. 2014;72:827–33. [PubMed] [Google Scholar]
- 26.Casse R, Rowe CC, Newton M, Berlangieri SU, Scott AM. Positron emission tomography and epilepsy. Mol Imaging Biol. 2002;4:338–51. doi: 10.1016/s1536-1632(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 27.Ismet Sarikaya. PET studies in epilepsy. Am J Nucl Med Mol Imaging. 2015;5:416–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhl E, Engel J, Phelps E, Selin C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol. 1980;18:348–60. doi: 10.1002/ana.410080403. [DOI] [PubMed] [Google Scholar]
- 29.Engel J, Kuhl E, Phelps E, Crandall H. Comparative localization of epileptic foci in partial epilepsy by PCT and EEG. Ann Neurol. 198;12:529–37. doi: 10.1002/ana.410120605. [DOI] [PubMed] [Google Scholar]
- 30.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K. et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 31.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frölich L. et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 32.Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE. Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with age. J Nucl Med. 1995;36:1141–9. [PubMed] [Google Scholar]
- 33.Won HJ, Chang KH, Cheon JE, Kim HD, Lee DS, Han MH. et al. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am J Neuroradiol. 1999;20:593–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang SI, Kim JH, Park SW, Han MH, Yu IK, Lee SH. et al. Comparative analysis of MR imaging, positron emission tomography, and ictal single-photon emission CT in patients with neocortical epilepsy. Am J Neuroradiol. 2001;22:937–46. [PMC free article] [PubMed] [Google Scholar]
- 35.Fink GR, Pawlik G, Stefan H, Pietrzyk U, Wienhard K, Heiss WD. Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesi al structures. J Neurol Sci. 1996;137:28–34. doi: 10.1016/0022-510x(95)00323-t. [DOI] [PubMed] [Google Scholar]
- 36.Zubal IG, Avery RA, Stokking R, Studholme C, Corsi M, Dey H. et al. Ratio-images calculated from interictal positron emission tomography and single-photon emission computed tomography for quantification of the uncoupling of brain metabolism and perfusion in epilepsy. Epilepsia. 2000;41:1560–6. doi: 10.1111/j.1499-1654.2000.001560.x. [DOI] [PubMed] [Google Scholar]
- 37.Buch K, Blumenfeld H, Spencer S, Novotny E, Zubal IG. Evaluating the accuracy of perfusion/metabolism (SPET/PET) ratio in seizure localization. Eur J Nucl Med Mol Imaging. 2008;35:579–88. doi: 10.1007/s00259-007-0550-y. [DOI] [PubMed] [Google Scholar]
- 38.Desai A, Bekelis K, Thadani VM, Roberts DW, Jobst BC, Duhaime AC. et al. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–50. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 39.Drzezga A, Arnold S, Minoshima S, Noachtar S, Szecsi J, Winkler P. et al. 18F-FDG PET studies in patients with extratemporal and temporal epilepsy: evaluation of an observer-independent analysis. J Nucl Med. 1999;40:737–46. [PubMed] [Google Scholar]
- 40.Javeria N, Rajesh I, Seetharam R. Ictal PET in presurgical workup of refractory extratemporal epilepsy. Ann Indian Acad Neurol. 2013;16:676–7. doi: 10.4103/0972-2327.120475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siclari F, Prior JO, Rossetti AO. Ictal cerebral positron emission tomography (PET) in focal status epilepticus. Epilepsy Res. 2013;105:356–61. doi: 10.1016/j.eplepsyres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Engel J Jr, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol. 1982;12:518–28. doi: 10.1002/ana.410120604. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 44.Sieghart W. Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci. 1994;19:24–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Derry JM, Dunn SM, Davies M. Identification of a residue in the gammaaminobutyric acid type A receptor alpha subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J Neurochem. 2004;88:1431–8. doi: 10.1046/j.1471-4159.2003.02264.x. [DOI] [PubMed] [Google Scholar]
- 46.Goldfrank Lewis R. Goldfrank’s toxicologic emergencies. New York: McGraw-Hill; 2002. [Google Scholar]
- 47.Savic I, Persson A, Roland P, Pauli S, Sedvall G, Widén L. In-vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci. Lancet. 1988;2:863–6. doi: 10.1016/s0140-6736(88)92468-3. [DOI] [PubMed] [Google Scholar]
- 48.Heiss WD, Kracht L, Grond M, Rudolf J, Bauer B, Wienhard K. et al. [(11) C]Flumazenil/H(2)O positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke. 2000;31:366–9. doi: 10.1161/01.str.31.2.366. [DOI] [PubMed] [Google Scholar]
- 49.Mayer M, Koeppe RA, Frey KA, Foster NL, Kuhl DE. Positron emission tomography measures of benzodiazepine binding in Alzheimer´s disease. Arch Neuro. 1995;52:314–7. doi: 10.1001/archneur.1995.00540270110027. [DOI] [PubMed] [Google Scholar]
- 50.Pascual B, Prieto E, Arbizu J, Marti-Climent JM, Peñuelas I, Quincoces G. et al. Decreased carbon-11-flumazenil binding in early Alzheimer’s disease. Brain. 2012;135:2817–25. doi: 10.1093/brain/aws210. [DOI] [PubMed] [Google Scholar]
- 51.Litton JE, Neiman J, Pauli S, Farde L, Hindmarsh T, Halldin C. et al. PET analysis of [11C]flumazenil binding to benzodiazepine receptors in chronic alcohol-dependent men and healthy controls. Psychiatry Res. 1993;50:1–13. doi: 10.1016/0925-4927(93)90019-e. [DOI] [PubMed] [Google Scholar]
- 52.Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 1992;12:924–9. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maziere M, Hantraye P, Prenant C, Sastre J, Comar D. Synthesis of ethyl 8-fluoro-5,6-dihydro-5-[11C]methyl-6-oxo-4H-imidazo [1,5-a] [1,4]benzodiazepine-3-carboxylate (RO 15.1788-11C): a specific radioligand for the in vivo study of central benzodiazepine receptors by positron emission tomography. Int J Appl Radiat Isot. 1984;35:973–6. doi: 10.1016/0020-708x(84)90215-1. [DOI] [PubMed] [Google Scholar]
- 54.Savic I, Ingvar M, Stone-Elander S. Comparison of [11C]flumazenil and [18F] FDG as PET markers of epileptic foci. J Neurol Neurosurg Psychiatry. 1993;56:615–21. doi: 10.1136/jnnp.56.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boundy KL, Rowe CC, Black AB, Kitchener MI, Barnden LR, Sebben R. et al. Localization of temporal lobe epileptic foci with iodine-123 iododexetimide cholinergic neuroreceptor single-photon emission computed tomography. Neurology. 1996;47:1015–20. doi: 10.1212/wnl.47.4.1015. [DOI] [PubMed] [Google Scholar]
- 56.Beer HF, Bläuenstein PA, Hasler PH, Delaloye B, Riccabona G, Bangerl I. et al. In vitro and in vivo evaluation of iodine-123-Ro 16-0154: a new imaging agent for SPECT investigations of benzodiazepine receptors. J Nucl Med. 1990;31:1007–14. [PubMed] [Google Scholar]
- 57.Samson Y, Hantraye P, Baron JC, Soussaline F, Comar D, Mazière M. Kinetics and displacement of [11C]RO 15-1788, a benzodiazepine antagonist, studied in human brain in vivo by positron tomography. Eur J Pharmacol. 1985;110:247–51. doi: 10.1016/0014-2999(85)90218-3. [DOI] [PubMed] [Google Scholar]
- 58.Moerlein SM, Perlmutter JS. Binding of 5-(2’-[18F]fluoroethyl)flumazenil to central benzodiazepine receptors measured in living baboon by positron emission tomography. Eur J Pharmacol. 1992;218:109–15. doi: 10.1016/0014-2999(92)90153-u. [DOI] [PubMed] [Google Scholar]
- 59.Leveque P, Labar D, Gallez B. Biodistribution, binding specificity and metabolism of [18F]fluoroethylflumazenil in rodents. Nucl Med Biol. 2001;28:809–14. doi: 10.1016/s0969-8051(01)00251-7. [DOI] [PubMed] [Google Scholar]
- 60.Grunder G, Siessmeier T, Lange-Asschenfeldt C, Vernaleken I, Buchholz HG, Stoeter P. et al. [18F]Fluoroethylflumazenil: a novel tracer for PET imaging of human benzodiazepine receptors. Eur J Nucl Med. 2001;28:1463–70. doi: 10.1007/s002590100594. [DOI] [PubMed] [Google Scholar]
- 61.Mitterhauser M, Wadsak W, Wabnegger L, Mien L-K, Togel S, Langer O. et al. Biological evaluation of 2ʹ-[18F]fluoroflumazenil ([18F]FFMZ), a potential GABA receptor ligand for PET. Nucl Med Biol. 2004;31:291–5. doi: 10.1016/j.nucmedbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Chang YS, Jeong JM, Yoon YH, Kang WJ, Lee SJ, Lee DS. et al. Biological properties of 2ʹ-[18F]fluoroflumazenil for central benzodiazepine receptor imaging. Nucl Med Biol. 2005;32:263–8. doi: 10.1016/j.nucmedbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Ryzhikov NN, Seneca N, Krasikova RN, Gomzina NA, Shchukin E, Fedorova OS. et al. Preparation of highly specific radioactivity [18F] flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl Med Biol. 2005;32:109–16. doi: 10.1016/j.nucmedbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Vivash L, Gregoire MC, Lau EW, Ware RE, Binns D, Roselt P. et al. 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med. 2013;54:1270–7. doi: 10.2967/jnumed.112.107359. [DOI] [PubMed] [Google Scholar]


