Abstract
Purpose
The hypothesis that outdoor exposure might protect against myopia has generated much interest, although available data find only modest clinical efficacy. We tested the effect of outdoor rearing on form-deprivation myopia in chicks, a myopia model markedly inhibited by high-intensity indoor laboratory lighting.
Methods
Unilaterally goggled cohorts of White Leghorn chicks were maintained in a species-appropriate, outdoor rural setting during daylight hours to the extent permitted by weather. Control chicks were reared indoors with incandescent lighting. Besides ocular refraction and ultrasound, we determined dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) content in retina and vitreous and measured mRNA expression levels of selected clock and circadian rhythm-related genes in the retina/RPE.
Results
Myopia developed in the goggled eyes of all cohorts. Whereas outdoor rearing lessened myopia by 44% at 4 days, a protective effect was no longer evident at 11 days. Outdoor rearing had no consistent effect on retinal or vitreous content of dopamine or DOPAC. Conforming to prior data on form-deprivation myopia, retina and vitreous levels of DOPAC were reduced in goggled eyes. Compared with contralateral eyes, the retinal expression of clock and circadian rhythm-related genes was modestly altered in myopic eyes of chicks reared indoors or outdoors.
Conclusions
Outdoor rearing of chicks induces only a partial decrease of goggle-induced myopia that is not maintained, without evidence that retinal dopamine metabolism accounts for the partial myopia inhibition under these outdoor conditions. Although modest, alterations in retinal gene expression suggest that studying circadian signals might be informative for understanding refractive mechanisms.
Keywords: myopia, refraction, form deprivation, outdoors, weather, dopamine, DOPAC, clock genes, circadian rhythms, retina, vitreous
Despite evidence for a genetic contribution,1,2 the rapidly increasing myopia prevalence in many societies3–5 implies environmental and/or behavioral influences. However, the roles of many long-presumed causes of myopia, such as intensive reading, have become uncertain with modern epidemiology.1
Several experimental methods induce myopia in vertebrates that manifest anatomical and optical parallels to human myopia.6 A widely studied technique, blurring the retinal image by wearing an image-diffusing goggle reliably induces ipsilateral myopia in laboratory animals (so-called form-deprivation myopia). Chicks have excellent vision, and investigating chicks has proved useful in identifying biological mechanisms that have subsequently been recognized in human refractive development.7 The retina is now thought largely to control refractive development, and specific retinal neurons and signaling mechanisms are being identified that potentially govern postnatal refraction or cause myopia.6,7
A subject of much research, the retinal levels and release of the amacrine cell neurotransmitter dopamine increase during the day relative to night, and the daytime increase is inhibited in experimental myopia in laboratory settings with rearing under a light:dark cycle.8,9 As currently hypothesized, retinal dopamine seemingly interacts with the signaling pathway linking retinal activity to the regulation of eye growth and refraction, and disruption in diurnal dopamine cycling is thought to contribute to myopia development.8,9
Elevating the intensity of laboratory lighting above typical vivarium levels (e.g., 10,000–40,000 vs. 300 lux) markedly inhibits form-deprivation myopia. Although protocols vary, bright laboratory lighting inhibited form-deprivation myopia by at least 57% and sometimes completely in chicks.10–13 Bright laboratory lighting also reduced the mean myopic response in form-deprived tree shrews by almost 40%14 and blocked myopia from developing in six of eight form-deprived rhesus monkeys.15 It has been suggested that elevated light levels in schools also might exert an anti-myopia effect in children16; however, the light intensity of 100 lux of the control environment and the elevated light intensity of only some 400–600 lux in the intervention group are both low and complicate interpreting the mechanism of this initial clinical study. Nonetheless, the inhibition of form-deprivation myopia by bright laboratory lighting has revived clinical ideas first proposed in the 19th century that exposures to outdoors, perhaps from its high intensity light, might protect children against myopia.3,17–21
Most available contemporary cross-sectional human studies indeed suggest that increased time outdoors may protect against myopia onset.3,21 However, the effect is modest in magnitude, amounting in pooled analysis to some 2% reduced odds of myopia per hour per week outdoors; a few cross-sectional studies also have not found an anti-myopia effect of outdoor exposures.3,21 While varying in design, the few available prospective clinical studies also have yielded equivocal findings about the protective effects of outdoor exposures. Two studies found that increasing outdoor time in schoolchildren inhibited myopia onset by 9% and could be clinically significant if continuing and persisting to maturity.22,23 However, reduced progression of established myopia from outdoor exposures either was not demonstrable24,25 or has been disappointingly small. Of the two favorable prospective studies of the effects of outdoor exposure with cycloplegic refractions, one found 0.13 diopters (D) less myopic progression at 1 year, confounded by the concurrent use of atropine among both groups.23 The other found reduced progression of the myopic refraction by 0.056 D/y, but no statistically significant slowing of the rate of axial elongation over the 3 years of the study.22 Another prospective 10-month study found a modest slowing of axial growth of children with greater daily bright exposure amounting to 0.04–0.07 mm/y comparing the highest to the other two exposure levels.26 Unfortunately, cycloplegic refractions were not reported,26 but this level of axial growth slowing corresponds to some 0.11–0.19 D/y, assuming that a change of 1.0 mm in axial length corresponds to 2.7 D of power.27 Despite the enthusiasm accompanying these findings,28 the modest anti-myopia activity of outdoor exposures in available studies raises many questions about the long-term efficacy and possible persistence of effects from this interventional strategy.29 Reducing either myopia incidence and/or its progression would be clinically important, and developing behaviorally based mechanistic understandings and validated clinical therapies are attractive goals. The reports on the anti-myopia effects of outdoor exposures speculate that increased retinal dopamine metabolism and/or dopamine release by high intensity light may be the underling biological mechanism.
The limited anti-myopia efficacy of outdoor exposures in children contrasts with the more marked inhibition by bright indoor laboratory lighting against form-deprivation myopia described above in laboratory animals. This discrepancy suggests that bright artificial laboratory lighting may not be a suitable surrogate for outdoor lighting in studying juvenile eye development. The visual and light experience of indoor and outdoor activities are quite different.30 Further, many developmental, neural, and other responses can be dramatically affected by shifting experimental subjects from the laboratory setting to seminatural or field conditions.31 Accordingly, we directly tested a potential anti-myopia effect of outdoor exposures in chicks with unilateral form-deprivation myopia reared in a “real world” species-appropriate outdoor environment. We included refraction, ultrasound, biochemical, and molecular biology measurements.
Materials and Methods
Induction of Myopia
White Leghorn chicks (Charles River Laboratories, North Franklin, CT, USA) were housed at the avian facilities of New Bolton Center (Kennett Square, PA, USA). Located in a rural area, New Bolton Center is the large animal facility of the University of Pennsylvania's School of Veterinary Medicine. One of the chicken houses at New Bolton Center was designated as the indoor location. Outdoor light entered this chicken house through three plexiglass windows, facing east or south. Immediately after delivery, chicks were maintained within one of three identical custom-made portable cages, designed to fit through the door of the chicken house. Chicks were provided food (Purina Lab Chick Chow S-G #5065; PMI Nutrition International (LabDiet/TestDiet), St. Louis, MO, USA) and water ad libitum. Light intensities for the chicks was estimated with a lux meter positioned at chick eye level.
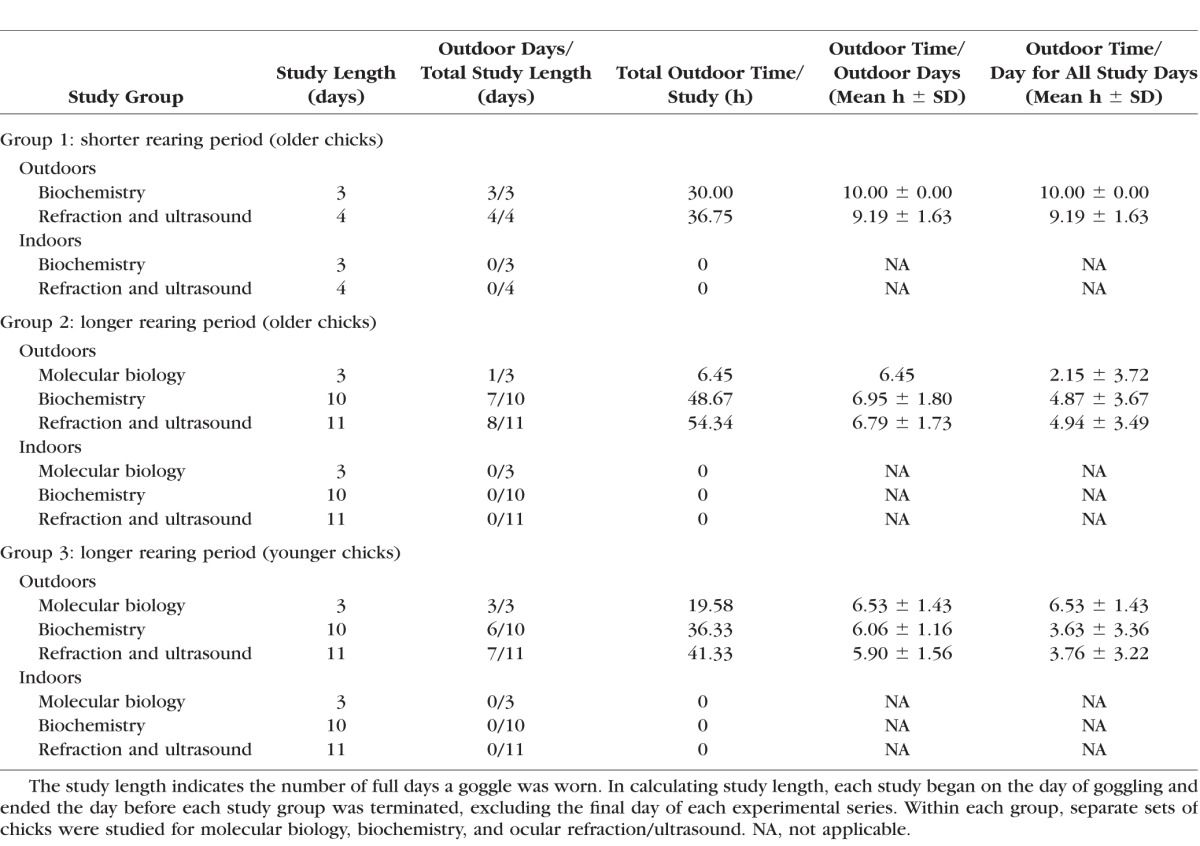
When the chicks were 5 or 9 days of age, we induced form-deprivation myopia by securing translucent white plastic goggles to one eye using cyanoacrylate glue, alternating the experimental eye between left and right. Even though we used a method that reliably secured goggles in the vivarium environment, a number of chicks lost their goggles in this study perhaps because of the high ambient humidity of the Eastern Pennsylvania summers. Any chick who lost a goggle was removed from the study, accounting for variable chick numbers in the outcome data. The chicks were anesthetized with intramuscular ketamine (20 mg/kg) and xylazine (5 mg/kg) for goggle applications and eye examinations. Two chicks in each study did not receive a goggle, as additional controls for molecular biology. Within each study group, separate sets of chicks were studied for retinal biochemistry, for retinal molecular biology, or for refraction/ultrasonography on the days indicated (Table 1). The studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Table 1.
Outdoor Exposure Durations of Chicks Raised Outdoors and the Corresponding Cohorts Reared Indoors
Outdoor Rearing
For outdoor cohorts, the cages with chicks were moved between indoors and outdoors from the designated chicken house. Initially kept indoors, the chicks were first placed outdoors on the day they received a goggle. The cages were maintained outside for as long as possible each day after goggling, with limits imposed by weather, ambient temperature, and personnel schedules. When outside, the chicks were placed on a grassy area approximately 8 ft in front of the chicken house, with views that included fields, stables, fences, distant buildings, a wooded region, and occasional horses. Reflective plexiglass panels, 3–6 in. wide, were secured to one end of each cage to provide a limited shaded area for chicks to move in and out of direct sunlight. To casual observation, the chicks did not show preference for sunny or shaded locations. Each night, the cages were moved inside the chicken house for temperature control and for protection against nocturnal predators or possible rain. During the day, the cages also were moved inside if needed to protect the chicks from rain or cool outside temperatures. When inside the chicken house, each cage was placed under one of the windows.
Light intensity varied considerably during these experiments. The intensity of direct sunlight on a clear day typically is considered to be 100,000–120,000 lux, although the upper limit of our lux meter was 20,000 lux. Outside under cloudy conditions, the illuminance at chick eye level measured from 1500 to 3300 lux depending on cloud thickness and the direction of gaze. Inside the chicken house during the day, light levels were less regular and were affected by the position of the sun throughout the day, the cloud cover density and gaze direction. On a rainy day, the inside illuminance at chick eye level measured from 10 to 3300 lux. When the chicks were inside because of cool outside temperatures as distinct from rain, approximately one-third of each cage potentially received several hours of direct sunlight in the morning or mid-day when there was no cloud cover.
For chicks in the outdoor cohorts, no artificial lights were used inside the chicken house during either day or night; at night, only natural outdoor lighting (starlight and moonlight) was transmitted through the windows, which were noncovered. Mounted from the ceiling of the chicken house was a temperature-regulated propane-fired brooder to provide heat as needed, depending on the ambient temperature. When ignited to generate heat, the brooder's flame delivered approximately 0.2–0.3 lux at chick eye level.
On the final experimental day, the chicks were moved to the Avian Medicine Division's laboratory and maintained outside in their cages: in the sun if possible or, if raining, on a landing with open sides and a roof adjacent to the laboratory. In isolating retina/RPE for biochemistry or molecular biology, the chicks were selected in random order; and the dissections began when the chicks had received at least 4 hours of outdoor light exposure and continued until the all dissections for the day were completed approximately 2 hours later.
Outdoor Exposures and the Pennsylvania Weather
Typically, the weather in Pennsylvania is quite variable, even within a single day. To illustrate the variability of the sky and associated weather, we summarized hourly meteorologic data (cloud cover, wind properties, and precipitation events) during each day for one of the outdoor rearing periods (Supplementary Fig. S1). Further, the need to protect chicks from rain and cold affected the outdoor exposure durations between days and cohorts. When reared in a vivarium for 5 days, 2 h/day of intense laboratory lighting (15,000 lux) had no protective effect on form-deprivation myopia in chicks; but 5 h/day of exposure to intense lighting inhibited form-deprivation myopia by 70%, with longer exposures having no additional effect.13 Further, increasing myopia protection in chicks correlates with increased laboratory light intensity.12 Despite the unavoidable daily variability of outdoor exposures in our study groups, the high sunlight intensity during outdoor exposures, the number of outdoor days, and the mean hours spent outdoors on outdoor days (Table 1) all support the notion that the outdoor exposures attained here are a useful test of a potential influence of a natural outdoor environment on refraction.
Indoor Rearing Conditions
The cohorts reared indoors were age matched to the outdoor cohorts and experienced the same protocols, except for the locations for rearing and the type of light exposure. The same cages were kept inside the same chicken house. Before the chicks were delivered for indoor rearing, the windows were blocked with black plastic and remained blocked continuously for the experiments' durations. For these chicks, the only natural light inside the chicken house comprised slight light leaks through a ceiling vent and an occasional crack in the wall; light leaks from outside sources measured <0.1 lux at chick eye level at mid-day. Before and after receiving a goggle, the indoor control cohorts were maintained on their respective light:dark cycles with a single 100-W incandescent light bulb suspended above the center of each cage; the illuminance measured 190–510 lux at chick eye level, depending on the chick's location in the cage. The incandescent lighting was maintained on a light:dark cycle, with the onset/offset timing closely matching the sunrise/sunset times of the corresponding outdoor cohorts (Table 1). With the cage placement for the indoor cohorts, the brooder heater delivered approximately 0.1 lux at chick eye level when ignited. The indoor cohorts were not exposed to outdoor lighting at any time during the entire protocol, including their transfer on the final day to the Avian Medicine laboratory where the cages were maintained under an incandescent bulb that delivered ∼500 lux to chick eye level at the cage center. The timing of retina/RPE dissections for biochemistry or molecular biology from indoor reared chicks followed as closely as possible the protocols for the outdoor reared chicks.
Assays for Retinal Dopamine and 3,4-Dihydroxyphenylacetic Acid
After 3 or 10 days of goggle wear, chicks were decapitated without anesthesia (11–20 chicks per cohort). The eyes were rapidly enucleated, placed on ice and opened just anterior to the equator. On opening, clear liquid (presumably liquid vitreous, extracellular choroidal fluid, and perhaps aqueous humor) drained from the eye as collected in a previous study32; for simplicity, this fluid is termed “liquid vitreous.” The volume of liquid vitreous was measured. The formed vitreous gel was removed and is termed the “vitreous gel.” The retina/RPE was excised from the eyecup, and each preparation was placed in preweighed screw-top tubes. The liquid vitreous, the vitreous gel, and the retina/RPE were frozen individually on dry ice as quickly as possible and stored on dry ice for shipping to Emory for further processing. There, tubes containing samples were reweighed to estimate the wet weight of the samples, and the contents of dopamine and its principal metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) were determined as described.33 External standards of dopamine and DOPAC were analyzed in each experiment.
Retinal Molecular Biology Assays
After 3 days of goggle wear, chicks were decapitated without anesthesia (six experimental plus two control chicks per cohort). The eyes were rapidly enucleated, placed on ice, and were opened just anterior to the equator. The retina/RPE was excised, immediately frozen on dry ice, and subsequently stored in liquid nitrogen until processed for RNA isolation and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as described.34 Based on prior findings in experimental lens-induced myopia,34 we used qRT-PCR to assay expression of the following clock and circadian rhythm-related genes: ARNTL (aryl hydrocarbon receptor nuclear translocator-like protein 1; also known as BMAL1), CLOCK (circadian locomotor output cycles kaput), NPAS2 (neuronal PAS domain-containing protein 2) PER3 (period 3), and CRY1 (cryptochrome-1) and the transcript expression of OPN4 (melanopsin) and MTNR1A (melatonin receptor 1A). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a housekeeping gene. The efficiencies of the expressions of GAPDH and the genes of interest were determined to be the same, and GAPDH expression was unaltered across the eyes within each experimental cohort. For primers, we purchased QuantiTect Primer Assays (Qiagen, Germantown, MD, USA): Gg_ARNTL_1_SG; Gg_CLOCK_1_SG; Gg_NPAS2_1_SG; Gg_PER3_2_SG; Gg_CRY1_1_SG; Gg_OPN4_1_SG; Gg_MTNR1A_1_SG; and Gg_GAPDH_1_SG.
Ocular Measurements
After 4 or 11 days of goggle wear, ocular measurements were obtained on anesthetized chicks as described (10–20 chicks per cohort).35 Three independent measurements of refraction and ultrasonography were obtained on each eye, with the data analysis and results utilizing the mean values. Ocular refractions, measured with a calibrated Hartinger-type refractometer, are reported in diopters as spherical equivalents (sphere plus one-half cylinder). Axial dimensions were measured with A-scan ultrasonography. These chicks then received CO2 euthanasia.
Statistical Methods
Ocular measurements and assays for retinal dopamine and DOPAC were summarized by rearing location (i.e., daytime outdoor exposure versus strict indoor rearing) and reported as means and SEMs for the goggled eyes, the contralateral eyes, and the differences between the goggled and contralateral eyes. Bar graphs were used to describe the distributions of the differences between the goggled and contralateral eyes for each parameter, rearing location, and experimental group. Retinal molecular biology assays were reported as the ratio of the goggled to contralateral eye and were summarized in scatterplots. Two-sample t-tests were used to test the differences between rearing locations within an experimental group, and paired t-tests were used to test the differences between goggled and contralateral eyes within a rearing location. We calculated the Pearson correlation coefficient to assess the strength of the linear relationship between the differences in refraction and the differences in axial and vitreous cavity lengths. Individual parameters were treated as independent variables, all tests were two-sided, and P < 0.05 was considered significant.
Results
Refraction and Ocular Growth
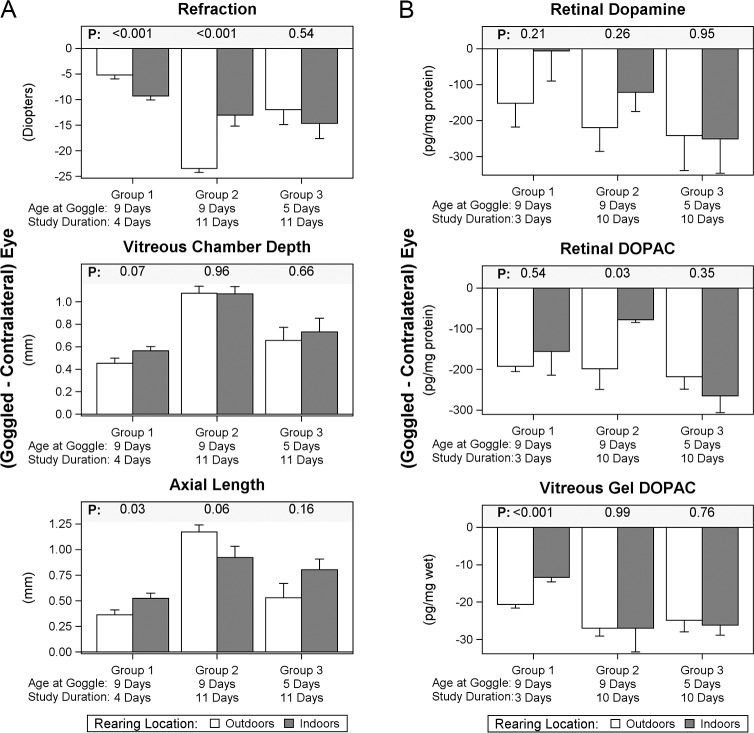
Myopic refractions developed in the goggled eyes of all cohorts of chicks (Fig. 1A). In the cohorts goggled at 9 days of age, outdoor rearing reduced the mean induced myopia by approximately 4.1 D in experimental versus contralateral eyes (44% reduction) at 4 days compared with the indoor-reared control cohort (group 1). By 11 days, however, the chicks reared outdoors actually had 10.4 D greater relative myopia than contralateral eyes (80% more) than those reared indoors (group 2). In the cohorts goggled at 5 days of age, the induced myopia was equivalent at 11 days in the indoor and outdoor cohorts (group 3). Supplementary Table S2 contains complete refraction and ultrasound data.
Figure 1.
Effects of rearing location on ocular measurements and biochemical assays. (A) Ocular measurements. Refraction (top) and ultrasound results (middle and bottom) are shown for the differences between goggled and contralateral nongoggled eyes. (B) Dopamine and DOPAC assays. The differences between goggled minus nongoggled eyes in each of the experimental groups are shown for retinal dopamine (top), retinal DOPAC (middle), and vitreous gel DOPAC (bottom). Complete refraction, ultrasound, and biochemical data for all studies, including sample sizes and liquid vitreous DOPAC levels, appear in Supplementary Tables S1 and S2. Means ± SEM. P values compare intereye differences for corresponding outdoor versus indoor cohorts; two-sample t-test.
By ultrasound, unilateral form deprivation induced elongation of the vitreous chamber and overall greater axial length in the goggled versus nongoggled eyes of all cohorts, whether reared indoors or outdoors (Fig. 1A). Conforming to the refractive effects after 4 days of goggle wear (group 1), the goggle-induced axial elongation in the outdoor cohort was less than that for the cohort reared strictly indoors, with a similar trend for the outdoor cohort to develop less vitreous chamber elongation than the indoor cohort. At 11 days of goggle wear, however, there were no statistically significant differences in the vitreous chamber lengthening, but the cohort with greater myopia in the outdoor cohort showed a trend toward longer axial length (group 2). There were no differences in the vitreous chamber and axial length responses comparing outdoor with indoor rearing in the cohorts goggled at the younger age (group 3).
To assess these ocular responses further, we estimated the correlations between changes in refraction and changes in axial length or vitreous cavity depth, the major anatomical changes accounting for experimental myopias. For the entire study, there was a strong linear relationship between the changes in refraction and the changes in both axial length (r = −0.77; P < 0.001) and vitreous cavity length (r = −0.71; P < 0.001). For individual cohorts, the group goggled at the younger 5 days of age reared indoors or outdoors (group 3), and the chicks goggled at the older age and reared indoors (groups 1 and 2) also showed moderate or strong correlations between changes in refraction and changes in axial length or vitreous cavity depth (r = −0.59 to −0.77; P= 0.07–0.002). In contrast, both cohorts goggled at the older 9 days of age and reared outdoors (groups 1 and 2) behaved differently, showing weak correlations for these comparisons (r = −0.26 to −0.31; P values, not significant). These differences may imply that coordinated growth of the ocular components may vary with age and rearing location.
Of the other components of refraction, inconsistent changes in anterior chamber depth or lens thickness were observed in some, but not all, cohorts (Supplementary Table S2). In assessing the effect of location on anterior chamber depth differences between goggled and contralateral eyes, only group 2 chicks demonstrated an effect of outdoor versus strictly indoor location. The deeper anterior chamber in this outdoor-reared group may have resulted from corneal steepening and/or posterior displacement of the lens. Not measured here, greater corneal curvature could have contributed to their greater myopia. If the deeper anterior chamber resulted only from posterior lens displacement, however, it would have altered the net refraction by approximately +2 D,36 shifting the refraction away from myopia and not toward it. Outdoor rearing had no effect on lens thickness.
Retinal Dopamine and DOPAC Biochemistry
Summaries of the dopamine and DOPAC levels appear in Tables 2 and 3 and Figure 1B; Supplementary Table S3 provides complete data. For dopamine and DOPAC, the essential questions are (1) whether outdoor rearing affects their content either within goggled (i.e., myopic) eyes or within contralateral nongoggled control eyes compared with their levels with indoor rearing and (2) whether outdoor rearing alters the biochemical response occurring in goggled myopic eyes relative to contralateral nongoggled eyes.
Table 2.
Effect of Rearing Location on Dopamine and DOPAC Levels
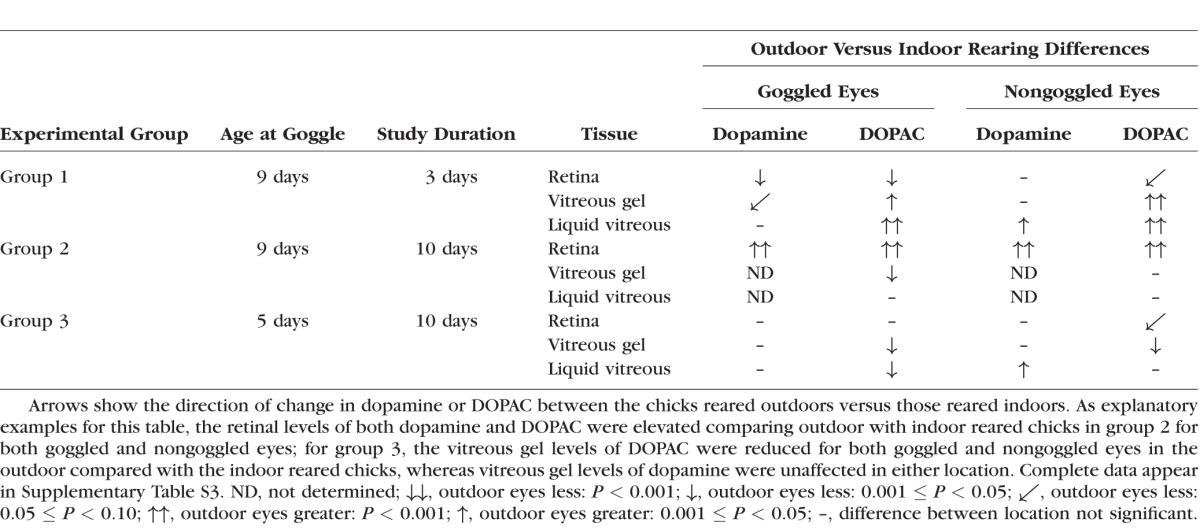
Table 3.
Effect of Goggle Wear on Dopamine and DOPAC Levels Within Chicks
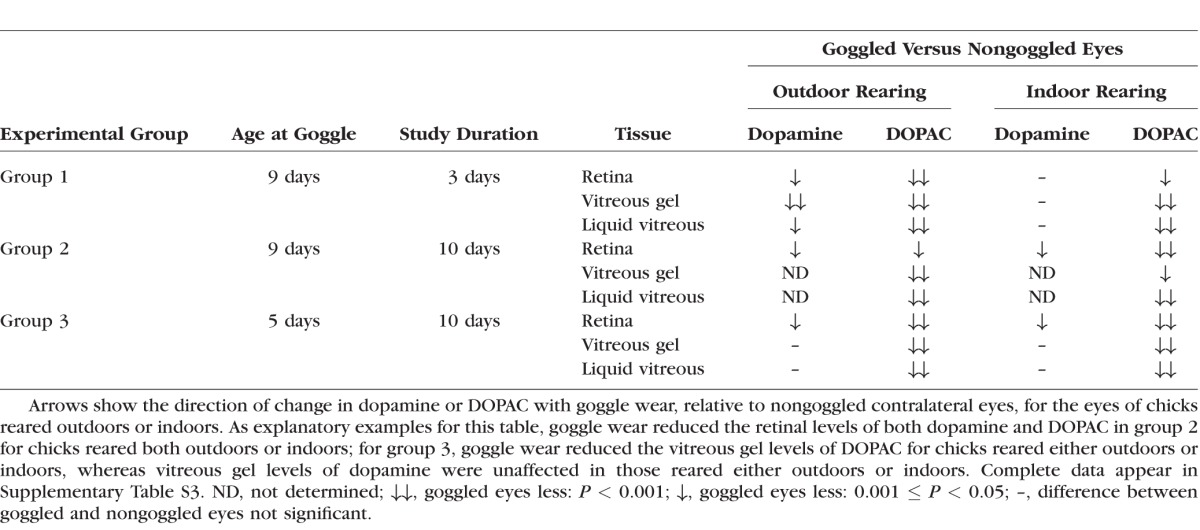
The effect of rearing location on levels of dopamine and DOPAC varied considerably between tissues, chick age, duration of goggle wear, and whether the eyes were goggled or not (Fig. 1B; Table 2; Supplementary Table S3). The retinal levels of both dopamine and DOPAC were elevated in group 2 chicks reared outdoors compared with those reared indoors, but the retinal content of each was either reduced or unaffected by outdoor rearing in the other two groups (Table 2; Supplementary Tables S3A, B). Technical problems in the assays impaired complete assessments of the vitreous dopamine levels in group 2; these specific data are omitted, but vitreous DOPAC levels for group 2 are reported. The dopamine level of the vitreous gel of goggled eyes was depressed in the outdoor cohort of group 1, but there were no other statistically significant effects in the available dopamine levels of the vitreous gels comparing rearing locations (Table 2; Supplementary Table S3C). Relative to indoor rearing, the dopamine levels in the liquid vitreous of nongoggled eyes of chicks reared outdoors were elevated in the two available samples (groups 1 and 3) but were unaffected by rearing location in the goggled eyes (Table 2; Supplementary Table S3E). The levels of the more robust marker, DOPAC, in both vitreous samples was elevated outdoors in group 1, but DOPAC was reduced or unaffected in the other two groups (Table 2; Supplementary Tables S3D, F). These variabilities thus preclude identifying a consistent influence of outdoor versus indoor rearing on these biochemical parameters for goggled or for nongoggled eyes.
In contrast, goggle wearing consistently reduced DOPAC levels in the retina and in both vitreous samples in all groups compared with contralateral nongoggled eyes (Fig. 1B; Table 3; Supplementary Tables S3B, D, F). While not uniformly reaching statistical significance in all comparisons, dopamine levels in goggled eyes also tended to be reduced relative to their contralateral eyes, particularly in the eyes of chicks reared outdoors (Fig. 1B; Table 3; Supplementary Tables S3A, C, E). For retina and vitreous assays, the tendencies of the ratios of goggled/nongoggled eyes to be more depressed for DOPAC than for dopamine conforms to prior reports.8,9
Retinal/RPE Gene Expression
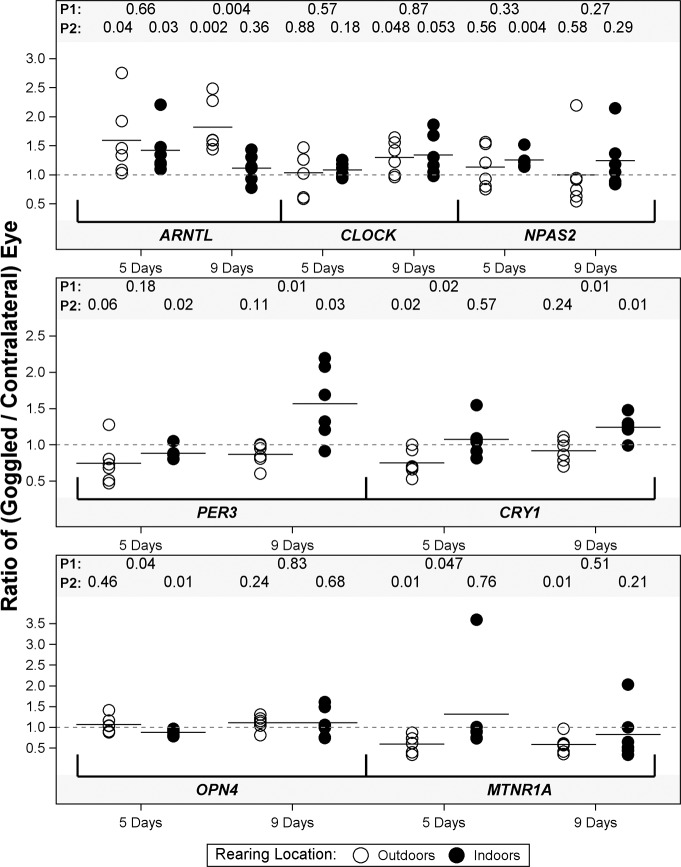
After 3 days of goggle wear, we used qPCR to study the retinal/RPE expression of five clock genes (ARNTL, CLOCK, NAPS2, PER3, and CRY1) and two circadian rhythm-related genes (OPN4 and MTNR1A).34 As previously noted in experimental myopias,37 the alterations in retinal gene expression were small in magnitude when comparing either cohorts reared indoors versus outdoors or goggled versus nongoggled eyes (Fig. 2). Altered retinal/RPE expression of each of these genes developed under one or more conditions, but inconsistent results between cohorts precluded other general conclusions from these data.
Figure 2.
Gene expression in the combined retina/RPE. The ratio of mRNA expression in the retina/RPE for the goggled versus contralateral control eye is shown for individual birds goggled at either 5 or 9 days of age and assayed after 3 days of goggle wear following either outdoor or indoor rearing. Six chicks were assayed for each cohort. We assayed the mRNA expression of the clock genes ARNTL (aryl hydrocarbon receptor nuclear translocator-like protein 1), CLOCK (circadian locomotor output cycles kaput), NPAS2 (neuronal PAS domain-containing protein 2) PER3 (period 3), and CRY1 (cryptochrome-1) and the transcript expression of OPN4 (melanopsin) and MTNR1A (melatonin receptor 1A) using qRT-PCR. Horizontal bars, mean ratio for each cohort; P1, P values comparing outdoor versus indoor cohorts using independent sample t-tests with log transformations; P2, P values comparing the goggled versus contralateral eyes within each cohort using paired t-tests with log transformations.
Differences in the expression levels for indoor versus outdoor cohorts were noted for five genes (ARNTL, PER3, CRY1, OPN4, and MTNR1A) at one or both ages of goggle application; however, the direction of the effect of rearing location depended on the age at goggle application and on the specific gene being analyzed (Fig. 2). For chicks goggled at 5 days of age, the gene expression level was lower for CRY1 and MTNR1A but was higher for OPN4 in the retina/RPE of chicks reared outdoors compared with those reared indoors. For chicks goggled at 9 days of age, the retinal/RPE expression level was lower for PER3 and CRY1 and was higher for ARNTL for chicks reared outdoors compared with those reared indoors.
In addition, goggle wear affected the retinal/RPE expression of each of the assayed genes relative to that of contralateral nongoggled eyes, but the effect of goggle wear depended on the age at goggle application, the rearing location, and the specific gene (Fig. 2). For chicks goggled at 5 days of age, the gene expression level not significantly different for CLOCK, was reduced for PER3, CRY1, OPN4, and MTNR1A, and was elevated for ARNTL and NPAS2 in the retina/RPE of goggled eyes relative to that of contralateral nongoggled eyes for chicks reared outdoors and/or indoors. For chicks goggled at 9 days of age, the gene expression level was reduced for MTNR1A but was elevated for ARNTL, CLOCK, PER3, and CRY1 in the retina/RPE of goggled eyes compared with contralateral nongoggled eyes for chicks reared outdoors and/or indoors.
Discussion
The inhibitory activity of intense laboratory lighting against form-deprivation myopia in experimental animals,14 including chicks,10 and the long-held hypothesis that outdoor exposures might inhibit childhood myopia3,17–21 motivated this investigation. We found that outdoor exposures exerted only partial and transient inhibition of form-deprivation myopia in chicks (Fig. 1A). In fact, one outdoor cohort had greater myopia than its corresponding indoor-reared cohort (Fig. 1A, group 2). The anatomical hallmark of both clinical and experimental myopia is excessive axial length of the eye, mostly from vitreous chamber elongation. Similarly, there was somewhat less axial growth stimulation and a trend toward less vitreous cavity elongation in goggled eyes reared outdoors after 4 days, but any “protective” effect of outdoors on these dimensions did not persist at 11 days. More frequent sampling than possible in the present studies is needed to determine whether slower progression or delayed onset accounted for the transient myopia inhibition. The extent to which uncontrollable variations in the weather between cohorts influenced the results cannot be determined from the present study design.
Most laboratory protocols find partial inhibition of form-deprivation myopia from high intensity lighting after 4–7 days of goggle wear in chicks10–13 and after 11 days of form deprivation in tree shrews14; these results are analogous to our outdoor findings in chicks after 4 days of goggle wear. The highest indoor laboratory light intensity of 40,000 lux studied in chicks for 7 days completely inhibited onset and progression of goggle-induced myopia.12 In rhesus monkeys, exposure to 25,000 lux completely blocked form-deprivation myopia in six monkeys but not in two other animals in the same report.15 Given the differences between the 4- and 11-day outdoor cohorts in our study, more research is needed to understand the relation of outdoor rearing to the intensity and duration of high intensity lighting indoors.
Well established for indoor rearing, unilateral goggle wear in chicks reared outdoors also induces a robust ipsilateral myopia response. The refractions in form-deprivation myopia are expected to demonstrate a more variable distribution than those of control eyes,38 and we observed higher variability in the refractions of goggled eyes than in their contralateral eyes (Supplementary Table S2A). Nonetheless, the induction of myopia in all cohorts confirms that a visual feedback mechanism regulates refractive development in a “real world” species-appropriate outdoor environment,6,39 even though the precise properties of the visual or optical signals regulating eye growth outdoors remain controversial.30
There are a number of uncertainties in our findings. We cannot explain the high myopia response in the outdoor cohort goggled at 9 days of age (group 2) or the weaker correlations between refractive changes and axial/vitreous chamber lengths in the older chicks reared outdoors. There exist no prior comparable outdoor experiments in chicks for comparison or perspective; and besides age, perhaps some yet-to-be defined feature of the outdoor environment may contribute to the excessive myopia or weaker correlations. Because corneal power was not measured, we cannot conclude whether outdoor rearing or any of the complexities of a natural environment independently affect corneal morphology in either form-deprived or contralateral control eyes.
Another experimental paradigm, the wearing of a minus spectacle lens, stimulates ipsilateral ocular growth to maintain the focal position of distant images at the retinal photoreceptors; the eye demonstrates a myopic refractive error with vitreous cavity and axial elongation when the minus lens is removed. While showing analogous anatomical changes and other similarities, form-deprivation and lens-induced myopias differ in the visual stimulus to eye growth (i.e., blur versus defocus) and in a number of other properties.40,41 Pertinent to the present study, bright vivarium lighting slows the rate of compensation to minus lenses but does not alter the final compensation to the imposed defocus in either chicks42 or tree shrews.14 In monkeys reared under a light:dark cycle, interrupting typical vivarium lighting with high intensity laboratory lighting for 6 h/day did not alter the compensation to −3.0 D lens wear.43 On the other hand, in a more complex protocol, interrupting vivarium lighting with an outdoor exposure of 3 h/day inhibited the compensation of monkeys to −3.0 D lens wear44; whether the incomplete compensation in this latter study represents a reduced myopic response from an outdoor exposure or an incomplete compensation to defocus (i.e., failure of emmetropization to imposed defocus) is an inherent ambiguity in the lens-induced myopia model.
Retinal dopamine modulates many diurnal phenomena in the retina, and both light and the biological clock regulate the daily cycling of dopamine levels and release.45 Because retinal dopamine also impacts the signaling mechanism linking vision to refractive development,8 many of the publications addressing refractive effects of high intensity laboratory lighting or of outdoor exposures in children3,10–13,21 have speculated that increased retinal dopamine metabolism and release might provide a mechanism for any purported anti-myopia action.
To our knowledge, the current study is the first to measure retinal and vitreous dopamine/DOPAC in experimental myopia under species-appropriate outdoor conditions. We assayed retinal dopamine and DOPAC and also two different preparations previously used for vitreous DOPAC content, a robust marker for dopamine release from retina.32,46 Relative to indoor rearing, the effects of outdoors on dopamine/DOPAC were complex and variable within and between groups, with no consistent evidence that outdoor exposures per se consistently increase retinal dopamine or its release in goggled or nongoggled eyes, despite sample sizes that we found adequate in our previous studies of chicks reared in vivaria (Supplementary Table S3). The depression of DOPAC levels in retina and both vitreous preparations were similar in goggled versus contralateral nongoggled eyes in each cohort, whether the chicks were reared inside or outside and including the group with the briefest goggle wear where goggle-induced myopia was partially inhibited outdoors. In contrast to the results here, bright laboratory lighting partly blunted the reduction in vitreous DOPAC levels following monocular goggle wear in chicks.47 Dissimilar effects of outdoor exposures versus bright indoor lighting, as well as differences such as chick age or sampling time, are considerations that perhaps contributed to the differences from our study. For outdoor rearing, we thus confirm the many past reports of reduced retinal dopamine turnover in experimental myopia.8,9 We conclude that retinal dopamine metabolism outdoors is consistent with form-deprivation myopia, not with its inhibition, and we question a role for retinal dopamine in any inhibitory effect of outdoor exposures on myopia.
In contrast to the more readily controlled setting of laboratory housing, uncontrollable and changing environmental conditions complicate interpreting the mechanisms of biological responses in natural conditions,31 such as the present study. The Pennsylvania weather is very variable, even within a single day (Supplementary Fig. S1), and cannot be predicted reliably before planning outdoor experiments. Besides the changeability of Pennsylvania weather shown in Supplementary Figure S1, other differences of outdoor exposures to vivarium rearing include temperature, humidity, gradual light-dark transitions, and light spectral composition, including ultraviolet light. Rather than a limitation of our study, however, uncontrollable environmental features should be viewed as core strengths in seeking relevance to children outdoors. The interactions of the sun's movement with the cloud cover, the shifting spectrum of outdoor light throughout the day,48 the pronounced effects of gaze direction on light intensity, the changeability of the weather, the need for shelter to protect against inclement weather, and the complexities of the visual scene30 are all features that would influence outdoor visual experiences of both experimental animals and children. None of these parameters are fully controllable in “real world” situations. As found in other research areas,31 our results do not fully substantiate current translational hypotheses from the laboratory; and natural research settings may provide an alternative means to develop hypotheses to test refraction mechanisms in children.
The contemporary epidemiology on environmental exposures in myopia, despite uncertainties and contradictions,30,49 does suggest that some feature(s) of the outdoor environment might be protective against myopia,3,21,50 but the protective feature(s) appears not to be participation in outdoor sporting activities.51 The available prospective studies have been conducted in children during the years that refractive errors develop and have not yet stabilized. The modest favorable results from these relatively shorter-term clinical investigations could reflect delayed onset, reduced myopia end point, slowed progression and/or truly reduced occurrence, as these effects could be manifested by reduced clinical myopia at an intermediate developmental stage. Because we find reduced myopia magnitude only at an intermediate stage in a robust chick myopia model with proven clinical utility, understanding whether outdoor exposures exert meaningful long-term protective effects on myopia in children will require considerably more investigation.29 The current study clearly points to conundrums in available clinical data.
Related to outdoor exposures, experimental and clinical investigations increasingly address the effects of light. The contemporary lighting patterns in developed societies can weaken or otherwise distort circadian entrainment signals.52 Indoor artificial daytime lighting differs from outdoor lighting by low intensity and restricted chromaticity.53 At night, artificial indoor lighting shortens the dark phase and provides lighting intensity above natural night. Medical problems from potential light-related circadian disruptions are being increasingly recognized.54–57
We previously identified altered retinal/RPE expression of clock and circadian rhythm-related genes in lens-induced myopia,34 and we included those genes in the current study. In lens-induced myopia with vivarium rearing, the retinal/RPE expression levels of each clock and circadian rhythm-related gene were modestly reduced in goggled eyes relative to nongoggled contralateral eyes. Despite the low magnitude and nonuniform directions of the altered gene expression levels in the assays here, the present findings in form-deprivation myopia expand prior results from lens-induced myopia.34 While the assay techniques were parallel in the two studies, practical constraints of conducting the outdoor experiments mandated some procedural differences in assay timings relative to lens or goggle application in the two studies. It is not known whether differences in timing, differences in the visual effects of goggle versus lens wear, differences in the rearing environments or some yet undefined parameter could account for nonuniform or only partially overlapping results comparing our gene expression studies. Nonetheless, the expression changes, identified here in each gene chosen for assay, further buttress the hypothesis that the endogenous retinal clock may participate in the mechanism regulating postnatal eye development,7 although the clock's precise role in refractive development is unknown.
Whether anti-myopia therapies directed toward augmenting circadian signals to the retina might prove more robust than the available results of outdoor exposure is unknown and requires direct study. Although we found only a minimum protective effect of the outdoors against form-deprivation myopia in chick, the role of light as the primary zeitgeber for circadian entrainment, the roles of the retina in both light sensing and refractive development, the increasing recognition of medical effects from weakened circadian signals from contemporary lighting patterns, the exploding myopia prevalence in developed societies and the evolving evidence implicating circadian rhythm-related signals in refraction all suggest that studying circadian phenomena may prove productive in better understanding eye development.
Supplementary Material
Acknowledgments
The authors thank Jiayan Huang for assistance in the preliminary data analysis and Frank Schaeffel and Marita P. Feldkaemper for helpful discussions.
Supported by National Institutes of Health Grants R01 EY022342, R01 EY016435, R01EY004864, and P30EY006360; the Department of Veterans Affairs; a Rehab R&D Service Research Career Scientist Award (MTP), and the Paul and Evanina Bell Mackall Foundation Trust (RAS) and Research to Prevent Blindness.
Disclosure: R.A. Stone, Santen, Inc. (R), P; Y. Cohen, None; A.M. McGlinn, None; S. Davison, None; S. Casavant, None; J. Shaffer, None; T.S. Khurana, Guidepoint Global (R); M.T. Pardue, None; P.M. Iuvone, None
References
- 1. Sherwin JC,, Mackey DA. Update on the epidemiology and genetics of myopic refractive error. Expert Rev Ophthalmol. 2013; 8: 63–87. [Google Scholar]
- 2. Wojciechowski R,, Hysi PG. Focusing in on the complex genetics of myopia. PLoS Genet. 2013; 9: e1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. French AN,, Ashby RS,, Morgan IG,, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 4. Koh V,, Yang A,, Saw SM,, et al. Differences in prevalence of refractive errors in young Asian males in Singapore between 1996–1997 and 2009–2010. Ophthalmic Epidemiol. 2014; 21: 247–255. [DOI] [PubMed] [Google Scholar]
- 5. Vitale S,, Sperduto RD,, Ferris FL., III. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009; 127: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 6. Wallman J,, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 7. Stone RA,, Pardue MT,, Iuvone PM,, Khurana TK. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013; 114: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldkaemper M,, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 9. Stone RA,, Lin T,, Laties AM,, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashby R,, Ohlendorf A,, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354. [DOI] [PubMed] [Google Scholar]
- 11. Backhouse S,, Collins AV,, Phillips JR. Influence of periodic vs continuous daily bright light exposure on development of experimental myopia in the chick. Ophthalmic Physiol Opt. 2013; 33: 563–572. [DOI] [PubMed] [Google Scholar]
- 12. Karouta C,, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2015; 56: 299–309. [DOI] [PubMed] [Google Scholar]
- 13. Lan W,, Feldkaemper M,, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS One. 2014; 9: e110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norton TT,, Siegwart JT., Jr. Light levels, refractive development, and myopia—a speculative review. Exp Eye Res. 2013; 114: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EL, III,, Hung L-F,, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hua W-J,, Jin J-X,, Wu X-Y,, et al. Elevated light levels in schools have a protective effect on myopia. Ophthal Physiol Opt. 2015; 35: 252–262. [DOI] [PubMed] [Google Scholar]
- 17. Carter RB. Eyesight: Good and Bad. London: Macmillan and Co.; 1880.
- 18. Cohn HL. The Hygiene of the Eye in Schools. Turnbull WP, London: Simpkin, Marshall and Co.; 1886. [Google Scholar]
- 19. Harman NB. The Eyes of our Children. London: Methuen & Co. Ltd.; 1916. [Google Scholar]
- 20. Hobday R. Myopia and daylight in schools: a neglected aspect of public health? Perspect Public Health. 2016; 136: 50–55. [DOI] [PubMed] [Google Scholar]
- 21. Sherwin JC,, Reacher MH,, Keogh RH,, Khawaja AP,, Mackey DA,, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012; 119: 2141–2151. [DOI] [PubMed] [Google Scholar]
- 22. He M,, Xiang F,, Zeng Y,, et al. Effect of time spend outdoors at school on the developmentof myopia among children in China: a randomized clinical trial. JAMA. 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 23. Wu P-C,, Tsai C-L,, Wu H-L,, Yang Y-H,, Kuo H-K. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 24. Li S-M,, Li H,, Li S-Y,, et al. Time outdoors and myopia progression over 2 years in Chinese children: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci. 2015; 56: 4734–4740. [DOI] [PubMed] [Google Scholar]
- 25. Scheiman M,, Zhang Q,, Gwiazda J,, et al. Visual activity and its association with myopia stabilisation. Ophthal Physiol Opt. 2013; 34: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Read SA,, Collins MJ,, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 27. Bennett AG,, Rabbetts RB. Clinical Visual Optics. 2nd ed. Oxford, UK: Butterworth Heinemann, Ltd.; 1989: 485–501. [Google Scholar]
- 28. Dolgin E. The myopia boom. Nature. 2015; 519: 276–278. [DOI] [PubMed] [Google Scholar]
- 29. Repka MX. Prevention of myopia in children. JAMA. 2015; 314: 1137–1139. [DOI] [PubMed] [Google Scholar]
- 30. Flitcroft DI. The complex interactions of retinal optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 31. Calisi RM,, Bentley GE. Lab and field experiments: are they the same animal? Horm Behav. 2009; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 32. Cohen Y,, Peleg E,, Belkin M,, Polat U,, Solomon AS. Ambient illuminance retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 33. Pozdeyev N,, Tosini G,, Li L,, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008; 27: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stone RA,, McGlinn AM,, Baldwin DA,, Tobias JW,, Iuvone PM,, Khurana TS. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci. 2011; 52: 5765–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stone RA,, Lin T,, Desai D,, Capehart C. Photoperiod, early post-natal eye growth, and visual deprivation. Vision Res. 1995; 35: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 36. Schaeffel F,, Howland HC. Visual optics in normal and ametropic chickens. Clin Vision Sci. 1988; 3: 83–98. [Google Scholar]
- 37. Stone RA,, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010; 50: 2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flitcroft DI. Is myopia a failure of homeostasis? Exp Eye Res. 2013; 114: 16–24. [DOI] [PubMed] [Google Scholar]
- 39. Wallman J. Retinal control of eye growth and refraction. Prog Retinal Res. 1993; 12: 133–153. [Google Scholar]
- 40. Kee C-s,, Marzani D,, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001; 42: 575–583. [PubMed] [Google Scholar]
- 41. Morgan IG,, Ashby RS,, Nickla DL. Point-counterpoint. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt. 2013; 33: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ashby RS,, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 43. Smith EL, III,, Hung L-F,, Arumugam B,, Huang J. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y,, Ding H,, Stell WK,, et al. Exposure to sunlight reduces the risk of myopia in Rhesus monkeys. PLoS One. 2015; 10: e0127863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zawilska JB,, Bednarek A,, Berezińska M,, Nowak JZ. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem. 2003; 84: 717–724. [DOI] [PubMed] [Google Scholar]
- 46. Megaw P,, Morgan I,, Boelen M. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release. J Neurochem. 2001; 76: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 47. Lan W,, Yang Z,, Feldkaemper M,, Schaeffel F. Changes in dopamine and ZENK during suppression of myopia in chicks by intense illuminance. Exp Eye Res. 2016; 145: 118–124. [DOI] [PubMed] [Google Scholar]
- 48. Björn LO. Natural light. : Björn LO. Photobiology: The Science of Life. Dordrecht, The Netherlands: Klewer Academic Publishers; 2002: 87–93. [Google Scholar]
- 49. Ngo C,, Saw S-M,, Dharani R,, Flitcroft I. Point-counterpoint. Does sunlight (bright lights) explain the protective effects of outdoor activity against myopia? Ophthalmic Physiol Opt. 2013; 33: 368–372. [DOI] [PubMed] [Google Scholar]
- 50. Jones LA,, Sinnott LT,, Mutti DO,, Mitchell GL,, Moeschberger ML,, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones-Jordan LA,, Sinnott LT,, Cotter SA,, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53: 7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wright KP, Jr,, McHill AW,, Birks BR,, Griffin BR,, Rusterholz T,, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013; 23: 1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wyszecki G,, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd ed. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 54. Cho Y,, Ryu SH,, Lee BR,, Kim KH,, Lee E,, Choi J. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015; 32: 1294–1310. [DOI] [PubMed] [Google Scholar]
- 55. Kantermann T,, Roenneberg T. Is light-at-night a health risk factor or a health risk predictor? Chronobiol Int. 2009; 26: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 56. Stevens RG,, Brainard GC,, Blask DE,, Lockley SW,, Motta ME. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am J Prev Med. 2013; 45: 343–346. [DOI] [PubMed] [Google Scholar]
- 57. Zelinski EL,, Deibel SH,, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014; 40: 80–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.